Abstract
Wild relatives of wheat are an outstanding source of resistance to both abiotic and biotic stresses. In the present study, we evaluated the activity of four antioxidant enzymes—superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX) and guaiacol peroxidase (GPX)—along with photosynthetic pigments and shoot biomass in 12 Aegilops–Triticum accessions with different genomic constitutions and two tolerant and sensitive control varieties under well-watered (WW; 90% FC), moderate (MS; 50% FC) and severe (SS; 25% FC) water stress treatments. The analysis of variance for measured traits indicated highly significant effects of the water stress treatments, accessions, and their interactions. The 12 domesticated and wild relatives of wheat exhibited more variability and greater activity in the expression of antioxidative enzymes than cultivated wheats. While domesticated forms of wheat, T. aestivum (AABBDD) and T. durum (AABB) seem to have a functionally active antioxidant mechanism, other accessions with alien genomes—Ae. umbellulata (UU), Ae. crassa (MMDD), Ae. caudata (CC), Ae. cylindrica (DDCC) and T. boeoticum (AbAb)—respond to water stress by increasing enzymatic antioxidants as the dominant mechanism that contributes to the retention of oxidative balance in the cell. Furthermore, abovementioned accessions with alien genomes had higher photosynthetic pigment contents (chlorophyll a, chlorophyll b, total chlorophyll, and carotenoid) under water stress than well-watered conditions. Hence, these accessions could be used in future breeding programs to combine beneficial stress-adaptive characters of alien genomes into synthetic hexaploid wheat varieties in the field, even at limited water supply.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Water deficit is an environmental stress that has dramatic effects on plant growth and agricultural productivity in many parts of the world (Comas et al. 2013), and can simultaneously affect agro-morphological, physiological and physiological changes in plant tissues, which ultimately reduce yield performance (Cochard et al. 2002). To improve crop productivity in drought-prone regions, the various mechanisms of complex plant responses to water deficit need to be understood. The drought-induced production of reactive oxygen species (ROS)—including singlet oxygen (1O2), superoxide (O2−), hydroxyl radical (OH) and hydrogen peroxide (H2O2)—affects cell membranes, proteins and DNA, leading to cell death (Ruelland et al. 2009; Yadav 2010). Plants exposed to drought have some defense strategies to detoxify ROS and protect themselves from the negative effects of oxidative stress by producing different types of antioxidants. Antioxidants can be divided into two major classes: (1) enzymatic [monodehydroascorbate reductase (MDHAR), catalase (CAT), superoxide dismutase (SOD), ascorbate peroxidase (APX), peroxidase (POX), glutathione reductase (GR) and dehydroascorbate reductase (DHAR)], and (2) non-enzymatic [ascorbate acid (AsA), glutathione (GSH), carotenoids and tocopherols], which together alleviate the effects of oxidative stress (Ashraf 2009; Verma et al. 2014). Antioxidants can directly or indirectly induce drought tolerance in wheat. For example, Singh et al. (2012) identified a direct association between sustained yields in tolerant wheat genotypes and better antioxidant activities. In addition, Farooq et al. (2009a) reported that increased levels of antioxidants might improve drought tolerance in wheat by scavenging ROS. Furthermore, the accumulation of soluble phenolic and free proline is a key strategy for reducing stress-induced losses in plants (Farooq et al. 2009a, b). Moreover, increased accumulation of antioxidants in plants under stress conditions will result in ROS scavenging through hydrogen bonding of its hydroxyl groups to phosphate groups of membranes and the polar groups of proteins (Farooq et al. 2017).
The genetic diversity of crop wild relatives (CWR) is ideal for producing new wheat varieties; breeding lines and cultivated varieties have a narrow genetic base for developing drought-tolerant varieties (Pour-Aboughadareh et al. 2017a). The drought tolerance potential of CWR of wheat is being investigated through several drought-adaptive traits including root system architecture (Bektas et al. 2017), cell membrane stability (Suneja et al. 2017), relative water content and stomatal conductance (Pour-Aboughadareh et al. 2017b), water use efficiency (WUE) (Peleg et al. 2005), osmotic adjustment and carbohydrate remobilization (Reynolds et al. 2007), and proline and ABA contents (Kurahashi et al. 2009). Several studies have identified the antioxidant system as a surrogate for breeding selection at the seedling stage (Esfandiari et al. 2007; Osipova et al. 2011; Varga et al. 2012; Arabbeigi et al. 2014; Kong et al. 2014). For instance, Zhang and Kirkham (1994) revealed that T. compactum had higher POD and MDA levels and a less efficient antioxidant system than T. dicoccum, T. dicoccoides, T. carthilicum, T. durum and T. monococcum. In a recent study, Suneja et al. (2017) investigated accessions of progenitor wheats—T. dicoccoides (AABB genome) and Ae. tauschii (DD genome)—in terms of the activity of important ROS scavenging enzymes such as CAT, SOD, GR and APX and related them to plant performance under drought stress. The authors stated that these progenitors would be excellent donors for combining beneficial drought-tolerant traits into a new synthetic hexaploid variety to improve wheat for drought-prone environments.
While some wild relatives and main progenitors of wheat respond well to water stress (refs), there is little information on the status of antioxidant activities of wild relatives in response to water stress. Hence, we investigated the degree of resilience in a set of landrace genotypes and wild relatives of wheat in terms of biomass, physiological parameters and ROS scavenging enzymes.
Materials and methods
Plant materials
This study examined 12 accessions of Triticum and Aegilops species along with two control varieties (T. aestivum cv. Sirvan as a tolerant control and T. aestivum cv. Darya as a sensitive control). The materials were selected based on the results of a previous study that evaluated 180 accessions under well-watered and severe drought-stressed conditions for key physiological and photosynthesis parameters at the seedling stage (Pour-Aboughadareh et al. 2017b). Detailed information on the genomic constitution and sampling regions of these accessions is in Table 1.
Growth conditions and experimental setup
A pot experiment was conducted in a glass house at the Department of Genetics and Plant Breeding, Imam Khomeini International University, Qazvin, Iran (36°19′21″N, 36°19′21″E) during 2016–2017. After breaking seed dormancy, seeds of each accession were sown in plastic pots filled with 3 kg of dry soil and sand. The soil was sieved before use and the pots filled with a soil:sand mixture in a ratio of 3:1. The experimental design was a factorial randomized complete block design with three replications. All accessions were grown from seed in a glasshouse maintained at an optimal photoperiod (16/8 h light/dark cycle) and growing temperature (25/20 °C day/night). The plants were well-watered every 1–2 days to maintain 90 ± 5% field capacity. The water stress treatment was initiated at the three-leaf stage of seedling growth. At this time, the pots were subjected to three water regimes: (1) well-watered (WW, FC = 90 ± 5%); (2) moderate water stress (MS, FC = 50 ± 5%) and (3) severe water stress (SS, FC = 25 ± 5%). The FC was determined by the gravimetric method detailed in Souza et al. (2000). Thirty days after the imposition of watering regimes, one seedling was maintained for recording shoot fresh and dry weights, and leaves of other seedlings were harvested and immediately frozen in liquid nitrogen prior to analysis. Shoot fresh and dry weights, some physiological parameters, and antioxidant enzyme activities were measured.
Shoot fresh weight (SFW), shoot dry weight (SDW) and photosynthetic pigments
The aboveground tissues of a single seedling were harvested, weighted for SFW determination, before being oven dried at 70 °C for 72 h and then weighed for SDW determination. Photosynthetic pigments were measured as described by Arnon (1949). Briefly, lyophilized leaves that harvested from other seedlings (100 mg) were incubated with 20 mL of 80% (v/v) acetone. The solution was centrifuged at 12,000g for 10 min. The supernatant was analyzed by spectrophotometry (4802 UV/VIS UNICO) at 480, 510, 663 and 645 nm to obtain the concentrations of chlorophyll a (Chl a), chlorophyll b (Chl b), total chlorophyll (Chl T), and carotenoid content (CAR) using the following formulas:
where M is the the amount of fresh weight tissue.
Antioxidant assays
Antioxidant enzymes were extracted as described by Kang and Saltveit (2002), with minor modifications. First, 0.5 g of fresh leaf tissue was homogenized at 4 °C in 1 ml of extraction buffer containing 1 mM EDTA, 3 mM MgCl2, 0.05 M Tris–HCl buffer (pH 7.5), and 1.5% w/v PVP. For APX assay, the extraction buffer contained 0.2 mM ascorbate; the homogenate was centrifuged at 12,000g for 20 min. Finally, the supernatant was used as the crude extract for the four enzyme estimations—SOD, APX, CAT, and GPX.
Superoxide dismutase (SOD)
SOD activity was measured by estimating its ability to inhibit photochemical reduction of nitro-blue-tetrazolium (NBT) using the method of Dhindsa et al. (1981). The 1565 µl reaction mixture contained 50 mM phosphate buffer (pH 7.8), 75 µM NBT, 13 mM methionine, 0.1 mM EDTA, 25 µl riboflavin, and 40 µl enzyme extract. According to the methodology described by Kang and Saltveit (2002), riboflavin was added last, and the tubes placed 30 cm below two 15 W fluorescent lamps and shaken for 15 min. The absorbance of the reaction mixture was read at 560 nm.
Ascorbate peroxidase (APX)
APX activity was analyzed as described by Chen and Asada (1989) with minor modifications. The reaction mixture included of 60 µl enzyme extract, 0.1 mM EDTA, 1.54 mM hydrogen peroxide, 1.7 ml phosphate buffer (pH 7.0), and 0.5 mM ascorbate. The oxidation of ascorbate was followed by a decline in absorbance at 290 nm.
Catalase (CAT)
CAT activity was determined following the method of Maehly and Chance (1959) by estimating the rate of disappearance of H2O2 in absorbance at 240 nm. The reaction mixture contained 250 µl of 1% H2O2, 50 µl extracted enzyme and 750 µl phosphate buffer (pH 7.4).
Guaiacol peroxidase (GPX)
GPX activity was measured as described by Upadhyaya et al. (1985). For this assay, the reaction mixture contained 20 µl extracted enzyme, 1.25 mM phosphate buffer (pH 6.1), 0.50 ml of 1% H2O2 and 0.50 ml of 1% guaiacol. The increase in absorbance at 420 nm was followed for 1 min.
Protein content
According to the method described by Bradford (1976), total protein content was measured using BSA (bovine serum albumin). All enzyme activity was estimated per milligram of protein per minute and expressed as a percentage of the control.
Statistical analysis
Analysis of variance (ANOVA) was done by PROC GLM using SAS software (SAS Institute, Cary, NC, USA). Differences among the means of accessions were tested using Duncan’s test. The relative changes caused by water stress on traits were calculated as follows:
where \({\overline {X} _{{\text{ww}}}}\) and \({\overline {X} _{{\text{ws}}}}\) are the means of a trait in a given accession under wellwatered and water stress treatments, respectively (Fayaz and Arzani 2011). To discover relationships between different physiological traits and antioxidant enzyme activities, a PCA-based biplot was drawn using the XLSTAT package (Addisonsoft XLSTAT, Paris).
Results
Water stress decreased shoot biomass and leaf protein content
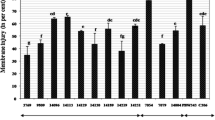
Water stress affected shoot fresh weight (SFW), shoot dry weight (SDW) and leaf protein content, with significant differences among accessions. The interaction between stress treatment and accession was significant (Table 2). Water stress reduced SFWs and SDWs in stressed plants by 20.88 and 33.43% in the MS treatment and 32.71 and 43.92% in the SS treatment, respectively (Table 2). The highest SFWs occurred in T. aestivum, T. durum, Ae. triuncialis and the tolerant control variety in the MS treatment, and Ae. caudata, T. aestivum, T. durum and the tolerant control variety in the SS treatment (Fig. 1a). The highest SDWs occurred in the tolerant control variety followed by T. aestivum, Ae. caudata and T. boeoticum in the MS treatment; and T. aestivum, T. durum, Ae. cylindrica and T. urartu in the SS treatment (Fig. 1b). Average leaf protein content declined by 17.88% in the MS treatment and 21.65% in the SS treatment. T. durum had the highest leaf protein content followed by the tolerant control variety, Ae. speltoides and Ae. umbellulata in the MS treatment, and T. urartu, T. durum, T. boeoticum and the tolerant control variety in the SS treatment (Fig. 4a).
Water stress changed photosynthetic pigments
The water stress treatments significantly affected Chl a, Chl b and Chl T contents and the amount of total CAR (Table 2). Differences in these pigments (except CAR) were observed among accessions. Furthermore, the interaction between water stress treatment and accession was significant for all traits. These photosynthetic pigments decreased as the water stress became more severe. The average Chl a content decreased by 11.38% in the MS treatment, more so in the tolerant control variety, Ae. umbellulata and Ae. triuncialis, and 42.19% in the SS treatment, more so in T. aestivum, T. boeoticum and Ae. umbellulata (Fig. 2a). Water stress reduced Chl b and Chl T contents by 21.86 and 16.14% in the MS treatment and 49.49 and 45.50% in the SS treatment, respectively (Table 2). The highest Chl b contents occurred in Ae. caudata, Ae. crassa, Ae. triuncialis and T. durum in the MS treatment and T. aestivum, T. boeoticum, Ae. triuncialis and Ae. umbellulata in the SS treatment (Fig. 2b). The highest Chl T contents occurred in Ae. caudata, Ae. crassa, Ae. triuncialis and T. durum in the MS treatment and T. boeoticum, T. aestivum, Ae. umbellulata and Ae. triuncialis in the SS treatment (Fig. 3a). Water stress reduced average CAR values by 15.30 and 34.62% in the MS and SS treatments, respectively (Table 2). The highest CAR values occurred in Ae. caudata, Ae. crassa, Ae. triuncialis and T. boeoticum in the MS treatment and T. aestivum, T. boeoticum and Ae. cylindrica in the SS treatment (Fig. 3b).
Water stress improved antioxidant activity
Significant differences occurred in the activity of all antioxidant enzymes between water stress treatments, accessions and their interaction. The water stress treatments significantly increased the antioxidant activities of APX, CAT, SOD and GPX (Table 2). SOD activity increased by 22 and 58% in the MS and SS treatments, respectively. The highest SOD activity occurred in T. boeoticum, Ae. tauschii, Ae. crassa and Ae. caudata in the MS treatment and T. aestivum, Ae. neglecta, Ae. cylindrica and Ae. caudata in the SS treatment (Fig. 4b). APX activity increased by 32 and 36% in the MS and SS treatments, respectively. The highest APX activity occurred in Ae. cylindrica, Ae. umbellulata, Ae. triuncialis and T. boeoticum in the MS treatment and Ae. crassa, Ae. cylindrica, Ae. speltoides and the tolerant control variety in the SS treatment (Fig. 5a). CAT activity increased by 88 and 108% in the MS and SS treatments, respectively. The highest CAT activity occurred in T. aestivum, T. durum, T. boeoticum and Ae. caudata in the MS treatment and Ae. umbellulata, Ae. triuncialis, Ae. neglecta and T. durum in the SS treatment (Fig. 5b). GPX activity increased by 22 and 57% in the MS and SS treatments, respectively. The highest GPX activity occurred in Ae. speltoides, Ae. cylindrica, T. urartu and the tolerant control variety in the MS treatment and Ae. cylindrica followed by Ae. speltoides, T. urartu and Ae. triuncialis in the SS treatment (Table 2; Fig. 6).
Association among physiological and antioxidant traits
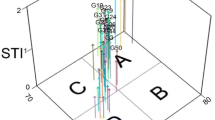
Principal component analysis (PCA) was used to discover relationships between different physiological traits and antioxidant enzyme activities. Associations among traits were considered from the angle between the trait vectors on the biplot. For instance, an acute angle displays a strong positive association, and an obtuse angle indicates a weaker relationship; a 180° angle indicates a negative correlation and a 90° angle indicates no correlation between indices. The results of the PCA-based biplot analyses are shown in Fig. 7a–c. The first two components in Fig. 7a accounted for 56.93% of the total variation in the WW treatment. In this treatment, shoot biomass was strongly correlated with GPX activity and protein content; chlorophyll components (Chl a, Chl b, Chl T) were positively correlated with APX and CAT activities; and CAR had a strong positive correlation with SOD activity. In the MS treatment, the first two components accounted for 48.83% of the total variation (Fig. 7b); all photosynthetic pigments were strongly correlated with each other and with CAT activity; CAT was positively correlated with SFW and SDW, and GPX activity was strongly correlated with SOD activity and CAR. In the SS treatment, the correlation patterns among traits differed from the other two treatments; the first two components accounted for 59.32% of the total variation; there was no strong correlation between photosynthetic pigments and shoot biomass; shoot biomass was weakly associated with protein content, and CAT activity had a strong positive association with GPX activity as did protein content and APX activity (Fig. 7c).
Discussion
Plants undergo a variety of changes when exposed to water deficit stress. In such situations, plants not only experience restrictions in growth and development, but also an increase in ROS that presents itself as a secondary stress. Accordingly, plant cells respond to this oxidative stress by actively scavenging excess ROS and preserving antioxidant defense compounds at levels that reflect ambient cellular metabolism (Suneja et al. 2017). Crop wild relatives are increasingly being used in wheat breeding programs as an ideal source of resistance to both abiotic and biotic stresses (Pour-Aboughadareh et al. 2017c). In this study, a representative set of Aegilops and Triticum accessions were dissected for their photosynthetic pigments and antioxidant potential under water stress at the seedling stage. Our results revealed that water stress treatments decreased shoot fresh and dry biomasses, total protein content and physiological traits in some accessions. In contrast, water stress significantly increased enzymatic activities. Furthermore, the response to water stress differed among accessions (Table 2). SFW increased in T. urartu, Ae. crassa, Ae. tauschii and Ae. umbellulata accessions in the MS and SS treatments, and Ae. speltoides in the MS treatment. SDW increased in Ae. umbellulata in the MS and SS treatments, Ae. crassa in the SS treatment, and Ae. cylindrica (DC genome) in the SS treatment (Table S1 and Fig. 1). Rahbarian et al. (2011) reported that increases in SFW and SDW might be related to a drought-adapted root system, such that more root growth enables water and nutrient uptake from deeper in the soil profile. Wild relatives and progenitors of wheat, specifically Aegilops species, widely studied for morphological characters and root system architecture to better understand the genetic potential of this germplasm. Understanding the relationships between different drought tolerance adaptive mechanisms and above- and below-ground biomass in wheat germplasm may help in the selection of ideal ideotypes for target conditions such as spring or winter growth habits, and short or long seasons (Bektas et al. 2017). Our findings are consistent with previous studies where drought stress increased seedling shoot and root biomass in wild relatives of wheat and barley (Barati et al. 2015; Pour-Aboughadareh et al. 2017b); this increasing trend during drought stress is a possible drought avoidance mechanism (Guo et al. 2002). The reduction in photosynthetic pigments due to water stress is mainly the result of damage to chloroplasts caused by ROS, such as O2 and H2O2, leading to lipid peroxidation, and consequently, chlorophyll destruction (Bouchemal et al. 2016). Several studies have reported damage to photosynthetic pigments as a result of water deficit (Kadkhodaie et al. 2014a, b; Bouchemal et al. 2016). Both Chl a and Chl b are susceptible to drought, which can affect plant performance and grain quality. Because higher chlorophyll contents have been linked to drought tolerance in plants, the selection of individuals based on increased or stable chlorophyll contents may inhibit growth and yield reductions under drought stress (Kadkhodaie et al. 2014a).
In the present study, water-stressed accessions of Ae. triuncialis, Ae. caudata and Ae. crassa had higher Chl a, Chl b and Chl T contents than the control, indicating that these accessions may succeed in future breeding programs. The higher chlorophyll contents under water stress in these accessions may be due to the increase in carotenoid content. Carotenoid plays a fundamental role in response to water deficiency conditions and may help plants to tolerate drought stress (Jaleel et al. 2009). The carotenoid content increased in Ae. triuncialis, Ae. caudata and Ae. crassa in the MS treatment and T. urartu, Ae. caudata, Ae. umbellulata and the tolerant control in the SS treatment, relative to the WW control treatment, suggesting that carotenoids play a role as a non-enzymatic antioxidant in the tolerance of wild wheat species to water deficit. The increased carotenoid content in these accessions is likely associated with the absorption of excessive light to avoid photooxidative damage to PSII (Deng et al. 2003). Furthermore, carotenoids are directly involved in reducing chlorophyll, which inhibits the generation of singlet oxygen and oxidative damage (Deng et al. 2003). Similarly, the carotenoid content in the leaves of other crops increased under water deficit stress (Kadkhodaie et al. 2014a, b; Bouchemal et al. 2016; Vieira et al. 2017).
Protein synthesis and accumulation are involved in water stress tolerance. Dehydrins (Dhn) and late embryogenesis abundant proteins (LEA) are two important protein chaperons that accumulate in response to water deficiency (Ennajeh et al. 2009). However, the extent of accumulation of compatible solutes such as protein and proline varies widely among plant species and genotypes (Kadkhodaie et al. 2014b). In our study, Ae. umbellulata and Ae. speltoides accessions accumulated more protein in their leaves in the MS treatment, and T. urartu accumulated more protein in the SS treatment than the other accessions (Fig. 4a). It appears that increasing protein content during water stress induction is an adaptive mechanism in this germplasm. Antioxidant production increases in response to water deficiency to help plants to cope with drought-induced raised levels of ROS in cells (Sharma et al. 2012). In the present study, the pattern of enzyme activity in the wild relatives of wheat varied under different levels of water stress. Water stress up-regulated the activity of the antioxidative defense system in all accessions and the two control varieties (Table S1). Superoxide dismutase (SOD) plays a central role in the defense against oxidative stress. This biochemical trait is an important parameter for evaluating the water tolerance status of plants (Suneja et al. 2017) and can be used as an indirect selection index for screening drought-resistant plants (Zaefyzadeh et al. 2009). In the current study, the tested accessions varied in their response to water stress. This indicates that different progenitors and wild relatives of wheat have discrete water stress thresholds. The highest SOD activity occurred in T. aestivum, Ae. tauschii and Ae. neglecta (MS and SS treatments), Ae. cylindrica (SS treatment) and T. boeoticum (MS treatment), which might protect them from water deficit (Fig. 4b).
Guaiacol peroxidase (GPX) is another major enzyme that has a key role in the defense against stress factors by scavenging H2O2 in chloroplasts and biosynthesizing lignin (Gill and Tuteja 2010). In our study, the highest GPX activity occurred in T. boeoticum, Ae. speltoides, Ae. triuncialis and Ae. crassa in both water stress treatments (MS and SS) (Table S1 and Fig. 6). Catalase (CAT) turns over rapidly in leaf cells, particularly under stress conditions, and is critical for the scavenging of H2O2 formed in the peroxisomes by photorespiration (Noctor et al. 2002). Higher CAT activity will reduce H2O2 levels in cells by breaking it down directly to form oxygen and water, and improve CO2 fixation and membrane stability because several enzymes in the Calvin cycle are very sensitive to H2O2 (Esfandiari et al. 2007). In our study, CAT activity increased in T. aestivum, T. boeoticum, Ae. crassa and Ae. caudata in the MS treatment and T. urartu, Ae. umbellulata, Ae. caudata and the tolerant control variety in the SS treatment (Table S1 and Fig. 5b). The variation in redox status in the wild relatives of wheat revealed different capabilities of these accessions to deal with oxidative damage by increasing enzyme activity (Pouresmael et al. 2015). In other words, the higher GPX and CAT activities in the accessions listed above revealed a superior capacity for H2O2 scavenging compared with the other accessions. Consistent with our results, several studies showed higher levels of GPX and CAT activities in response to water stress than the control (Mafakheri et al. 2010; Pouresmael et al. 2015; Suneja et al. 2017; Vieira et al. 2017). Furthermore, in a study conducted by Sheptovitsky and Brudvig (1996), CAT activity was associated with the photosystem II (PSII) membrane, which may be important to protect chloroplast proteins from damage and stabilize photosynthetic activities. In line with this result, our previous study (Pour-Aboughadareh et al. 2017b) showed that among cultivated varieties and their wild relatives, Ae. caudata and T. urartu had the highest photosynthetic activity, and were identified as superior candidate species in response to severe drought conditions. Ascorbate peroxidase is an important element of the ascorbate–glutathione (AsA–GSH) cycle, with a significant role in the control of intracellular ROS levels (Sharma et al. 2012). This enzyme is widely distributed in plant cells, and different isoforms are more efficient in scavenging H2O2 under stressful environments (Sharma et al. 2012). In our study, the highest APX activity occurred in Ae. umbellulata, Ae. tauschii, Ae. crassa and Ae. caudata in the MS treatment and T. durum, Ae. speltoides, Ae. crassa and Ae. caudata in the SS treatment (Table S1 and Fig. 5a). In a recent study on the activity of key ROS scavenging enzymes in a set of wheat progenitors, including diploid and tetraploid accessions, Suneja et al. (2017) found high levels of APX activity in Ae. tauschii in response to drought stress.
The PCA analysis identified correlations between enzyme activities and other measured traits. The significant positive association of CAT, SOD, and CAR with shoot biomass under water stress is noteworthy (Fig. 7). Modification of antioxidant enzyme expression due to environmental variability may help to sustain cellular homeostasis and maintain steady-state levels of ROS (Jaleel et al. 2009). Biochemical along with physiological plasticity demonstrated in the form of enzyme induction may contribute to improved performance under drought conditions.
Conclusion and prospects
Although we cannot claim that the differences in each trait are due to species, the main goal of the present study was to disclose the physiological diversity among selected accessions of wild relatives of wheat, and identify some alien genomes for more comprehensive studies. There is also limited knowledge on antioxidant activities and photosynthesis responses among the Triticeae tribe; hence, our work addressed interspecies variability. We showed that seedlings of the cultivated genotypes and their wild progenitors differed in their physiological and biochemical traits in response to water stress, and depended on the genomic constitution of the germplasm, thus confirming the existence of a genetically determined tolerance to water deficiency stress among different wild relatives of wheat. Consequently, some wild relatives that have key desirable genes and alleles are more adaptable for improving drought tolerance in cultivated wheat. The wild accessions used in this study exhibited more variability and greater activity in the expression of antioxidative enzymes than the cultivated wheats. While domesticated forms of wheat, T. aestivum and T. durum, seem to have a functionally active antioxidant mechanism that works constitutively to maintain cellular redox balance, other accessions with alien genomes, such as Ae. umbellulata (U genome), Ae. crassa (M D), Ae. caudata (C genome), Ae. cylindrica (DC genome) and T. boeoticum (Ab genome), respond to water stress by increasing enzymatic antioxidants as the dominant mechanism contributing to the retention of oxidative balance in the cell. We surmise that species-level differences among these wild relatives might result from different adaptation apparatuses developed over time to cope with unique aspects of environmental factors and local management. Hence, genetic variability in these physiological and biochemical traits could be exploited by crossing wild accessions with modern genotypes to develop new synthetic varieties. These neo-hexaploid forms will combine valuable stress-adaptive features of alien genomes (U, C, Ab, DM, and DC) into a unique genetic background. Such elite genetic material could be used in chromosome localization, genome mapping and cloning of new stress-responsive genes or alleles for deployment in commercial wheat varieties.
Author contribution statement
JA, AP and SF conceived the experiment. AM collected the seeds of the plant material. JA, as the team leader, supervised the process. AP and SF set up the experiment. AP and JA analyzed data and wrote the manuscript. KHMS critically reviewed the manuscript. All authors have read and approved the final manuscript.
Abbreviations
- WW:
-
Well-watered treatment
- MS:
-
Moderate water stress
- SS:
-
Severe water stress
- Chl a :
-
Chlorophyll a
- Chl b :
-
Chlorophyll b
- Chl T :
-
Total chlorophyll
- CAR:
-
Total carotenoid
- Pro:
-
Protein content
- SOD:
-
Superoxide dismutase
- CAT:
-
Catalase
- APX:
-
Ascorbate peroxidase
- GPX:
-
Guaiacol peroxidase
- SWF:
-
Shoot fresh weight
- SDW:
-
Shoot dry weight
References
Arabbeigi M, Arzani A, Majidi MM, Kiani R, Tabatabaei BES, Habibi F (2014) Salinity tolerance of Aegilops cylindrica genotypes collected from hyper-saline shores of Uremia Salt Lake using physiological traits and SSR markers. Acta Physiol Plant 36:2243–2251. https://doi.org/10.1007/s11738-014-1602-0
Arnon DI (1949) Copper enzymes in isolated chloroplasts, polyphenoxidase in Beta vulgaris. Plant Physiol 24:1–15. https://doi.org/10.1104/pp.24.1.1
Ashraf M (2009) Biotechnological approach of improving plant salt tolerance using antioxidants as markers. Biotechnol Adv 27:84–93. https://doi.org/10.1016/j.biotechadv.2008.09.003
Barati M, Majidi MM, Mirlohi A, Pirnajmodini F, Sharif-Moghaddam N (2015) Response of cultivated and wild barley germplasm to drought stress at different developmental stages. Crop Sci 55:2668–2681. https://doi.org/10.2135/cropsci2015.04.0229
Bektas H, Hohn CE, Waines JG (2017) Characteristics of the root system in the diploid genome donors of hexaploid wheat (Triticum aestivum L.). Genet Resour Crop Evol 64:1641–1650. https://doi.org/10.1007/s10722-016-0462-4
Bouchemal K, Bouldjadj R, Belbekri MN, Ykhlef N, Djekoun A (2016) Differences in antioxidant enzyme activities and oxidative markers in ten wheat (Triticum durum Desf.) genotypes in response to drought, heat and paraquat stress. Arch Agron Soil Sci 63:710–722. https://doi.org/10.1080/03650340.2016.1235267
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Chen GX, Asada K (1989) Ascorbate peroxidase in tea leaves: occurrence of two isozymes and the differences in their enzymatic and molecular properties. Plant Cell Physiol 30:987–998. https://doi.org/10.1093/oxfordjournals.pcp.a077844
Cochard H, Coll L, Roux XL, Amegilo T (2002) Unraveling the effects of plant hydraulics on stomatal closer during water stress in walnut. Plant Physio 128:282–290. https://doi.org/10.1104/pp.010400
Comas LH, Becker SR, Cruz VMV, Byrne PF, Dierig DA (2013) Root traits contributing to plant productivity under drought. Front Plant Sci 4:1–16. https://doi.org/10.3389/fpls.2013.00442
Deng X, Hu ZA, Wang HX, Wen XG, Kuang TY (2003) A comparison of photosynthetic apparatus of the detached leaves of the resurrection plant Boea hygrometrica with its non-tolerant relative Chirita heterotrichain response to dehydration and rehydration. Plant Sci 165:851–861. https://doi.org/10.1016/S0168-9452(03)00284-X
Dhindsa RS, Plump-Dhindsa P, Thorpe TA (1981) Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J Expt Bot 32:93–101. https://doi.org/10.1093/jxb/32.1.93
Ennajeh M, Vadel AM, Khemira H (2009) Osmoregulation and osmoprotection in the leaf cells of two olive cultivars subjected to severe water deficit. Acta Physiol Plant 31:711–721. https://doi.org/10.1007/s11738-009-0283-6
Esfandiari E, Shakiba MR, Mahboob SA, Alyari H, Toorchi M (2007) Water stress, antioxidant enzyme activity and lipid peroxidation in wheat seedling. J Food Agric Environ 11:1916–1922
Farooq M, Aziz T, Wahid A, Lee DJ, Siddique KHM (2009a) Chilling tolerance in maize: agronomic and physiological applications. Crop Pasture Sci 60:501–516. https://doi.org/10.1071/CP08427
Farooq M, Wahid A, Kobayashi N, Fujita D, Basra SMA (2009b) Plant drought stress: effects, mechanisms and management. Agron Sustain Dev 29:153–188. https://doi.org/10.1051/agro:200802
Farooq M, Hussain M, Nawaz A, Lee D, Alghamdi S, Siddique HMK (2017) Seed priming improves chilling tolerance in chickpea by modulating germination metabolism, trehalose accumulation and carbon assimilation. Plant Physiol Biochem 111:274–283. https://doi.org/10.1016/j.plaphy.2016.12.012
Fayaz N, Arzani A (2011) Moisture stress tolerance in reproductive growth stages in triticale (X Triticosecale Wittmack) cultivars under field conditions. Crop Breeding Journal 1:1–12
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930. https://doi.org/10.1016/j.plaphy.2010.08.016
Guo G, Liu SHQ, An S, Ren X, Lin RN (2002) Effect of limited water supply on root growth and development of winter wheat and the characters of soil moisture use before planting. J Appl Meteorol 13:621–626
Jaleel CA, Manivannan P, Wahid A, Farooq M, Somasundaram R, Panneerselvam R (2009) Drought stress in plants: a review on morphological characteristics and pigments composition. Int J Agri Biol 11:100–105
Kadkhodaie A, Razmjoo J, Zahedi M, Pessarakli M (2014a) Selecting sesame genotypes for drought tolerance based on some physiochemical traits. Agron J 106:111–118. https://doi.org/10.2134/agronj2013.0260
Kadkhodaie A, Zahedi M, Razmjoo J, Ressarakli M (2014b) Changes in some anti-oxidative enzymes and physiological indices among sesame genotypes (Sesamum indicum L.) in response to soil water deficits under field conditions. Acta Physiol Plant 36:641–650. https://doi.org/10.1007/s11738-013-1442-3
Kang HM, Saltveit ME (2002) Antioxidant enzymes and DPPH radical scavenging activity in chilled and heat shocked rice (Oryza sativa L.) seedling radicles. J Agric Food Chem 50:513–518. https://doi.org/10.1021/jf011124d
Kong L, Wang F, Si J, Feng B, Zhang B, Li S, Wang Z (2014) Increasing in ROS levels and callose deposition in peduncle vascular bundles of wheat (Triticum aestivum L.) grown under nitrogen deficiency. J Plant Interac 8:109–116. https://doi.org/10.1080/17429145.2012.712723
Kurahashi Y, Terashima A, Takumi S (2009) Variation in dehydration tolerance, ABA sensitivity and related gene expression patterns in d-genome progenitor and synthetic hexaploid wheat lines. Intl J Mol Sci 10:2733–2751. https://doi.org/10.3390/ijms10062733
Maehly AC, Chance B (1959) The assay of catalase and peroxidase. In: Glick D (ed) Methods of Biochemical Analysis. Inter science, New York, pp 357–425
Mafakheri A, Siosemardeh A, Bahramnejad B, Struik PC, Sohrabi Y (2010) Effect of drought stress on yield, proline and chlorophyll contents in three chickpea cultivars. Aust J Crop Sci 4:580–585
Noctor G, Veljovic-Jovanovic S, Driscoll S, Novitskaya L, Foyer CH (2002) Drought and oxidative load in the leaves of C3 plants: a predominant role for photorespiration. Ann Bot 89:841–850. https://doi.org/10.1093/aob/mcf096
Osipova SV, Permyakov AV, Peryakova MD, Pshenichnikova TA, Borner A (2011) Leaf dehydroascorbate reductase and catalase activity is associated with soil drought tolerance in bread wheat. Acta Physiol Plant 33:2169–2177. https://doi.org/10.1007/s11738-011-0756-2
Peleg Z, Fahima T, Abbo S, Krugman T, Nevo E, Yakir D, Saranga Y (2005) Genetic diversity for drought resistance in wild emmer wheat and its ecogeographical associations. Plant Cell Environ 28:176–191. https://doi.org/10.1111/j.1365-3040.2005.01259.x
Pour-Aboughadareh A, Ahmadi J, Mehrabi AA, Moghaddam M, Etminan A (2017a) Evaluation of agro-morphological diversity in wild relatives of wheat collected in Iran. J Agr Sci Tech 19:943–956
Pour-Aboughadareh A, Ahmadi J, Mehrabi AA, Etminan A, Moghaddam M, Siddique KHM (2017b) Physiological responses to drought stress in wild relatives of wheat: implications for wheat improvement. Acta Physiol Plant 39:106. https://doi.org/10.1007/s1173
Pour-Aboughadareh A, Mahmoudi M, Moghaddam M, Ahmadi J, Mehrabi AA, Alavikia SS (2017c) Agro-morphological and molecular variability in Triticum boeoticum accessions from Zagros Mountains, Iran. Genet Resour Crop Evol 64:545–556. https://doi.org/10.1007/s10722-016-0381-4
Pouresmael M, Khavari-Nejad RA, Mozafari J, Najafi F, Moradi F (2015) Diverse responses of tolerant and sensitive lines of chickpea to drought stress. Arch Agron Soil Sci 61:1561–1580. https://doi.org/10.1080/03650340.2015.1017721
Rahbarian R, Khavari-Nejad R, Ganjeali A, Bagheri A, Najafi F (2011) Drought stress effects on photosynthesis, chlorophyll fluorescence and water relations in tolerant and susceptible chickpea (Cicer arietinum L.) genotypes. Acta Biol Cracov Bot 53:47–56. https://doi.org/10.2478/v10182-011-0007-2
Reynolds M, Dreccer F, Trethowan R (2007) Drought adaptive traits derived from wheat wild relatives and landraces. J Exp Bot 58:177–186. https://doi.org/10.1093/jxb/erl250
Ruelland E, Vaultier MN, Zachowski A, Hurry V (2009) Cold signalling and cold acclimation in plants. Adv Bot Res 49:35–150. https://doi.org/10.1016/S0065-2296(08)00602-2
Sharma P, Jha AB, Dubey RS, Pessarakli M (2012) Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot 217037:1–26. https://doi.org/10.1155/2012/217037
Sheptovitsky YG, Brudvig GW (1996) Isolation and characterization of spinach photosystem II membrane-associated catalase and polyphenol oxidase. Biochemistry 35:16255–16263. https://doi.org/10.1021/bi9613842
Singh S, Gupta AK, Kaur N (2012) Differential responses of antioxidative defence system to long-term field drought in wheat (Triticum aestivum L.) genotypes difference in drought tolerance. J Agron Crop Sci 198:185–195. https://doi.org/10.1111/j.1439-037X.2011.00497.x
Souza CC, Oliveira FA, Silva IF, Amorim Neto MS (2000) Evaluation of methods of available water determination and irrigation management in “terra roxa” under cotton crop. Rev Bras Eng Agr Amb 4:338–342. https://doi.org/10.1590/S1415-43662000000300006
Suneja Y, Gupta AK, Bains NS (2017) Bread wheat progenitors: Aegilops tauschii (DD genome) and Triticum dicoccoides (AABB genome) reveal differential antioxidative response under water stress. Physiol Mol Biol Plants 23:99–114. https://doi.org/10.1007/s12298-016-0409-4
Upadhyaya A, Sankhla D, Davis TD, Sankhla N, Smith BN (1985) Effect of paclobutrazol on the activities of some enzymes of activated oxygen metabolism and lipid peroxidation in senescing soybean leaves. J Plant Physiol 121:453–461. https://doi.org/10.1016/S0176-1617(85)80081-X
Varga B, Janda T, Laszlo E, Veisz O (2012) Influence of abiotic stresses on the antioxidant enzyme activity of cereals. Acta Physiol Plant 34:849–858. https://doi.org/10.1007/s11738-011-0882-x
Verma KK, Singh M, Gupta RK, Verma CL (2014) Photosynthetic gas exchange, chlorophyll fluorescence, antioxidant enzymes, and growth responses of Jatropha curcas during soil flooding. Turk J Bot 38:130–140. https://doi.org/10.3906/bot-1212-32
Vieira EA, Silva MG, Moro CF, Laura VA (2017) Physiological and biochemical changes attenuate the effects of drought on the Cerrado species Vatairea macrocarpa (Benth.) Ducke. Plant Physiol Biochem 115:472–483. https://doi.org/10.1016/j.plaphy.2017.04.022
Yadav SK (2010) Cold stress tolerance mechanisms in plants. A review. Agron Sustain Dev 30:515–527. https://doi.org/10.1051/agro/200905
Zaefyzadeh M, Quliyev RA, Babayeva SM, Abbasov MA (2009) The effect of the interaction between genotypes and drought stress on the superoxide dismutase and chlorophyll content in durum wheat landraces. Turk J Biol 33:1–7. https://doi.org/10.3906/biy-0801-12
Zhang J, Kirkham MB (1994) Drought stress induced changes in activities of superoxide dismutase, catalase and peroxidase in wheat species. Plant Cell Physiol 35:785–779. https://doi.org/10.1093/oxfordjournals.pcp.a078658
Acknowledgements
This research was supported by the Iran National Science Foundation (INSF) No. 94010881.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H Peng.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ahmadi, J., Pour-Aboughadareh, A., Ourang, S.F. et al. Wild relatives of wheat: Aegilops–Triticum accessions disclose differential antioxidative and physiological responses to water stress. Acta Physiol Plant 40, 90 (2018). https://doi.org/10.1007/s11738-018-2673-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-018-2673-0