Abstract
The results of the current study represent the first report on an efficient regeneration protocol for Hypericum tomentosum L. hairy root cultures. Six out of ten hairy root clones of H. tomentosum obtained by Agrobacterium rhizogenes-mediated transformation differentiated shoots on Murashige and Skoog medium containing a urea-based cytokinin thidiazuron in combination with the auxin inhibitor p-chlorophenoxyisobutyric acid. The whole plant regeneration of this species in vitro was achieved by further cultivation of shoots on medium containing benzyladenine. All transformed plants were successfully acclimated to ex vitro conditions. Most of the adapted clones exhibited typical hairy root phenotype with stunted growth, small wrinkly leaves and shortened internodes. Increased number of dark nodules, the sites of hypericins accumulation, was observed in the leaves of all transgenic clones. The capability of naphthodianthrone production was also modulated leading to a significant 28- and 5-fold increase of total hypericin content in two transgenic clones. The qPCR analysis revealed seven rolC integrations in two transgenic clones and one integration in four clones. The clones with multiple rolC copies synthesized the highest and the lowest amount of naphthodianthrones, respectively. The chromosome number in all analysed samples was determined as 2n = 18 suggesting a revision of the cytogenetic characterization of H. tomentosum.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The genus Hypericum (Hypericaceae) encompasses almost 500 species of herbs, shrubs and trees which are classified into 36 sections (Crockett and Robson 2011). Members of the genus produce a complex of biologically active substances, from among them one of the most remarkable is the naphthodianthrone hypericin. This natural photoactive pigment has been extensively studied for its application in the photodynamic diagnosis and therapy of cancer (Castano et al. 2004; Jendželovská et al. 2016). Hypericin is also known for its wound healing, antimicrobial and anti-inflammatory activities (Öztürk et al. 2007; Huang and Chen 2012; Wölfle et al. 2014). The content of naphthodianthrones depends on harvesting time, temperature, light intensity, photoperiod and nutrient conditions (Couceiro et al. 2006) and varies between genotypes and even between the somaclones of the same genetic origin (Čellárová et al. 1994). Hypericins, comprising hypericin, pseudohypericin, their precursors and derivatives, are accumulated in glandular structures, also called “dark nodules”, occurring on the aboveground organs (Zobayed et al. 2006). Their distribution in hypericin-producing Hypericum species varies as shown in the recent paper of Kucharíková et al. (2016).
The most important and widely studied species of the genus is Hypericum perforatum L. due to its cosmopolitan distribution, the production of a broad spectrum of secondary metabolites and relatively high content of phloroglucinols and naphthodianthrones (Robson 2003; Nahrstedt and Butterweck 1997). However, along with H. perforatum, the pharmacological importance of several other members of this genus was recognized (Stojanović et al. 2013). H. tomentosum L., belonging to the section Adenosepalum Spach., is a perennial herb distributed mainly in the west Mediterranean region. The name “tomentosum” is derived from the densely white hairy appearance of aerial plant parts. Seeing that the content of hypericin in the leaves is relatively low corresponding with the presence of only one or two dark nodules at the leaf apex, the species is becoming interesting for naphthodianthrone elicitation studies during the vegetative period. However, H. tomentosum produces a plethora of dark nodules in the flowering stage (Robson 1996; Kucharíková et al. 2016). The presence of protruding dark nodules on the sepal cilia is the main taxonomic marker for H. tomentosum, distinguishing it from H. pubescens of similar phenotype and partially overlapping distribution. The literature sources also report different chromosome counts for these two species, 2n = 16 for H. tomentosum and 2n = 18 for H. pubescens (Nielsen 1924; Robson 1996; Matzk et al. 2003).
The biosynthetic capacity for secondary metabolites may be affected by transgene insertion as a consequence of Agrobacterium rhizogenes-mediated genetic transformation (Roychowdhury et al. 2013). The integration of rol genes into the plant genome may cause changes in metabolism of plant hormones or hormone signal perception in plant cells (Christey 2001). The rol genes are known to affect sensitivity to auxin which results in the altered “hairy root” phenotype of transgenic plants characteristic by reduced apical dominance, shortened internodes, increased branching, adventitious root production, wrinkled and wider leaves, late flowering, altered morphology of flower parts and impaired pollen and seed production (Spena et al. 1987; Casanova et al. 2005; Christey 2001). Integration of these genes may have an impact on biochemical processes in the transformed cells and result in secondary metabolism activation (Bulgakov 2008). Nevertheless, an efficient transformation procedure requires not only an effective protocol for genetic transformation and selection of transgenic cells, but also transformation conditions which should not interfere with plant regeneration (Birch 1997).
Agrobacterium-mediated transformation in the genus Hypericum is still largely limited (Hou et al. 2016), presumably because of the strong plant defence responses to Agrobacterium infection reducing bacterial viability (Franklin et al. 2008). In spite of this, efficient transformation systems for H. perforatum by A. rhizogenes have already been developed (Di Guardo et al. 2003; Vinterhalter et al. 2006; Tusevski et al. 2013). The hairy roots of H. perforatum regenerate spontaneously (Bertoli et al. 2008; Vinterhalter et al. 2015; Tusevski et al. 2014) and produce similar (Tusevski et al. 2017; Komarovská et al. 2010) or even higher amounts of naphthodianthrones than non-transgenic plants (Koperdáková et al. 2009, Tusevski et al. 2014). To date, up to 12-fold increase of naphthodianthrone content was observed in some of the regenerated transgenic clones of H. perforatum (Tusevski et al. 2014). The transformation of two other Hypericum spp., H. tomentosum and H. tetrapterum, was also achieved (Komarovská et al. 2010). However, in comparison to H. perforatum hairy roots, these cultures were recalcitrant to shoot regeneration. Since the biosynthesis of naphthodianthrones is strictly spatiotemporally regulated (Zobayed et al. 2006), the modulation of hypericins content can be studied only in aboveground parts of the plants. Therefore, the aims of the present study were:
-
1.
to develop a protocol for plant regeneration from H. tomentosum hairy roots previously established in our laboratory which show high level of recalcitrance towards shoot regeneration;
-
2.
to assess the effect of genetic transformation by wild-type A. rhizogenes strains A4 and ATCC 15834 on naphthodianthrone biosynthesis and formation of dark nodules in transgenic H. tomentosum plants;
-
3.
to contribute to the cytogenetic characterization of H. tomentosum by revision of the chromosome number.
Materials and methods
Plant material and culture conditions
Ten hairy root clones of H. tomentosum L. previously derived in our laboratory by Komarovská et al. (2009) via genetic transformation of root explants with wild-type agropine strains of Agrobacterium rhizogenes A4 (A4 clone 2, 5, 6, 7, 8) and ATCC 15834 (ATCC clone 1, 2, 4, 7, 8) were used in the experiments. Standard (non-transgenic) root culture of H. tomentosum was used as control.
The hairy roots were multiplied on solid medium (MS) containing 4.4 g l−1 Murashige–Skoog’s salt mixture (Murashige and Skoog 1962) with Gamborg’s B5 vitamins (Gamborg et al. 1968), 30 g l−1 sucrose, 7 g l−1 agar and 2 mg l−1 glycine with pH value adjusted to 5.6 before autoclaving for 15 min at 120 °C. Standard culture conditions were as follows: temperature 23 ± 1 °C, 30% relative humidity, continuous darkness. The subculture interval was 4–6 weeks.
Shoot regeneration
For shoot initiation, hairy roots from each transgenic clone were cut into 20–30 mm segments (Table 1) and cultured on MS medium containing 2 µmol l−1 thidiazuron (TDZ, Duchefa Biochemie, Haarlem, Netherlands) and 40 or 60 µmol l−1 p-chlorophenoxyisobutyric acid (PCIB, Sigma, St. Louis, Missouri, USA). The segments were kept under the same culture conditions as hairy roots with an exception of 16/8 h (light/dark) photoperiod of 85 µmol m−2 s−1 of PAR (photosynthetically active radiation). The shoot buds that surfaced from hairy root-derived callus were excised and transferred onto MS medium supplemented with 2.2 µmol l−1 6-benzyladenine (BA, Duchefa Biochemie, Haarlem, Netherlands) and further cultivated under the same culture conditions as for shoot induction.
Adaptation to ex vitro conditions
Transgenic (ATCC clone 1,4,7 and A4 clone 2,5,8) and control plantlets with differentiated root system cultivated in vitro were transferred to plastic vessels containing modified Knop’s solution consisting of 1 g l−1 Ca(NO3)2, 0.25 g l−1 KH2PO4, 0.25 g l−1 KNO3, 0.25 g l−1 MgSO4 and 0.01 g l−1 FeCl3. During 2 months, the vessels were gradually opened to lower the humidity from 100% to 30% (the relative humidity in the culture room) and transferred to plastic pots with sterile soil for further acclimation.
Determination of chromosome number
Mitotic chromosomes were counted in squashed preparations of root tips of in vitro cultured regenerated transgenic (ATCC clone 1,4,7 and A4 clone 2,5,8) and control plantlets. Roots were pre-treated with 0.002% (w/v) 8-hydroxyquinoline (Lachema, Brno, Czech Republic) at 4 °C for 4 h, followed by fixation in a mixture of 3:1 ethanol/glacial acetic acid for 16 h and subsequent hydrolysis in 1 mol l−1 HCl at 60 °C for 6 min (Murín 1960). The slides were then stained with 10% (v/v) Giemsa (Fluka, Seelze, Germany), pH 6.8 for 20 min. Three root tips from each transgenic clone and control plantlet were scored for chromosome counts with at least ten metaphases per root tip.
Detection and quantification of transgene integrations
Isolation of genomic DNA from the plants regenerated from each transformed clone and the control was performed according to Saghai-Maroof et al. (1984) by slightly modified cetyltrimethylammonium bromide (CTAB) method. As a negative control, the non-transformed DNA from H. tomentosum and as positive control the plasmid DNA from A. rhizogenes ATCC 15834 were used for polymerase chain reaction (PCR) analysis.
The presence of the rolA,B,C genes in the transformed plants was confirmed by PCR amplification using rolA,B,C-specific primers (Zdravković-Korać et al. 2003; Di Guardo et al. 2003). The amplification mixture of 30 µl total volume for each sample contained 30–50 ng of DNA template, 1 U Taq-DNA-polymerase, 1× PCR buffer with 1.5 mmol l−1 MgCl2, 0.2 mmol l−1 dNTP and 0.4 µmol l−1 of each primer. The PCR mixture was incubated in a DNA thermal cycler (MJ mini™ Personal Thermal Cycler, Bio-Rad, Dubai, United Arab Emirates). The reaction conditions were as follows: initial denaturation at 95 °C for 5 min, 40 cycles (94 °C 30 s, 60 °C 30 s, 72 °C 30 s) and final extension at 72 °C for 7 min. PCR amplification products were analysed by electrophoretic separation on 1.2% (w/v) agarose gel in TAE buffer (0.04 mol l−1 Tris acetate, 0.001 mol l−1 EDTA, pH 8.0) stained with 2.5% (v/v) GoodView (SBS Genetech, Beijing, China) and detected by fluorescence under UV light.
The copy number of integrated rol genes was assessed by quantitative real-time PCR (qPCR) of rolC gene and calculated according to Mason et al. (2002). PCR primers for rolC, GAPDH and TUB were designed according to Di Guardo et al. (2003) and Velada et al. (2014), respectively. The qPCR reactions were set up in 20 µl volume using PowerUp™ SYBR® Green Master Mix (Thermo Fisher Scientific, Waltham, Massachusetts, USA) containing 100 ng of DNA, 0.5 µmol l−1 primers and 1 × Master Mix. The amplification reactions were run on LightCycler® Nano Instrument (Roche Diagnostics GmbH, Mannheim, Germany) and performed as follows: denaturation at 95 °C for 3 min, 40 cycles (95 °C 30 s, 60 °C 30 s, 72 °C 30 s with a single fluorescence measurement), melting curve programme (55–95 °C with a heating rate of 0.1 °C per second and a continuous fluorescence measurement), and final cooling step to 40 °C. Two replicates were carried out for each clone. The relative amount of rolC gene was quantified and normalized to a geometric mean of two reference gene standards (GAPDH, TUB) to count the rolC/GAPDH, TUB ratio. The theoretical value for one integration of rolC (the virtual calibrator r i ) was calculated from the normalized amount of rolC in all transgenic clones. The number of integrations was estimated based on this value (r i rolC = 0.38).
Determination of hypericins and emodin content
The content of naphthodianthrones (protopseudohypericin, pseudohypericin, protohypericin and hypericin = total hypericin content) and their supposed intermediate emodin was determined in the aerial parts of the plants adapted to ex vitro conditions before they reached the stage of flowering by high-performance liquid chromatography (HPLC). The extracts were analysed in three replicates. 50 mg of air-dried homogenized material per sample was extracted with 1.5 ml of ethanol:methanol:acetone (1:1:1) mixture in an ultrasonic water bath for 60 min at 25 °C. The extract was centrifuged for 10 min at 14,000 rpm and 20 °C. The content of secondary metabolites was assessed by HPLC–DAD analysis according to Tolonen et al. (2003) by Agilent 1260 HPLC system (Agilent Technologies, Inc. California, USA). The separation of the substances was performed by means of Agilent Poroshell 120, EC-C18 column (3.0 × 50 mm, 2.7 µm) at the temperature of the column oven 40 °C and the injection volume of 10 µl. Naphthodianthrones and emodin were identified in the extracts of H. tomentosum by comparing the UV spectra of extracts and retention times of 4.3 min for protopseudohypericin, 4.8 min for pseudohypericin, 7.6 min for protohypericin, 8.3 min for hypericin and 4.5 min for emodin with the standards of hypericin (Carl Roth GmbH & Co. KG, Karlsruhe, Germany) and emodin (Fluka Chemie AG, Buchs, Switzerland). The naphthodianthrones were detected at 590 nm and emodin at 440 nm. The content of naphthodianthrones and emodin was calculated from the calibration curve of the respective standards.
Dark nodule analyses
Fifty fresh leaves from each transgenic clone and control adapted to ex vitro conditions (10 leaves from 5 plants) were excised and used for the analyses. Only leaves at the same developmental stage (4th to 5th leaf from the apex) were used for measurements. Dark nodules were observed and documented using Leica EZ4 D photo stereomicroscope (Leica Camera AG, Wetzlar, Germany). The acquired images were further analysed by ImageJ processing tool (NIH, Bethesda, Maryland, USA). Nodule count, average size of a nodule and average nodule coverage on the leaves were determined.
Statistical evaluation
The STATISTICA version 7.0 package program was used for all statistical assessments. The experimental data obtained by image processing (nodule count, average size of a nodule and average nodule coverage on the leaves) of the control plants and the transgenic clones were evaluated by paired t test (p = 0.01). The relationship between total hypericin content and average nodule coverage on the leaves was determined by Pearson’s correlation coefficient (p = 0.05).
Results
Shoot regeneration and plant adaptation to ex vitro conditions
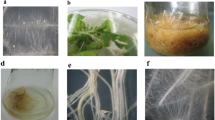
The standard shoot differentiation protocol routinely used for H. perforatum (Čellárová et al. 1992) could not be applied to H. tomentosum hairy roots. Since the exact physiological reasons for regeneration recalcitrance of H. tomentosum hairy roots upon the use of purine-based cytokinins are unknown, an alternative method had to be developed. The use of TDZ alone led to growth retardation and necrosis. However, the replacement of adenine-based cytokinins by the urea-based cytokinin TDZ in combination with an auxin inhibitor PCIB led to positive results. H. tomentosum transgenic shoots regenerated from hairy root segments on MS medium containing 2 µmol l−1 TDZ and 40 or 60 µmol l−1 PCIB under 16/8 h light/dark photoperiod (Fig. 1a–c). Root segments of the non-transformed control plants were also cultured and regenerated under the same conditions. From among ten different hairy root clones (5 derived by A. rhizogenes ATCC 15834 and 5 by A. rhizogenes A4-mediated transformation), six produced shoots on the regeneration medium. Three regenerated clones were obtained by transformation via A. rhizogenes ATCC 15834 and three via A. rhizogenes A4, respectively. Hairy root segments differed in the frequency of regeneration ranging from 10 to 80% (Table 1).
Series of steps from hairy roots to development of transgenic H. tomentosum plants. a H. tomentosum hairy root culture, b callus formation on medium containing TDZ and PCIB, c shoot buds emerging from the callus after the transfer to medium containing BA, d regenerated transgenic plants in vitro, e phenotype comparison of transgenic ATCC clone 7 (left) and non-transgenic control (right) in ex vitro conditions
The regeneration process was sporadic and long-lasting, but reproducible. Green shoot buds emerged from root-derived callus after 11 months of cultivation. The shoots were further transferred to MS medium containing 2.2 µmol l−1 BA to stimulate their growth and branching. Subsequent replacement of the TDZ and PCIB with 2.2 µmol l−1 BA resulted in whole plant regeneration (Fig. 1d).
The transgenic plantlets multiplied on MS medium with addition of 2.2 µmol l−1 BA showed typical hairy root phenotype with shortened internodes, dwarfing, reduced apical dominance and smaller wrinkly leaves.
All plantlets, both transgenic and control, tolerated the adaptation to ex vitro conditions, from the cultivation in Knop’s solution by subsequent lowering of the humidity and transfer to the soil.
The plants developed abundant root system and intensively differentiated adventive roots on the stems. Transgenic plants of ATCC clone 1 showed normal size and morphology similar to the plants regenerated form the control roots. All other clones maintained the features typical for plants regenerated from hairy roots (Fig. 1e).
Revision of H. tomentosum chromosome number
In spite of previously published data for H. tomentosum chromosome counts of 2n = 2x = 16, cytogenetic characterization of each of our samples revealed the chromosome number 2n = 2x = 18 in all root tips metaphases (Fig. 2a). Even though the phenotype of H. tomentosum closely resembles H. pubescens with 2n = 2x = 18, the main taxonomical marker for classification of both species is the placement of dark nodules on the sepals (Robson 1996). While in H. pubescens, the dark nodules do not protrude from the margin of the sepals and are even sunken, in our samples prominent dark nodules on peduncular cilia typical for H. tomentosum were observed (Fig. 2b, c).
Detection of rol gene integrations
Transgenic character of all H. tomentosum clones adapted to ex vitro conditions was confirmed by PCR amplification of the rolA,B,C genes. The PCR amplification was not performed for rolD and aux1,2 genes, since the integration of these genes has not been previously detected in hairy root cultures used in the experiments (Komarovská et al. 2009).
The transgenic clones differed in the number of rol genes integrations. Only one integration of rolC gene was present in ATCC clone 7 and A4 clone 2, 5 and 8. In contrast, seven rolC integrations for ATCC clone 1 and ATCC clone 4 are predicted (Table 2). For these clones, different insertion sites of T-DNA are presumed, since the clones arose from unique transformation events and the T-DNA integration into the plant genome is known to be random.
Comparison of number of dark nodules and hypericin content in control and transgenic plants
Hypericum tomentosum leaves commonly contain only one to two dark nodules on the leaf apex, and some leaves even lack nodules. These structures become more abundant on the flower parts during the reproductive stage of development. In transgenic plants, the dark nodules were present not only on leaf apices, but also on the margins of leaf laminas with an increased frequency in comparison with the controls. The highest number of nodules compared to the control was determined on the leaves of ATCC clone 1 (Fig. 3).
Although dark nodule count in all acclimated transgenic clones of H. tomentosum was significantly higher than in the controls, only the nodules on ATCC-transformed clones 1 and 7 were of a similar size as in the control plants. This resulted in higher nodule coverage on the leaves of these transgenic clones. Even though the quantity of nodules in ATCC clone 4 and A4 clones 2, 5, and 8 was higher, their size was significantly smaller than in the control plants resulting in no significant changes in nodule coverage on the leaves of these clones (p = 0.01) (Fig. 4a–c).
Quantitative characteristics of dark nodules in relation to hypericins content in acclimated H. tomentosum plants. Control—non-transgenic control plants regenerated and adapted at the same conditions as transgenic plants, ATCC clone 1, 4, 7—H. tomentosum transgenic clones regenerated from A. rhizogenes ATCC 15834 derived hairy roots, A4 clone 2, 5, 8—H. tomentosum transgenic clones regenerated from A. rhizogenes A4-derived hairy roots, *statistically significant difference compared to control (p = 0.01)
Low naphthodianthrone content in non-transgenic shoots of H. tomentosum adapted to ex vitro conditions corresponds to low nodule count [0.076 ± 0.005 mg of total hypericin g−1 of dry weight (DW)]. The plants with the highest nodule count and nodule coverage on the leaves contained the highest amount of hypericin, indicating possible positive correlation between these two variables (correlation coefficient 0.78 at p = 0.05) (Fig. 5). The highest total hypericins content (2.169 ± 0.061 mg g−1 of DW) was determined in ATCC clone 1 representing an about 28.5-fold increase compared to control. Significant increase (5.3-fold) of naphthodianthrones was also observed in ATCC clone 7, whereas hypericin content in A4 clone 2, 5 and 8 did not differ from that of non-transformed shoots. On the other hand, only trace amounts of naphthodianthrones were present in ATCC clone 4 (Fig. 4d).
The naphthodianthrones present in the samples were mainly pseudohypericin and its protoform; hypericin and protohypericin were detected only in trace amounts. The proposed hypericin precursor emodin was detected only in the extracts of H. tomentosum transgenic shoots with the highest naphthodianthrone content (ATCC clone 1 and 7). In other samples, the amount of emodin was under the HPLC detection limit (Table 3).
Discussion
Hypericum tomentosum is a perennial herb native to the Mediterranean region. The production of hypericins in this species is temporally regulated with an onset during the stage of flowering. Plants in the vegetative phase contain 0–2 dark nodules on the leaves and very low hypericin content (Robson 1996; Kucharíková et al. 2016). Despite that the Agrobacterium rhizogenes-mediated transformation system has already been developed in our laboratory (Komarovská et al. 2009), shoot regeneration from hairy root culture which would enable studying the regulation of hypericin biosynthesis remained unsolved for a longer time. The current study was therefore aimed at the development of a regeneration protocol for H. tomentosum hairy roots derived from transformation events mediated by A. rhizogenes A4 and ATCC 15834. Both strains are widely used for hairy root induction and secondary metabolites production in many plant species synthesizing profiling metabolites in the roots (Baskaran and Jayabalan 2009; Grzegorczyk et al. 2006). However, there is only scarce information on the hairy root-derived regeneration process in plant species accumulating bioactive constituents in the aerial parts (Sharafi et al. 2014). Within the genus Hypericum, transgenic hairy root cultures were successfully obtained from three species, H. perforatum (Di Guardo et al. 2003), H. tetrapterum and H. tomentosum (Komarovská et al. 2009). While the shoot differentiation from hairy roots in H. perforatum, a model species of the genus Hypericum, is spontaneous (Di Guardo et al. 2003; Vinterhalter et al. 2006; Tusevski et al. 2014), in H. tetrapterum neither spontaneous nor induced regeneration process was possible. Similarly, H. tomentosum is highly recalcitrant towards regeneration. BA as a very efficient cytokinin for shoot differentiation in H. perforatum (Čellárová et al. 1992; Pretto and Santarem 2000) was ineffective for H. tomentosum hairy roots. Therefore, the adenine-based cytokinin was replaced by urea-based TDZ. TDZ was previously used in the regeneration of H. perforatum plants from hypocotyls with great success (Murch et al. 2000). The use of TDZ alone did not induce shoot differentiation, but resulted in retardation of root growth and necrosis. Hairy root cultures often exhibit an increased sensitivity towards auxins leading to extensive root growth (Roychowdhury et al. 2013). Due to the properties of PCIB, it can be used as a competitive auxin inhibitor (Katekar and Geissler 1980; Oono et al. 2003). The combination of TDZ with auxin inhibitor PCIB induced shoot differentiation from hairy roots and led to successful regeneration of H. tomentosum transgenic shoots, although the regeneration process was sporadic and long-lasting. Six out of ten clones of H. tomentosum irrespective of the bacterial strain used for transformation produced shoot buds on medium containing 2 µmol l−1 TDZ and 40 or 60 µmol l−1 PCIB under 16/8 photoperiod after 11 months of cultivation. Since the clones used in the experiments arose from unique transformation events and the T-DNA integration into the plant genome is random (Kim et al. 2007), different clones may respond to the same hormone signals differently. For example, some Centaurium erythraea lines were capable of spontaneous regeneration, while the other did not regenerate even on medium with an addition of phytohormones (Subotić et al. 2004, 2009). Subsequent replacement of the TDZ and PCIB with 2.2 µmol l−1 BA resulted in the whole plant regeneration of H. tomentosum. The shoots of all transgenic H. tomentosum clones showed phenotypic changes typical for plants regenerated from hairy roots. Similar phenotypes were described in transgenic H. perforatum plants obtained by Di Guardo et al. (2003) and Bertoli et al. (2008). On the contrary, transgenic H. perforatum plants established by Vinterhalter et al. (2006, 2015) and Tusevski et al. (2014) did not differ from the controls.
Ability of transgenic plants of Hypericum spp. to survive the acclimation process varies. Transgenic plants of H. tomentosum seem to be highly tolerant to acclimation because of the abundant root system of this species. Contrary to our results, Komarovská et al. (2010) showed that only 3 out of 11 transgenic clones of H. perforatum were adapted due to poor rooting and a fragile root system of the plants. Also, Franklin et al. (2007) successfully adapted 87.5% of transgenic H. perforatum clones into soil.
All transgenic clones of H. tomentosum have integrated rolA,B,C genes (Komarovská et al. 2009). Along with the site of insertion, the copy number may also have an impact on gene expression. While the quantification by real-time PCR revealed only one integration of rolC gene into the genome of H. tomentosum ATCC clone 1, A4 clone 2, 5 and 8, seven transgene integrations were estimated for ATCC clones 1 and 4. Komarovská et al. (2010) attempted to correlate the number of transgene insertions with the phenotype of transgenic H. perforatum plants. While the clones with high transgene copy number resembled the controls, the plants with only one to two rol genes integrations showed the phenotype typical for plants regenerated from hairy roots. Interestingly, all H. tomentosum transgenic plants with the exception of ATCC clone 1 retained hairy root phenotype in outdoor conditions. Komarovská et al. (2010) proposed that the high copy number resulting in post-transcriptional silencing was responsible for the resemblances of clones with higher number of transgene integrations to controls. However, single transgene integration events can also lead to gene silencing as documented by Elmayan and Vaucheret (1996), who found that single-copy transgenes of N. tabacum were expressed in the seedlings but silenced during later developmental stages.
The naphthodianthrone content in Hypericum spp. is determined genetically and the biosynthesis may be affected by endogenous and environmental parameters (Briskin and Gawienowski 2001). Both the production of naphthodianthrones and dark nodules count in transgenic plants of H. tomentosum described in this study differed from non-transgenic controls. The cause for altered phenotype might be attributed to genetic transformation and also to the stress caused by the in vitro culture conditions during the process of regeneration.
Genetic transformation might lead to enhanced production of secondary metabolites, even when other elicitation attempts are not effective. Increased levels of secondary compounds were determined in numerous plant species after transformation with wild-type A. rhizogenes and also by single rol genes integration or their combinations [reviewed in Bulgakov (2008), Tian (2015), Matveeva and Sokorna (2016)]. However, the mechanism of modulation of secondary metabolism is still poorly understood. The effect of rol gene expression on naphthodianthrones production in Hypericum spp. is greatly variable. The highest amount of total naphthodianthrones, 28 times exceeding the content in the controls was observed in H. tomentosum ATCC clone 1. Similarly, transgenic shoots of H. perforatum derived by A. rhizogenes A4-mediated transformation contained 12-fold higher total naphthodianthrone content in comparison with the control (Tusevski et al. 2014). On the other hand, H. tomentosum ATCC clone 4 with the same number of estimated rol genes integrations as the ATCC clone 1 contained only trace amounts of hypericins. This might indicate that the sites of the integrations need to be taken into consideration. In comparison, regenerated transgenic plants of H. perforatum showed lowered naphthodianthrone content compared to the control (Komarovská et al. 2010; Tusevski et al. 2017). The biosynthesis of hypericins was also lowered in transgenic A. rhizogenes ATCC 15834-derived H. perforatum plants acclimated to ex vitro conditions by Koperdáková et al. (2009).
The biosynthesis of hypericins is proved to be related to the formation of dark nodules (Zdunek and Alfermann 1992; Briskin and Gawienowski 2001; Zobayed et al. 2006). Our results also indicate positive correlation between hypericins content and nodule coverage on the leaves. Increased number of nodules in H. tomentosum leaves was also observed after the regeneration of plants from cryopreserved explants (Bruňáková and Čellárová 2016). These results indicate that biosynthesis of hypericins is affected by stress conditions. Elevated content of naphthodianthrones was found after alteration of physical culture conditions such as temperature and light intensity (Odabas et al. 2009) and osmotic stress (Cui et al. 2010). For regeneration of plants from H. tomentosum hairy roots, we used TDZ in combination with PCIB and subsequently BA. Besides the shoot differentiation role of TDZ and BA, they were proved to also modulate hypericin biosynthesis. The production of hypericins was positively affected by BA treatment in H. perforatum (Gadzovska et al. 2005; Liu et al. 2007), H. triquetrifolium (Karakas et al. 2009) and H. perforatum by TDZ (Murch et al. 2000). On the contrary, TDZ had a negative effect on hypericin biosynthesis in H. hirsutum, H. maculatum (Coste et al. 2011) and H. sampsonii (Liu et al. 2007).
Hypericum tomentosum belongs to Hypericum species which are taxonomically well characterized, but data on phytochemical profile or pharmacological activities are very limited. Earlier cytogenetic data characterize this species as diploid with the chromosome number 2n = 16 (Nielsen 1924; Robson 1996; Matzk et al. 2003). Cytogenetic analyses of our samples revealed that all regenerated plants, both transgenic and control, differed in this attribute with the chromosome counts of 2n = 18. This number is characteristic for H. pubescens, a species closely related to H. tomentosum. The important characteristic discriminating H. pubescens from H. tomentosum is the position of the dark nodules on the sepals. Sepals of H. tomentosum have acute to shortly aristate and usually glandular apex and the margins contain prominent peduncular glands or glandular cilia. On the other hand, sepals of H. pubescens are described by entire to subentire margins with usually eglandular apex (Robson 1996). The plants described in this study showed typical sepals of H. tomentosum, though the chromosome number does not correspond with previously published records (Nielsen 1924; Robson 1996; Matzk et al. 2003). If the position of dark nodules on the sepals represents a decisive taxonomical marker, then the previously published results on the chromosome number 2n = 16 should be revised.
Conclusion
In this study, we report on an efficient regeneration protocol from H. tomentosum hairy root cultures. The shoot differentiation was achieved by using the urea-based cytokinin TDZ in combination with the auxin inhibitor PCIB. Further cultivation of shoots on MS medium containing the adenine-based cytokinin BA led to the first successful whole plant regeneration of this species in vitro. The analysis of transgenic plants of H. tomentosum revealed the possibility of modulating naphthodianthrone biosynthesis by a significant 28- and 5-fold increase of total hypericin content, respectively. Furthermore, the number of dark nodules accumulating hypericins was multiplied in all transgenic plants. Also, we contributed to the cytogenetic characterization of H. tomentosum suggesting a revision of the chromosome number, which was determined as 2n = 18 in all analysed samples.
Author contributions statement
JH performed the experiments, analysed the data and drafted the manuscript. EC conceived the project, designed the experiments, interpreted the data and revised the manuscript.
References
Baskaran P, Jayabalan N (2009) Psoralen production in hairy roots and adventitious roots cultures of Psoralea corylifolia. Biotechnol Lett 31:1073–1077
Bertoli A, Giovannini A, Ruffoni B, Di Guardo A, Spinelli G, Mazzetti M, Pistelli L (2008) Bioactive constituent production in St. John’s Wort in vitro hairy roots regenerated plant lines. J Agric Food Chem 56:5078–5082
Birch RG (1997) Plant transformation: problems and strategies for practical application. Annu Rev Plant Physiol Plant Mol Biol 48:297–326
Briskin DP, Gawienowski MC (2001) Differential effects of light and nitrogen on production of hypericins and leaf glands in Hypericum perforatum. Plant Physiol Biochem 39:1075–1081
Bruňáková K, Čellárová E (2016) Conservation strategies in the genus Hypericum via cryogenic treatment. Front Plant Sci 7:1–12
Bulgakov VP (2008) Functions of rol genes in plant secondary metabolism. Biotechnol Adv 26:318–324
Casanova E, Trillas MI, Moysset L, Vainstein A (2005) Influence of rol genes in floriculture. Biotechnol Adv 23:3–39
Castano AP, Demidova TN, Hamblin MR (2004) Mechanisms in photodynamic therapy: part one photosensitizers, photochemistry and cellular localization. Photodiagn Photodyn Ther 1:279–293
Čellárová E, Kimáková K, Brutovská R (1992) Multiple shoot formation and phenotypic changes of R0 regenerants in Hypericum perforatum L. Acta Biotechnol 12:445–452
Čellárová E, Kimáková K, Halušková J, Daxnerová Z (1994) The variability of hypericin content in the regenerants of Hypericum perforatum. Acta Biotechnol 14:267–274
Christey MJ (2001) Use of ri-mediated transformation for production of transgenic plants. In Vitro Cell Dev Biol 37:687–700
Coste A, Vlase L, Halmagyi A, Coldea G (2011) Effects of plant growth regulators and elicitors on production of secondary metabolites in shoot cultures of Hypericum hirsutum and Hypericum maculatum. Plant Cell Tiss Organ Cult 106:279–288
Couceiro MA, Afreen F, Zobayed SMA, Kozai T (2006) Variation in concentrations of major bioactive compounds of St. John’s wort: effects of harvesting time, temperature and germplasm. Plant Sci 170:128–134
Crockett SL, Robson NKB (2011) Taxonomy and chemotaxonomy of the genus Hypericum. Med Aromat Plant Sci Biotechnol 5:1–13
Cui XH, Murthy HN, Wu CH, Paek KY (2010) Sucrose-induced osmotic stress affects biomass, metabolite, and antioxidant levels in root suspension cultures of Hypericum perforatum L. Plant Cell Tiss Organ Cult 103:7–14
Di Guardo A, Čellárová E, Koperdáková J, Pistelli L, Ruffoni B, Allavena A, Giovannini A (2003) Hairy root induction and plant regeneration in Hypericum perforatum L. J Genet Breed 57:269–278
Elmayan T, Vaucheret H (1996) Expression of single copies of a strongly expressed 35S transgene can be silenced post-transcriptionally. Plant J 9:787–797
Franklin G, Oliveira M, Dias ACP (2007) Production of transgenic Hypericum perforatum plans via particle bombardment-mediated transformation of novel organogenetic cell suspension cultures. Plant Sci 172:1193–1203
Franklin G, Conceição LFR, Kombrink E, Dias ACP (2008) Hypericum perforatum plant cells reduce Agrobacterium viability during co-cultivation. Planta 227:1401–1408
Gadzovska S, Maury S, Ounnar S, Righezza M, Kascakova S, Refregiers M, Spasenoski M, Joseph C, Hagège D (2005) Identification and quantification of hypericin and pseudohypericin in different Hypericum perforatum L. in vitro cultures. Plant Physiol Biochem 43:591–601
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50:151–158
Grzegorczyk I, Królicka A, Wysokińska H (2006) Establishment of Salvia officinalis L. hairy root cultures for the production of rosmarinic acid. Z Naturforsch C 61:351–356
Hou W, Shakya P, Franklin G (2016) A perspective on Hypericum perforatum transformation. Front Plant Sci 7:879
Huang L, Chen S (2012) Hypericin in Hypericum: chemistry, botanical sources and biological activities. JCPS 21:388–400
Jendželovská Z, Jendželovský R, Kuchárová B, Fedoročko P (2016) Hypericin in the light and in the dark: two sides of the same coin. Front Plant Sci 7:560
Katekar GF, Geissler AE (1980) Auxin transport inhibitors. Plant Physiol 66:1190–1195
Kim SI, Veena, Gelvin SB (2007) Genome-wide analysis of Agrobacterium T-DNA integration sites in the Arabidopsis genome generated under non-selective conditions. Plant J 51:779–791
Komarovská H, Giovannini A, Košuth J, Čellárová E (2009) Agrobacterium rhizogenes-mediated transformation of Hypericum tomentosum L. and Hypericum tetrapterum Fries. Z Naturforsch C 64:864–868
Komarovská H, Košuth J, Giovannini A, Smelcerovic A, Zuehlke S, Čellárová E (2010) Effect of the number of rol genes integrations on phenotypic variation in hairy root-derived Hypericum perforatum L. plants. Z Naturforsch C 65:701–712
Koperdáková J, Komarovská H, Košuth J, Giovannini A, Čellárová E (2009) Characterization of hairy root-phenotype in transgenic Hypericum perforatum L. clones. Acta Physiol Plant 31:351–358
Kucharíková A, Kimáková K, Janfelt C, Čellárová E (2016) Interspecific variation in localization of hypericins and phloroglucinols in the genus Hypericum as revealed by desorption electrospray ionization mass spectrometry imaging. Physiol Plant 157:2–12
Liu NX, Zhang XQ, Sun JS (2007) Effects of cytokinins and elicitors on the production of hypericins and hyperforin metabolites in Hypericum sampsonii and Hypericum perforatum. Plant Growth Regul 53:207–214
Mason G, Provero P, Vaira AM, Accotto GP (2002) Estimating the number of integrations in transformed plants by quantitative real-time PCR. BMC Biotech 2:20
Matveeva T, Sokornova S (2016) Agrobacterium rhizogenes-mediated transformation of plants for improvement of yields of secondary metabolites. In: Pavlov A, Bley T (eds) Bioprocessing of plant in vitro systems. Springer International Publishing AG, Switzerland, pp 1–42
Matzk F, Hammer K, Schubert I (2003) Coevolution of apomixis and genome size within the genus Hypericum. Sex Plant Reprod 16:51–58
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15:473–497
Murch SJ, Choffe KL, Victor JMR, Slimmon TY, KrishnaRaj S, Saxena PK (2000) Thidiazuron-induced plant regeneration from hypocotyl cultures of St. John’s wort (Hypericum perforatum. cv ‘Anthos’). Plant Cell Rep 19:576–581
Murín A (1960) Substitution of cellophane for glass covers to facilitate preparation of permanent squashes and smears. Stain Technol 35:351–353
Nahrstedt A, Butterweck V (1997) Biologically active and other chemical constituents of the herb Hypericum perforatum L. Pharmacopsychiatry 30:129–134
Nielsen N (1924) Chromosome numbers in the genus Hypericum. Hereditas 5:378–382
Odabas MS, Radusiene J, Camas N, Janulis V, Ivanauskas L, Cirak C (2009) The quantitative effects of temperature and light intensity on hyperforin and hypericins accumulation in Hypericum perforatum L. J Med Plants Res 3:519–525
Oono Y, Ooura C, Rahman A, Aspuria ET, Hayashi K, Tanaka A, Uchimiya H (2003) p-Chlorophenoxyisobutyric acid impairs auxin response in Arabidopsis root. Plant Physiol 133:1135–1147
Öztürk N, Korkmaz S, Öztürk Y (2007) Wound-healing activity of St. John’s Wort (Hypericum perforatum L.) on chicken embryonic fibroblasts. J Ethnopharmacol 111:33–39
Pretto FR, Santarem ER (2000) Callus formation and plant regeneration from Hypericum perforatum leaves. Plant Cell Tiss Org 62:107–113
Robson NKB (1996) Studies in the genus Hypericum L. (Guttiferae) 6. Sections 20. Myriandra to 28. Elodes. Bull Nat Hist Mus Lond (Bot) 26:75–271
Robson NKB (2003) Hypericum botany. In: Ernst E (ed) Hypericum: the genus Hypericum. Taylor and Francis, New York, pp 1–22
Roychowdhury D, Majumder A, Jha S (2013) Agrobacterium rhizogenes-mediated transformation in medicinal plants: prospects and challenges. In: Chandra S, Lata H, Varma A (eds) Biotechnology for medicinal plants. Springer, Berlin, Heidelberg, pp 29–68
Saghai-Maroof MA, Soliman KM, Jorgensen RA, Allard RW (1984) Ribosomal DNA spacer-length polymorphisms in barley: mendelian inheritance, chromosomal location, and population dynamics. Proc Natl Acad Sci USA 81:8014–8018
Sharafi A, Sohi HH, Azadi P, Sharafi AA (2014) Hairy root induction and plant regeneration of medicinal plant Dracocephalum kotschyi. Physiol Mol Biol Plants 20:257–262
Spena A, Schmulling T, Koncz C, Schell JS (1987) Independent and synergistic activity of rolA, B and C loci in stimulating abnormal growth in plants. EMBO J 6:3891–3899
Stojanović G, Ðorđević A, Šmelcerović A (2013) Do other Hypericum species have medical potential as St. John’s Wort (Hypericum perforatum)? Curr Med Chem 20:2273–2295
Subotić A, Budimir B, Grubišić D, Momčilović I (2004) Direct regeneration of shoots from hairy root cultures of Centaurium erythraea inoculated with Agrobacterium rhizogenes. Biol Plant 47:617–619
Subotić A, Jevremović S, Grubišić D (2009) Influence of cytokinins on in vitro morphogenesis in root cultures of Centaurium erythraea—valuable medicinal plant. Sci Hortic-Amsterdam 120:386–390
Tian L (2015) Using hairy roots for production of valuable plant secondary metabolites. Adv Biochem Eng Biotechnol 149:275–324
Tolonen A, Hohtola A, Jalonen J (2003) Fast high- performance liquid chromatographic analysis of naphthodianthrones and phloroglucinols from Hypericum perforatum extracts. Phytochem Anal 14:306–309
Tusevski O, Petreska Stanoeva J, Stefova M, Kungulovski D, Atanasova-Pancevska N, Sekulovski N, Panov S, Gadzovska Simic S (2013) Hairy roots of Hypericum perforatum L.: a promising system for xanthone production. Cent Eur J Biol 8:1010–1022
Tusevski O, Petreska Stanoeva J, Stefova M, Pavokovic D, Gadzovska Simic S (2014) Identification and quantification of phenolic compounds in Hypericum perforatum L. transgenic shoots. Acta Physiol Plant 36:2555–2569
Tusevski O, Vinterhalter B, Milošević DK, Soković M, Ćirić A, Vinterhalter D, Zdravković Korać S, Petreska Stanoeva J, Stefova M, Gadzovska Simic S (2017) Production of phenolic compounds, antioxidant and antimicrobial activities in hairy root and shoot cultures of Hypericum perforatum L. Plant Cell Tiss Organ Cult 128:589–605
Velada I, Ragonezi C, Arnholdt-Schmitt B, Cardoso H (2014) Reference genes selection and normalization of oxidative stress responsive genes upon different temperature stress conditions in Hypericum perforatum L. PLoS One 9:e115206
Vinterhalter B, Ninković S, Cingel A, Vinterhalter D (2006) Shoot and root culture of Hypericum perforatum L. transformed with Agrobacterium rhizogenes A4M70GUS. Biol Plant 50:767–770
Vinterhalter B, Zdravković-Korać S, Mitić N, Bohanec B, Vinterhalter D, Savić J (2015) Effect of sucrose on shoot regeneration in Agrobacterium transformed Hypericum perforatum L. roots. Acta Physiol Plant 37:39–42
Wölfle U, Seelinger G, Schempp CM (2014) Topical application of St. John’s Wort (Hypericum perforatum). Planta Med 80:109–120
Zdravković-Korać S, Ćalić D, Druart PH, Lj Radojević (2003) The horse chestnut lines harboring the rol genes. Biol Plant 47:487–491
Zdunek K, Alfermann W (1992) Initiation of shoot organ cultures of Hypericum perforatum and formation of hypericin derivatives. Planta Med 58:621–625
Zobayed SMA, Afreen F, Goto E, Kozai T (2006) Plant–environment interactions: accumulation of hypericins in dark glands of Hypericum perforatum. Ann Bot (Lond) 98:793–804
Acknowledgements
The research was funded by the Slovak Research and Development Agency APVV-14-0154, the Scientific Grant Agency of Slovak Republic VEGA 1/0090/15 and P. J. Šafárik University grant for young researches VVGS-PF-2015-479. The authors would like to thank Dr. Ján Košuth and Dr. Katarína Nigutová for the consultations on evaluation of the qPCR data, Dr. Linda Petijová for the help with statistical analyses and Zdenka Lacková for the preparation of microscope slides. The advice of Dr. Nicolai Nürk, University of Bayreuth, Germany, on taxonomic markers is greatly appreciated.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by J. Van Staden.
Rights and permissions
About this article
Cite this article
Henzelyová, J., Čellárová, E. Modulation of naphthodianthrone biosynthesis in hairy root-derived Hypericum tomentosum regenerants. Acta Physiol Plant 40, 82 (2018). https://doi.org/10.1007/s11738-018-2664-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-018-2664-1