Abstract
Hairy root-regenerated clones of Hypericum perforatum L. grown in vitro similarly to those successfully adapted to ex vitro conditions showed phenotype features typical for plants transformed with Agrobacterium rhizogenes T-DNA. These included reduced apical dominance, increased branching, dwarfing and reduced fertility. Transgenic clones differed in ability to develop root system as a necessary condition for transfer to the soil. One of the profiling characters, capability of hypericin biosynthesis was altered as well. Dark glands as the sites of hypericin accumulation and/or synthesis exhibited significantly higher densities on both, leaves and petals of transgenic clones comparing to controls. In the genome of transgenic clones, rolABC genes were detected. Both clones harboured similar copy number of individual rol genes. However, copy numbers descended from rolA to rolC gene in both clones.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
St. John’s wort (Hypericum perforatum L.) is a medicinal plant used for treatment of mild to moderate depression. Many of the pharmacological activities appear to be attributable to the phloroglucinol derivative hyperforin (Beerhues 2006). Interest is shown also on naturally occurring photosensitizer hypericin as potential clinical anticancer and antiviral agent (Kubin et al. 2005).
The content of secondary metabolites is considerably affected by genetic and environmental factors. In addition, reproduction peculiarities of this facultatively apomictic species can lead to several different ploidy levels in the progeny. The high pharmacological significance of H. perforatum has promoted an effort to search, along with traditional ways, for biotechnological approaches towards an increase of their content. Production of secondary metabolites can also be affected by Agrobacterium-mediated transformation as rol genes act as potential activators of secondary metabolism via transcriptional activation of defence genes in transformed plant cells (Bulgakov 2008). The efficient hairy root induction and plant regeneration in H. perforatum using the natural vector system of Agrobacterium rhizogenes with wild agropine type plasmid and Ri plasmid containing GUS construct, respectively, have been reported so far (Di Guardo et al. 2003; Vinterhalter et al. 2006). Transgenic plants were also produced via particle bombardment-mediated transformation of organogenic suspension cultures (Franklin et al. 2007).
The aim of this work was to characterize hairy root-regenerated H. perforatum plants grown in vitro at morphological, physiological and biochemical levels; to detect copy number of integrated T-DNA genes; to adapt them to ex vitro conditions; and to survey morphological characteristics and hypericin content in ex vitro-grown transgenic plants.
Materials and methods
Plant material
Shoots of two transgenic clones, B and L, of H. perforatum L. grown in vitro and the non-transformed clone K were used. The clone K was in vitro propagated wild H. perforatum genotype from Italy. Wild agropine type A. rhizogenes strain ATCC 15834 was used to transform leaf and root cuttings of the clone K by Di Guardo et al. (2003). Developed hairy roots were isolated and further cultivated on hormone-free media, where transgenic shoots differentiated spontaneously. Clone B was derived from a hairy root developed on root cutting while clone L from that on leaf cutting. All samples represented tetraploid plants.
Culture media
Transgenic plantlets and non-transformed controls were kept on basal medium containing mineral salts according to Linsmaier and Skoog (1965), B5 vitamins according to Gamborg et al. (1968), 100 mg L−1 myo-inositol, 2 mg L−1 glycine, 30 g L−1 sucrose and 0.6% agar, with pH adjusted to 5.6 before autoclaving. For hydroponics modified Knop’s solution (Pastírová et al. 2004) with pH adjusted to 5.2 was used.
Adaptation to ex vitro conditions
After 2-year in vitro culture transgenic and control plantlets with differentiated and roots were transferred to the aerated hydroponic vessels placed in the plastic container. During 2–3 weeks the containers were gradually opened to lower humidity until the plants were able to grow under laboratory conditions. Plants were transferred to autoclaved soil after 4 weeks of hydroponic cultivation.
Culture conditions
In vitro cultures of shoots, hydroponic cultures of transgenic clones, non-transformed controls and ex vitro adapted plants were kept in the culture room at 23°C with 16/8 h photoperiod using fluorescent lighting of 33 μmol m−2 s−1 and 70% relative humidity. The shoot cultures were subcultured at 6-week interval. Hydroponic solution was supplied on 14th day of cultivation.
Detection and quantification of T-DNA integrations
Total genomic DNA was isolated from 100 mg of fresh plant material using the DNeasy Plant Mini Kit (Qiagen, Valencia, CA, USA).
The presence of the integrated genes in the genome of the transformed H. perforatum plants was determined by PCR amplification with gene-specific primers for rolABD described by Zdravkovic-Korac et al. (2004), or rolC as described by Di Guardo et al. (2003) or designed according to published sequence of aux1,2 genes of pRi ATCC 15834 strain of A. rhizogenes (GenBank accession no. DQ782955). The number of integrated rolABC genes was measured by quantitative real time PCR (qPCR) with SYBR Green I detection using iCycler iQ Real Time PCR Detection System (BioRad, Hercules, CA, USA). For template normalization, genomic fragment of hyp-1 gene was used as internal control. PCR primers for amplification of the hyp-1 gene fragment were designed based on the published H. perforatum specific sequence (GenBank accession no. AY148090). qPCR was performed in duplicates in 30 μL reaction volume containing: 1× iQ™ SYBR Green Supermix (0.2 mM dNTP, 3 mM MgCl2), 0.5 μM primers and 50 ng of DNA. The reaction conditions were as follows: 3 min at 95°C, 35 cycles (30 s at 94°C, 30 s at 60°C (rolABC), respectively 52°C (hyp-1), 30 s at 72°C), 7 min at 74°C followed by melting curve analysis.
The relative amount of the rolABC and hyp-1 gene was evaluated by the method of standard curve obtained by the amplification of a serially diluted mixture of DNA samples (twofold dilutions), with five dilution points, each one in two replicates. The quantities of hyp-1-normalized copy number of integrated rolABC genes were calculated according to Mason et al. (2002).
Evaluation of morphological characters
Ex vitro cultivated plants were scored for plant height, number of stems, number of axillary shoots, number and length of internodes. Altogether, 24 plants were evaluated—9 of the clone B and 15 of the control plants in the first vegetation period, from which only five transgenic and three non-transformed plants survived until second vegetation period. Several parameters—petal area, length and width of sepals, number and length of stamens, length and width of anthers, and length of pistils on 21 fully opened flowers from each clone were measured.
Leaf area and number of hypericin containing dark glands on leaf lamina were determined in 40 in vitro grown shoots of each clone. Leaves at similar developmental stage (five uppermost fully developed leaf pairs) from each shoot were used. These traits were also evaluated on the first five leaf pairs just below the inflorescence of three ex vitro cultivated plants of both, transformed and non-transformed clones. Number of dark glands was determined also on petals of ex vitro grown plants. The length and width of dark glands were measured on leaves and petals of ex vitro grown plants. More than 900 glands on petals and 400 dark glands on leaves of both clones were sized.
Digital photographs of the leaves and flower parts were taken to measure their size or area using UTHSCSA Image Tool 3.00. The number of dark glands was determined by light microscopy. Length (l) and width (w) of dark glands were measured using light microscopy. Area of the gland was calculated as ¼ × π × l × w. Gland density was calculated as quotient of number of glands on leaf/petal and its area. Moreover, percentage of the leaf/petal area covered by dark glands was determined.
Hypericin assay
Total hypericin content in shoots of different clones grown in vitro after 6-week subculture and in the upper half of the flowering aerial parts of plants growing ex vitro was quantified in extracts by spectrophotometry as follows: 100 mg of dried and homogenized plant material were extracted twice with 30 mL chloroform in a sonicator and filtered, than extracted with 30 mL methanol in a sonicator, filtered and evaporated in a water bath. This was followed by addition of 10 mL of chloroform; the supernatant was discarded and the solid phase containing hypericin and its derivatives was dissolved in methanol and filtered. The total content of hypericins was quantified by spectrophotometer Spectronic 20 Genesys (Spectronic Inst., Rochester, NY, USA) at 592 nm in ten replicates of each clone grown in vitro and in three replicas of each ex vitro grown plant. The content of hypericins was calculated from measured absorbance on the base of hypericin standard (Roth, Karlsruhe, Germany) curve. As spectrophotometric measurement does not enable separation hypericin from pseudohypericin and their protoforms, the sum of all hypericins was determined.
Statistical analyses
The non-parametric test of Kruskal–Wallis was used for evaluation of differences in morphological and biochemical characteristics between the clones. This was followed by multiple range analysis. The results of analyses were evaluated at 0.001 significance level using Statgraphics® statistical software.
Results and discussion
Phenotype of in vitro grown plantlets
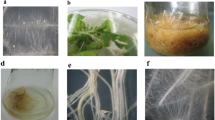
The appearance of hairy root-regenerated plantlets of H. perforatum was typical for plants transformed by A. rhizogenes T-DNA (or its part)—dwarfing, reduced apical dominance, shortened internodes and smaller leaves (Fig. 1). As transgenic clones were derived from different transformation events and types of tissues, their habit differed with more or less pronounced hairy root-syndrome. Contrary to these results in recently published study of Vinterhalter et al. (2006), transgenic H. perforatum plants regenerated from hairy roots derived by A. rhizogenes strain A4M70GUS showed normal phenotype; nevertheless, differences in several growth parameters were observed.
The leaf area (Fig. 2), number of dark glands per leaf and their density differed significantly among the clones (Table 1). Although non-transgenic clone had much greater leaf area, its gland density was the smallest. St. John’s wort leaves contain two types of glands: translucent glands distributed across the leaf lamina and dark glands located mainly along the leaf margins. The accumulation of hypericins is associated with the dark glands (Briskin and Gawienowski 2001; Zobayed et al. 2006).
The analyses of total hypericin content were repeatedly performed four times in 1-year interval of in vitro cultivation (Table 1). Despite the fact that in vitro culture under defined conditions is free from environmental changes, hypericin content in all the clones varied over the studied period of time. However, the clone B plants maintained 1.5 up to three times higher hypericin content than the other clones over the surveyed period. The highest hypericin content was detected in transgenic clone with the highest number and density of dark glands on its leaves. Hypericin content of the other clones was comparable although these clones differed in number and density of glands. Concentration of hypericins depends not only on the number of dark glands, their size and area (Zobayed et al. 2006) but also on their structure (Kornfeld et al. 2007). Moreover, different integration sites of rol genes and activation of their expression can explain differences in hypericin content in transgenic clones.
The process of rooting comprising rooting ability, rate of rooting, site of root differentiation and branching of roots was studied during 8-week period on 60 shoot cuttings from each clone on basal medium. Shoots of the clone L differentiated roots more slowly than shoots of the other clones. Almost one-third of shoots of the clone L, leaf originating clone, formed no roots (Fig. 3). The site of root differentiation also varied. Shoots of the clone B, root originating clone, often differentiated several adventitious roots mainly from the axils above the culture medium. On the other hand shoots of the other clones formed roots only on the wounded site. While the roots of the clone B branched abundantly, the roots of the clone L branched poorly and were rather fragile. These results suggest that the origin of the cell being transformed, including epigenetic changes leading to its differentiation, could have long-term effect on regenerated plant behaviour.
Differences in the process of root differentiation in transgenic clones were reported by other authors as well. Increased formation of adventitious roots on the stem has been observed on transgenic tobacco plants expressing rolB gene (Schmülling et al. 1988). On the contrary, rolA, B and C genes induced shorter internode length and a more fibrous root system typical for rol-transformed plants, but were not useful for increasing the rooting potential of a transgenic walnut hybrid (Vahdati et al. 2002). Antirrhinum majus plants regenerated from hairy root clone showed poorly developed (Senior et al. 1995) or an abundant root system (Handa 1992). The differences in rooting ability of the transformed clones that were observed also in our experiments probably reflect different insertion sites and differences in copy number of the foreign T-DNA in the genome of plants, which arose from a unique transformation event from different type of cuttings.
Detection and quantification of T-DNA integrations
Only the insertion of three of the root organizing loci (rolABC genes) was confirmed in the studied H. perforatum transgenic clones. Neither rolD nor aux1,2 integration in transgenic clones was detected. Quantification of copy number of the integrated genes had shown very similar copy number of insertions in both clones with descending tendency towards the rolC gene (Table 1). Since the T-DNA is integrated into the plant genome randomly and individual transgenic clones arose from unique transformation events different insertion sites are presumed.
Agropine plasmid, which was used for transformation, contains two independently transferable T-DNA regions (Huffman et al. 1984)—TL DNA and TR DNA. TR DNA harbours genes for opine synthesis and auxin biosynthesis, TL DNA rolABCD genes corresponding to open reading frames 10, 11, 12 and 15 (Slightom et al. 1986). The presence and copy number of evaluated genes suggest that only part of TL DNA was integrated into genome of transgenic clones.
Ex vitro acclimation
Acclimation of the clones from in vitro conditions required maintaining of sufficient humidity in the first step of adaptation. Therefore, hydroponic system with subsequent transfer into soil was used for acclimation of in vitro growing shoots with differentiated roots to ex vitro conditions. Ability of the clones to survive process of acclimation was different (Table 2). Plantlets of the clones K and B tolerated hydroponics well with survival rate higher than 80%. On the contrary, only 20% of the clone L shoots survived as long as they could be transferred into the soil where they soon withered and died. Transfer to the soil did not affect control plantlets but was critical for the B clone ones as their number decreased to 29%. Moreover, long term ex vitro cultivation reduced their number to less than 5%.
During acclimation to ex vitro conditions plants must adapt to severe changes of environment. Plants undergo substantial changes in leaf morphology and anatomy, including development of cuticle and effective stomatal regulation of transpiration, differentiation of leaf mesophyll and changes of chloroplast number and structure (Pospíšílová et al. 1999). The process of acclimation of transgenic plants is even more complicated by expression of transgenes often leading to decreased vigour. Sevón et al. (1997) reported on successful transfer of 13% of in vitro grown transgenic clones of Hyoscyamus muticus to greenhouse conditions. Their adaptation to soil was considered difficult or extremely difficult. Acclimatization of Angelonia salicariifolia plantlets transformed with wild A. rhizogenes strains was also problematic, only 20% of plantlets without apparent hyperhydric appearance could have been transplanted into greenhouse (Koike et al. 2003). Similarly as in other species, acclimatization of transgenic H. perforatum plants to ex vitro in our experiment has been also shown to be tricky.
Morphological and biochemical traits of plants ex vitro
Only plants of the clone B and control plants were subjected to evaluation of morphological and biochemical traits, as only these plants were successfully adapted to ex vitro conditions. During ex vitro cultivation transgenic plants exhibited bushy dwarfed phenotype (Fig. 4). They were significantly smaller than the control plants in the first and second year of cultivation. Both clones had comparable number of axillary shoots. While both clones had similar number of internodes, clone K internodes were more than four times longer than that of transgenic clone (Table 3).
The studied transgenic plants had smaller leaves which were no more wrinkled during ex vitro cultivation. Leaf senescence was observed much earlier on transgenic than on non-transformed plants. Both clones developed significantly greater leaves during ex vitro cultivation than under in vitro conditions (Tables 1, 3). Number of glands on leaves increased almost 1.5 times on the clone B plants and almost two times on control plants in ex vitro conditions. Dark gland density was reduced more than two times on leaves of both clones in ex vitro. Contrary to this study, Kirakosyan et al. (2004) found no difference in the number of dark glands per leaf in tissue culture and under greenhouse conditions in several cultivars of H. perforatum.
Several studies dealt with the number and dark gland density on H. perforatum leaves. Regenerated plants described by Čellárová et al. (1994) had similar gland density of 0.20–0.29 on leaves as non-transformed control in our study. Density of dark glands on the top leaves of plants from different Australian populations varied from 0.33 to 0.50 glands per mm2 of leaf area (Southwell and Campbell 1991) and that of northwestern USA populations from 0.61 to 0.85 (Walker et al. 2001). On the other hand, Cirak et al. (2007) reported on several times higher gland density on leaves. The other available data provide total counts per leaf that vary between 11.12 and 19.93 (Briskin and Gawienowski 2001) or 10.3–15.2 (Kirakosyan et al. 2003), but do not indicate gland density.
Transgenic H. perforatum plants had also reduced number and size of flowers. Flowers of both clones contained equal number of floral whorls; only stamen number was reduced in clone B (55.55 ± 1.30) comparing to the control one (66.14 ± 2.69). All evaluated floral parts of transgenic clone were significantly smaller than those of the control clone (Table 4). Number of dark glands on petals varied between the clones. Density of dark glands was higher on clone B petals (Table 4). While control plants produced seeds, transgenic ones were sterile.
Dark gland size differed between the clones. Dark glands on petals were greater than on leaves in both clones (Table 5). Dark glands covered 0.4% of leaf lamina in the clone B but only 0.3% in the control. Percentage of dark gland area on petals was higher resembling 0.58% on clone B and 0.6% on clone K. Similar results reported by Zobayed et al. (2006) show that dark glands cover approximately two times greater area of petals than leaves of plants cultured under controlled conditions.
The ability of hypericin biosynthesis in transgenic clone B was significantly reduced in ex vitro environment when compared with control plants (Table 5). Unlike in vitro-grown shoots, ex vitro cultivated plants were flowering. As flowers contain higher amounts of secondary metabolites than shoots (Couceiro et al. 2006), hypericin content was supposed to be higher in the ex vitro grown plants. Surprisingly, hypericin content of ex vitro clone B plants was even lower than in vitro grown plants (Table 1). On the other hand, total hypericin content of control plants increased in ex vitro conditions. The hypericin content is largely affected by the stage of plant development and also by proportion of plant parts or tissues in the analysed sample (Couceiro et al. 2006). As control and transgenic plants significantly differed in proportion of flowers and leaves in the upper half of the flowering aerial parts, additional hypericin analyses were performed using stem, leaf and flower tissues, respectively. Even in these samples, hypericin content was much lower in transgenic tissues (data not shown).
Recent studies showed that hypericins are almost exclusively present in the dark-gland tissues located on the aerial parts of a plant, especially on the leaves and floral parts. Concentration of hypericin and pseudohypericin in various organs of this species depends on the number of dark glands, their size or area (Zobayed et al. 2006). Reduced number of dark glands on leaves and petals as well as their smaller size could explain decreased hypericin content in transgenic clone ex vitro. Our results also indicate that hypericin content is not attributed only to gland density or their size in the sample analysed. Stage of development, inner structure and metabolic activity of these multicellular glands should also be taken into consideration. Overall, transgenic plants in ex vitro conditions had decreased vigour which could also affect development of dark glands as well as accumulation of hypericin.
To our knowledge, little information is available on secondary metabolite production in hairy-root regenerated medicinal plants. Several authors report on non-changed or reduced production of secondary metabolites in transgenic plants (Oksman-Caldentey et al. 1991; Saito et al. 1992). On the other hand, transgenic Duboisia myoporoides × D. leichardtii plants with the strongest hairy root phenotype had the highest content of scopolamine. However, the overall yield of scopolamine and hyoscyamine was reduced in transformed plants in comparison with the control plants (Celma et al. 2001).
To summarize, integration of rolA, B and C genes into genome of the clone B and L has been proved while integration of rolD and aux genes was not confirmed by PCR amplification. The clones varied just slightly in the copy number of integrated genes but the sites of transgene integration were different. Both transgenic clones exhibited hairy root syndrome and transformation events lead to significantly increased hypericin production in one of the clones in vitro conditions. The acclimation process was difficult and only few plants of one transgenic clone were successfully adapted to ex vitro conditions. Even in ex vitro setting these plants exhibited hairy root syndrome with reduced viability and fertility. Moreover, hypericin content was significantly reduced not only comparing to control plants ex vitro, but also to the same clone cultivated under in vitro conditions, thereby indicating that transgenic plants tested ex vitro in this study do not provide a viable alternative for production of hypericins.
References
Beerhues L (2006) Hyperforin. Phytochemistry 67:2201–2207
Briskin DP, Gawienowski MC (2001) Differential effects of light and nitrogen on production of hypericins and leaf glands in Hypericum perforatum. Plant Physiol Biochem 39:1075–1081
Bulgakov VP (2008) Functions of rol genes in plant secondary metabolism. Biotechnol Adv 26:318–324
Čellárová E, Daxnerová Z, Kimáková K, Halušková J (1994) The variability of the hypericin content in the regenerants of Hypericum perforatum. Acta Biotechnol 14:267–274
Celma CR, Palazon J, Cusido RM, Pinol MT, Keil M (2001) Decreased scopolamine yield in field-grown Duboisia plants regenerated from hairy roots. Planta Med 67:249–253
Cirak C, Radusien J, (Saglam) Karabuk B, Janulis V (2007) Variation of bioactive substances and morphological traits in Hypericum perforatum populations from Northern Turkey. Biochem Syst Ecol 35:403–409
Couceiro MA, Afreen F, Zobayed SMA, Kozai T (2006) Variation in concentrations of major bioactive compounds of St. John’s wort: effects of harvesting time, temperature and germplasm. Plant Sci 170:128–134
Di Guardo A, Čellárová E, Koperdáková J, Pistelli L, Rufonni B, Allavena A, Giovannini A (2003) Hairy root induction and plant regeneration in Hypericum perforatum L. J Genet Breed 57:269–278
Franklin G, Oliveira M, Dias ACP (2007) Production of transgenic Hypericum perforatum plants via particle bombardment-mediated transformation of novel organogenic cell suspension cultures. Plant Sci 172:1193–1203
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cultures. Exp Cell Res 50:151–158
Handa T (1992) Genetic transformation of Antirrhinum majus L. and inheritance of altered phenotype induced by Ri T-DNA. Plant Sci 81:199–206
Huffman GA, White FF, Gordon MP, Nester EW (1984) Hairy-root inducing plasmid: physical map and homology to tumor-inducing plasmids. J Bacteriol 157:269–276
Kirakosyan A, Gibson DM, Sirvent T (2004) A comparative study of Hypericum perforatum plants as sources of hypericins and hyperforins. J Herbs Species Med 10:73–88
Koike Y, Hoshino Y, Mii M, Nakano M (2003) Horticultural characterization of Angelonia salicariifolia plants transformed with wild-type strains of Agrobacterium rhizogenes. Plant Cell Rep 21:981–987
Kornfeld A, Kaufman PB, Lu CR, Gibson DM, Bolling SF, Warber SL, Chang SC, Kirakosyan A (2007) The production of hypericins in two selected Hypericum perforatum shoot cultures is related to differences in black gland structure. Plant Physiol Biochem 45:24–32
Kubin A, Wierrani F, Burner U, Alth G, Grünberger W (2005) Hypericin—the facts about a controversial agent. Curr Pharm Design 11:233–253
Linsmaier EM, Skoog F (1965) Organic growth factor requirements of tobacco tissue cultures. Physiol Plant 18:100–127
Mason G, Provero P, Vaira AM, Accotto GP (2002) Estimating the number of integrations in transformed plants by quantitative real-time PCR. BMC Biotechnol 2:20–29
Oksman-Caldentey K-M, Kivela O, Hiltunen R (1991) Spontaneous shoot organogenesis and plant-regeneration from hairy root cultures of Hyoscyamus muticus. Plant Sci 78:129–136
Pastírová A, Repčák M, Eliášová A (2004) Salicylic acid induces changes of coumarin metabolites in Matricaria chamomilla L. Plant Sci 167:819–824
Pospíšílová J, Tichá I, Kadleček P, Haisel D, Plzáková Š (1999) Acclimatization of micropropagated plants to ex vitro conditions. Biol Plant 42:481–497
Saito K, Yamazaki M, Anzai H, Yoneyama K, Murakoshi I (1992) Transgenic herbicide-resistant Atropa belladonna using an Ri binary vector and inheritance of the transgenic trait. Plant Cell Rep 11:219–224
Schmülling T, Schell J, Spena A (1988) Single genes from Agrobacterium rhizogenes influence plant development. EMBO J 7:2621–2629
Senior I, Holford P, Cooley RN, Newbury HJ (1995) Transformation of Antirrhinum majus using Agrobacterium rhizogenes. J Exp Bot 46:1233–1239
Sevón N, Dräger B, Hiltunen R, Oksman-Caldentey K-M (1997) Characterization of transgenic plants derived from hairy roots of Hyoscyamus muticus. Plant Cell Rep 16:605–611
Slightom JL, Durand-Tardif M, Jouanin L, Tepfer D (1986) Nucleotide sequence analysis of TL-DNA of Agrobacterium rhizogenes agropine type plasmid. Identification of open reading frames. J Biol Chem 261:108–121
Southwell IA, Campbell MH (1991) Hypericin content variation in Hypericum perforatum in Australia. Phytochemistry 30:475–478
Vahdati K, McKenna JR, Dandekar AM, Leslie CA, Uratsu SL, Hackett WP, Negri P, McGranahan GH (2002) Rooting and other characteristics of a transgenic walnut hybrid (Juglans hindsii x J. regia) rootstock expressing rolABC. J Am Soc Hortic Sci 127:724–728
Vinterhalter B, Ninkovic S, Cingel A, Vinterhalter D (2006) Shoot and root culture of Hypericum perforatum L. transformed with Agrobacterium rhizogenes A4M70GUS. Biol Plant 50:767–770
Walker L, Sirvent T, Gibson D, Vance N (2001) Regional differences in hypericin and pseudohypericin concentrations and five morphological traits among Hypericum perforatum plants in the northwestern United States. Can J Bot 79:1248–1255
Zdravkovic-Korac S, Calic D, Druart PH, Radojevic L (2004) The horse chestnut lines harboring the rol genes. Biol Plant 47:487–491
Zobayed SMA, Afreen F, Goto E, Kozai T (2006) Plant-environment interactions: accumulation of hypericin in dark glands of Hypericum perforatum. Ann Bot (Lond) 98:793–804
Acknowledgments
This work was supported by the Slovak Research and Development Agency under contracts No APVT-20-003704, VVCE-0001-07 and 0321-07 and by Scientific Grant Agency of the Slovak Republic 1/2326/05 and 1/0049/08. Authors would like to thank Bc. Ľudmila Majsniarová, Mrs. Alžbeta Maková and Mrs. Terézia Medvecová for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Stobiecki.
Rights and permissions
About this article
Cite this article
Koperdáková, J., Komarovská, H., Košuth, J. et al. Characterization of hairy root-phenotype in transgenic Hypericum perforatum L. clones. Acta Physiol Plant 31, 351–358 (2009). https://doi.org/10.1007/s11738-008-0241-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-008-0241-8