Abstract
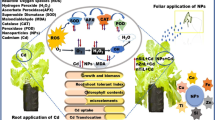
Contamination of agricultural land by chromium (Cr) can inhibit physiological and biochemical processes in plants, leading to reduced crop productivity and food/feed safety. Owing to their fine size, large surface area, and high adsorption affinity for metals, nanomaterials have shown a potential for phytoremediation of heavy metal-contaminated soils. Nanomaterials enhance fitness of plants under metal stress through their modifying effects on plant physiology and biochemistry. The aim of this study was to assess the performance of sunflower (Helianthus annuus) plants grown in soil spiked with hexavalent chromium (Cr IV; 0, 75 and 150 ppm) and the potential role of nano-zerovalent iron (Fe0 nanoparticles; 0, 1 and 2%) to ameliorate Cr toxicity. Results revealed that the Cr uptake decreased by increasing the concentration of Fe0 nanoparticles, causing a significant enhancement in plant morphological and physiological attributes. Treatment with Fe0 nanoparticles reduced bioaccumulation factor (BAF) (in both root and shoot tissues) and translocation factor (TF); however, the magnitude of BAF and TF decreased significantly by increasing the level of Cr(VI). Chromium stress increased the activities of antioxidant enzymes, which further increased by Fe0 nanoparticle application, resulting in improved growth traits. A significant positive correlation was found between growth, BAF and TF of seedlings treated with Fe0 nanoparticles (both 1 and 2%) upon Cr exposure (75 and 150 ppm). The results demonstrated the potential of Fe0 nanoparticles to improve performance of sunflower plants under Cr toxicity through reducing their Cr uptake, which was accompanied by enhanced activity of detoxification enzymes (SOD, CAT, POX, and APX) in cells.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
An increase in urbanization rate and changes in urban lifestyle imply an increase in the proportion of urban population and multiple changes in industrial policy, economic growth model, and household consumption pattern, which greatly increased environmental pollutions. Increasing pollution created by human activities has a negative effect on the biophysical environments and ecosystems. Management of a contaminated area is a complicated issue with several social, engineering and environmental consequences. The problem of heavy metals/and or semi-metals such as cadmium (Cd), mercury (Hg), nickel (Ni), lead (Pb), and chromium (Cr) accumulation in different environments is not new. Pollution levels in environmental platforms are due to farming and semi-industrial activities, energy provision, mining or waste disposal (Asgari Lajayer et al. 2017a; Prasad and Strzaka 2002).
Cr, the seventh most abundant elements on earth’s crust is released in soil during extensive industrial processes (Panda and Choudhury 2005). Leather industry, steel production, tile and well drilling release industrial wastewater into flowing freshwater and contribute to Cr pollution (Sundaramoorthy et al. 2010). Cr may be found predominantly in all environments including soil, water and air, but soil is the major source for released Cr, as it can bond with soil particles and accumulate in it (Shanker et al. 2005). In the nature, Cr can be found in different oxidized forms, but its most stable forms are three-valent (III) and six-valent (VI) Cr. These two forms have completely different chemical properties and effects (Barnhart 1997). However, Cr(VI) is remarkably more toxic than Cr(III) and has been known as one of the most powerful carcinogenic agents in human and animal health (Cohen et al. 1993; Zayed et al. 1998). On the other hand, Cr(III) is less toxic and less mobile which mainly bonds with organic matter of the soil and aqueous media (Barton et al. 2000; Bishnoi et al. 1993). It has also been acknowledged that Cr(VI) represents in many regions in Europe one of the groundwater pollutants of major concern mostly due to its high toxicity, even synergistically enhanced in the presence of other groundwater contaminants and inorganic species such as nitrate and heavy metals/metalloids (Vilardi et al. 2017).
As heavy metals negatively affect all groups of biocenosis and ecosystem processes, environmental monitoring and application of proper refinery technologies are necessary. The capacity and efficiency removal of heavy metal methods depends on the employed technique, level of pollution and its type in the platform as well as presence of other pollutants (their interaction, changes in pH and redox potential). In contrary to organic compounds (which can be possibly degraded or mineralized with final products of CO2 and H2O) heavy metals can be degraded to simpler forms and hence they will never be completely removed from the contaminated platforms. However, they can be immobilized or be absorbed by plants through a process called phytoremediation (Jabeen et al. 2009; Juwarkar et al. 2010; Asgari Lajayer et al. 2017a, b). This is a biological tool in which species or varieties of the plants are selected to accumulate inorganic pollutants or degrade the organic contaminants (Vamerali et al. 2010). Majority of the plant species show a low capability to extract specific elements from the rhizosphere zone (Marschner 1995). Therefore, they absorb unnecessary and even highly toxic elements. Adsorption of pollutants/heavy metals by the plants and their accumulation in plant tissues has found considerable interest in recent years, but not only due to their negative impact on human health in the environment. Enhanced resistance of some crop plant species to toxic elements can effectively extract heavy metals from contaminated soil, urban wastewater and water which have introduced a highly promising tool in phytoremediation process (Kumar et al. 1995; Song et al. 2018; Asgari Lajayer et al. 2019). However, plants have developed various mechanisms to overcome the toxic effects of heavy metals (Zenk 1996) such as avoidance, detoxification and non-selective resistance approaches (for example, increased biosynthesis of free amino acids like proline) (Mehta and Gaur 1999; Alia and Matysik 2001). Most of the studies have emphasized on the physiological mechanisms, but relatively little is known regarding the genetic basis of specific species (Macnair et al. 2000; Liang et al. 2019). Plant resistance toward toxic metals/metalloids is mainly dependent on the efficiency of its detoxification mechanisms in intracellular fluid (Piechalak et al. 2002; Clemens 2001). Heavy metal-contaminated soils may be subjected to various engineering remediation techniques. One of the new strategies to improve the efficiency of phytoremediation is the use of nanoparticles. Presence of nanoparticles in rhizosphere stimulates plants’ growth and increases their resistance to the pollutants and act as an important agent in increasing the efficiency of phytoaccumulation/phytodegradation technique.
Our studies showed that the sunflower has a high potential for Cr accumulation, and its treatment with proper productive agents such as Fe0 nanoparticles can prevent Cr absorption. Thus, the aim of the present study was to investigate the application of Fe0 nanoparticles and their effects on morpho-physiological performance in sunflower (Helianthus annuus L.) as well as their possible impact on improvement of antioxidants’ capacity to prevent the toxicity induced by Cr(VI).
Materials and methods
Plant materials, experimental design and treatments
Sterilization of sunflower seeds was conducted using 1% sodium hypochlorite (NaOCl) for 5 min followed by their complete rinsing by distilled water. The seeds then experienced 10 min soaking in distilled water. Plastic pots (containing 4 kg of soil) were employed to sow the seeds. Irrigation was conducted using tap water. The pH and EC of the examined soil were measured to be 7.20 and 1.65 dS m−1, respectively. The studied soil was classified as silt loam texture [(a mixture of silt (56%), sand (32%) and clay (12%)] with average organic matter of 0.78%, and total N, P and K concentrations of 0.094, 9.3, and 5.8 mg kg−1, respectively. For the first 2 weeks, the plants were only irrigated with tap water which was then thinned to five per pot prior to application of any treatment. The applied treatments comprised different Cr concentrations (0, 75 and 150 ppm) and Fe0 nanopowders (0, 1 and 2%). The first set of the seedlings with the age of 3 weeks were treated with various Cr concentrations, while the second set was considered as control group which received no Cr treatment (treated with the same volume of distilled water). The treatments were conducted in triplicates where the pots were randomly arranged in a greenhouse. The plants’ growth condition involved the light/dark temperatures of 28/18 °C, humidity of 75% and 16/8 h light/dark photoperiod with light intensity of 255 ± 25 μmol m−2s−1.
Properties of nano-zerovalent iron
Nano Fe0 was supplied from Nanosany, an Iranian nanomaterials pioneers company, (Mashhad, Iran). The supplied materials were further analyzed by experimental procedures. The specific surface area (SSA) of the nano Fe0 was about 8–14 m2 g−1, and its purity was more than 99%. The Fe0 NPs size was determined as 35–45 nm using a scanning electron microscope (SEM) apparatus (Fig. 1a). X-ray diffraction (XRD) (XPert PRO MPD, PANalytical) was also employed to evaluate the crystal structure of nano Fe0 particles. The mentioned instrument operated in the 2θ range of 30°–120°, voltage of 40 kV and current of 40 mA using Ni-filtered Cu Kα (λ = 0.15406 nm) radiation (Fig. 1b).
Sample preparation and measurement processes
Determination of root and shoot dry weight
In the 8th week of cultivation (corresponding to growth stage of BBCH 36–37), the plants were carefully removed from the pots and washed until no soil adhered to all the parts of roots. Shoot and roots were gently isolated to measure their weights. For assessing their dry matter, double-distilled water was used to wash/rinse the shoots and roots followed by drying at 70 °C. Some of the samples were also kept to determine their physiological indices.
Estimation of Cr concentration
The ground and sieved samples (0.25 g) were digested by concentrated nitric acid and 30% H2O2. Then, 5 mL of concentrated HNO3 was added to 75-mL digestion tubes and kept overnight. The digestion tubes were put in a heating block (Tucker 1974) where they were heated to 60 °C, then 3 mL of H2O2 was added and the samples were left to digest for 3 h at 150 °C. The products were cooled down, diluted, and filtered prior to the analyses. Atomic absorption spectrometry (AA-7000 Shimadzu, Japan) was used to assess chromium concentration in the digests.
Soil analyses were conducted after harvesting of sunflower seedlings. The soils were first air dried and sieved (using a 2-mm sieve). Then, 2.0 g of the soil sample was digested for 30 min by a solution containing HNO3 and 30% H2O2. Afterwards, they underwent a 15-min heating with concentrated HCl without boiling. The products were then cooled down, filtered and diluted to 50 mL using distilled water (Radojevic and Bashkin 1999).
Accumulation and translocation of heavy metals
The bioaccumulation and translocation factors can be applied to evaluate the phytoextraction efficiency of plants. The bioaccumulation factor is an indicator of plant efficiency to accumulate a metal within its tissues from its environment (Ladislas et al. 2012). This parameter was calculated by Zhuang et al. (2007):
where Charvested and Csoil indicate the target metal concentrations of the harvested plant tissue and soil (substrate), respectively.
Translocation factor is the criterion measuring the plant efficiency in translocation of the accumulated metal from its roots to shoots, which can be determined by Padmavathiamma and Li (2007):
where Cshoot denotes the metal concentration in plant shoots, whereas Croot shows the metal concentration within the plant roots.
Enzyme assays
The frozen shoot sample (0.5 g) was homogenized in 0.05 M phosphate buffer (pH 7.8) within an ice bath using the mortar grinding and a pestle with liquid nitrogen. The homogenized samples were then centrifuged (15,000 × g) for 15 min at 4 °C. The obtained supernatants were kept at 4 °C to assess various antioxidant enzyme activities. The method of Bradford (1976) was employed to evaluate protein content using bovine serum albumin (BSA) as standard.
POX (EC 1.11.1.7) activity was measured at 470 nm in which the samples’ guaiacol–tetraguaiacol conversion ability was tested (extinction coefficient 26.6 mM−1 cm−1) as described by Chance and Maehly (1955).
H2O2 reduction was measured (extinction coefficient 39.4 mM−1 cm−1) at 240 nm to assess the CAT (1.11.1.6) activity based on the method previously described by Aebi (1984).
APX (EC 1.11.1.11) activity was evaluated through 1-min examination of A290 decline (extinction coefficient 2.8 mM−1 cm−1) in 1 mL of a reaction mixture as developed by Nakano and Asada (1981). In the mentioned procedure, the reaction mixture contained 100 mM potassium phosphate buffer (pH 7.0), 2 mM H2O2, 0.5 mM ASC and a given level of enzyme extract. The reaction started by addition of the crude enzyme extract. Corrections were made considering the low non-enzymatic reduction of ascorbate (ASC) by H2O2.
The SOD (1.15.1.1) activity was conducted by the procedure as described by Dhindsa et al. (1980). This method relies on spectrophotometric evaluation of inhibition in the photochemical reduction of nitroblue tetrazolium (NBT) at 560 nm. For this purpose, an action mixture containing 50 mM K phosphate buffer (pH 7.8), 13 mM methionine, 75 μM NBT, 0.1 μM EDTA, 4 μM riboflavin and a specific amount of enzyme extract was used. The reaction initiated upon addition of riboflavin to the solution and 15 min exposure of the tubes to fluorescent (15 W) irradiation. An enzyme-free reaction mixture which resulted in maximum color was employed as the control, while the blank sample was a non-irradiated complete reaction mixture. One unit of SOD activity is defined as the amount of enzyme needed for 50% inhibition in the reduction of NBT at 560 nm, as evaluated based on Giannopolitis and Ries’ method (1977).
Statistical analysis
The obtained data were analyzed by analysis of variance (ANOVA) using SAS statistical software version 6, which involved three replicates (n = 3) of an RCBD-based (randomized complete block design) factorial experiment. Pearson’s correlation coefficient was applied for correlation of the studied traits using SPSS version 16 (SPSS Inc., Chicago, United States). Analysis of mean (ANOM) was carried out using Duncan’s multiple range test (DMRT) at P < 0.01.
Results and discussion
Growth parameters
Data analyses (ANOVA) revealed the significant (P < 0.01) effect of different concentrations of Cr and nanoparticles on root and shoot dry weights (Table 1). As shown, significant difference was observed between the seedlings grown under Cr stress treatment compared to the non-stress conditions. Dry weight of root and shoot organs exhibited 31.68% and 44.33% reduction under stress conditions, respectively (Table 2). However, application of Fe0 nanoparticles improved root and shoot tissues growth of sunflower plants grown under Cr stress and non-stress conditions (Table 2). Exposure of the plant to a high concentration of Cr resulted in serious damages to metabolic activities and decrease in growth. High levels of heavy metals may inhibit enzymatic activities (Gadd 2007), inactivate photosystems (Sandmann and Boger 1980) and disturb the nutritional balance of the plant (Janas et al. 2010). Given the high biomass of sunflower plant, it could be a proper choice in phytoremediation for rapid elimination of heavy metals (Forte and Mutiti 2017).
In the present study, a significant decline was observed in growth of root and shoot tissues of sunflower grown under Cr stress which was more profound in the case of roots. Studies also revealed that Cr affects the division and elongation of cells which finally led to decline in growth rate (Sing et al. 2013). These results are in line with previous reports showing that Cr treatment will decrease the growth of the plants (Maiti et al. 2012; Amin et al. 2013). Presence of iron nanoparticles improved the growth condition of sunflowers under Cr stress which could be due to improvement of photosynthesis system, plastid pigments and carbohydrate metabolism (Malik et al. 2011, 2012).
Our previous study showed that exposure to Cr may increase oxidative damage and therefore trigger morpho-physiological damages through increasing H2O2 and MDA, while iron nanoparticles remarkably declined H2O2 and MDA contents (Mohammadi et al. 2018). Enhanced efficiency of plants in terms of growth, physiology and yield has been observed in response to the application of engineered nanomaterials under normal and heavy metal stress conditions. According to Singh and Lee (2016) TiO2 nanoparticles confined Cd toxicity and enhanced growth, relative water and chlorophyll contents and photosynthetic rate in heavy metal-exposed soybean. Similarly, improved growth, nutrient assimilation, photosynthesis and activities of antioxidant enzymes have been observed in response to the application of Si nanoparticles to wheat plants against Cr(VI) stress (Tripathi et al. 2015).
Cr translocation and bioaccumulation
Cr content in root and shoot tissues of sunflower increased with increasing Cr concentration from 0 to 150 ppm (Table 2). The highest Cr content was observed in the shoot (0.799 mg g−1 DW) and root (0.381 mg g−1 DW), respectively.
Bioaccumulation and translocation factors have been proven as the appropriate tools to evaluate plants’ ability in absorbing heavy metals (Brooks 1998). Bioaccumulation results showed that sunflower may accumulate higher concentrations of Cr in its shoot organs compared to the roots. Cr translocation from the soil to shoot organs resulted in BAF > 1 in low soil Cr concentrations with an average of 1.42 (from soil to root) and 2.94 (from soil to shoot). Further increase of Cr in soil to 150 ppm led to BAF enhancement (Table 3). As BAF > 1, therefore, sunflower has high potential in accumulating heavy metal (Zu et al. 2005). Hence, it can be used to clean Cr-contaminated soils.
Cr translocation to plant tissues (TF) showed that sunflower is able to transport Cr into its tissues which increased by enhancement of Cr concentration. As both BAF and TF values are more than one, sunflower revealed high capacity for phytoextraction process when the soil was spiked with hexavalent Cr.
Although phytoremediation is a promising approach to clean the soils contaminated with heavy metals, it also suffers from several limitations including contamination of the nutritional system. Application of zerovalent iron nanoparticles (Fe0), in particular at a concentration of 2%, significantly (P < 0.01) decreased BAF and TF values (Table 3), which could be attributed to immobilization of Cr by nanoparticles and/or improvement of sunflower defense system due to application of iron nanoparticles.
Nanoparticles are very effective in remediation of different metal ions from aqueous as well as soil ecosystems and among the different nanomaterials tested for the remediation purpose, Fe0 has been successfully utilized to trim down the concentrations of arsenic (As-III) and chromium (Cr-VI) (Bhowmick et al. 2014; Wang et al. 2014; Poguberovic et al. 2016). Application of Fe0 as a reducing agent is an extensively utilized tool for the environmental remediation purpose during the recent years (Shi et al. 2011; Qu et al. 2013). High surface energy together with the reaction activity of these Fe0 allows their utilization for the swift decontamination of a number of pollutants including the deadly heavy metals (Sun et al. 2006). Furthermore, Fe0 reduces heavy metal bioavailability in the soil and improves the biomass of plants (Nasiri et al. 2013; Tafazoli et al. 2017). The explicit elimination mechanisms involved in the remediation of the heavy metals by the application of Fe0 is primarily determined by the standard redox potential of the metal contaminant. Metals with similar or more negative standard redox potential (e.g., Cd and Zn) to Fe can be removed following adsorption with Fe0, while metals with positive standard redox potential (e.g., Pb and Ni) are removed by both adsorption as well as reduction. Similar to our result, Watanabe et al. (2009) reported that application of Fe0 (0.01%) to a Cd-spiked soil significantly reduces the accumulation of Cd in rice leaves and seeds. Adsorption of Cd on the nanoparticles’ surface helps in reducing the Cd toxicity mainly due to the reduced bioavailability. Moreover, support also comes from the studies of Liu et al. (2008) who observed that supplementation of soils with Fe nanoparticles immobilizes Cd, thereby reducing its bioavailability to plants. Houben and Sonnet (2010) have also reported that application of powdered Fe nanoparticles reduce the amount of Cd and Zn in the soils by 45–63%, respectively.
Antioxidant defense enzymes
The activities of SOD, CAT, POX, and APX enzymes increased in shoot organs of plants grown under 75 ppm of Cr treatment. In comparison to control, 6.23, 11.74, 6.05 and 0.8% increase was observed in the activities of CAT, POX, APX and SOD enzymes, respectively. However, the activities of CAT, POX and SOD enzymes exhibited 8.87, 8.47 and 40.51% reduction under Cr stress conditions, respectively (Fig. 2). Iron nanoparticles also enhance the activity of CAT, POX, APX and SOD enzymes which finally resulted in improvement of seedlings’ antioxidant system (Fig. 2), which can be subsequently observed in growth of roots and shoot organs.
In the present study, activities of CAT, POX, APX and SOD increased under the effect of Cr (Table 4) which are consistent with previous reports expressing that Cr may enhance the activities of antioxidant enzymes (Ali et al. 2011). Other studies also reported that ROS generated due to stress may induce tolerance mechanisms in the plants (Apel and Hirt 2004; Baiazidi-Aghdam et al. 2016; Ghorbanpour and Hatami 2015; Hatami et al. 2019). It has been revealed that some of the antioxidant enzymes can control ROS contents in intracellular levels, but CAT, APX, POX and SOD play a major role under such conditions (Noctor and Foyer 1998; Tian et al. 2018; Ghorbanpour and Hadian 2015). As the sunflower seedlings decreased the growth of both shoot and root parts under Cr stress treatments, it seems that the defense mechanisms failed to detoxify ROS and decline their accumulation. Application of Fe0 under Cr stress, however, managed to improve the growth through increasing antioxidant enzyme activities and even succeeded to reduce TF and BAF contents and hence their accumulation.
When heavy metal concentration in the environment exceeds the toxicity threshold of the plants (depending on its species), overaccumulation of heavy metals within the cells will cause numerous toxic effects including rapid growth inhibition, leaf chlorosis and early aging of the leaves due to reduced photosynthesis, decline in root elongation and prevention of seeds’ germination (Borowiak et al. 2012; Obrouchova et al. 1998). Heavy metals interfere with active sites of many enzymes including ATPase, phosphatase and antioxidants enzymes (e.g., SOD, CAT, GR, and APX) which will prevent from natural activity of the mentioned enzymes (Van Assche and Clijsters1990; Verma and Dubey 2003). However, antioxidative enzymes’ function synergistically or cooperatively during environmental perturbations helps the plants to overcome oxidative stress.
Pearson’s correlation analysis among studied traits in sunflower plants treated with different concentrations of Cr and Fe0 is presented in Table 4. Data show that root and shoot dry weights were significantly negatively correlated with translocation factor (TF) under all employed treatments. Among Fe0 concentrations, the strongest correlation coefficient (r0.01 = − 0.999) was observed between shoot Cr content and root bioaccumulation factor (BAFr) under application of 1% Fe0, followed by root and shoot bioaccumulation factor under 0% Fe0 treatment. There was negative and significant correlation between CAT activity and TF (r0.01 = − 0.815), BAFr (r0.01 = − 0.875) and BAFr (r0.01 = − 0.880) upon exposure to 2% Fe0 (Table 4).
Conclusions
Our results suggest that the Cr(VI) targets root and shoot growth, antioxidant enzyme activities, however, application of Fe0 nanoparticles ameliorated the Cr(VI)-induced toxic effects. The results indicated that sunflower has a high ability to accumulate a significant amount of Cr(VI) in its roots and shoots. However, the accumulation of Cr in plant shoot was more than the root. The value of BAF for Cr was higher than one for the treatment of Cr at 150 ppm, indicating its ability for metal ion uptake. In addition, the value of the TF of Cr decreased by increasing the levels of Cr in soil, however, this reduction was more in treatment with Fe0 nanoparticles. Stress amelioration of Cr with exogenous application of Fe0 nanoparticles to sunflower seedlings reached maximum value through enhancement of enzymatic antioxidant defense system which was accompanied by changing BAF and TF.
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Ali S, Bai P, Zeng F, Cai S, Shamsi IH, Qiu B, Wu F, Zhang G (2011) The ecotoxicological and interactive effects of chromium and aluminum on growth, oxidative damage and antioxidant enzymes on two barley genotypes differing in Al tolerance. Environ Exp Bot 70(2):185–191
Alia Mohanty P, Matysik J (2001) Effect of proline on the production of singlet oxygen. Amino Acids 21:195–200
Amin H, Arain BA, Amin F, Surhio MA (2013) Phytotoxicity of chromium on germination, growth and biochemical attributes of Hibiscus esculentus L. Am J Plant Sci 4:2431–2439
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Asgari Lajayer B, Ghorbanpour M, Nikabadi S (2017a) Heavy metals in contaminated environment: destiny of secondary metabolite biosynthesis, oxidative status and phytoextraction in medicinal plants. Ecotoxicol Environ Saf 145:377–390
Asgari Lajayer H, Savaghebi G, Hadian J, Hatami M, Pezhmanmehr M (2017b) Comparison of copper and zinc effects on growth, micro and macronutrients status and essential oil constituents in pennyroyal (Mentha pulegium L.). Braz J Bot 40:379–388
Asgari LB, Khadem Moghadam N, Maghsoodi MR, Ghorbanpour M, Kariman Kh (2019) Phytoextraction of heavy metals from contaminated soil, water and atmosphere using ornamental plants: mechanisms and efficiency improvement strategies. Environ Sci Pollut Res 26:8468
Baiazidi-Aghdam MT, Mohammadi H, Ghorbanpour M (2016) Effects of nanoparticulate anatase titanium dioxide on physiological and biochemical performance of Linum usitatissimum (Linaceae) under well watered and drought stress conditions. Braz J Bot 39:139–146
Barnhart N (1997) Chromium and its soils in the proximity of the old tannery waste lagoon. Int Agrophys 15:121–124
Barton LL, Johnson AG, Wagener BM (2000) Inhibition of ferric chelate reductase in alfalfa roots by cobalt, nickel, chromium and copper. J Plant Nutr 23:1833–1845
Bhowmick S, Chakraborty S, Mondal P, Van Renterghem W, Van den Berghe S, Roman-Ross G, Chatterjee D, Iglesias M (2014) Montmorillonite-supported nanoscale zero-valent iron removal of arsenic from aqueous solution: kinetics and mechanism. Chem Eng J 243:14–23
Bishnoi NR, Chugh LK, Sawhney SK (1993) Effect of chromium on photosynthesis, respiration and nitrogen fixation in pea (Pisum sativum L.) seedlings. J Plant Physiol 142:25–30
Borowiak K, Drzewiecka K, Magdziak Z, Gasecka M, Mleczek M (2012) Effect of Ca/Mg ions ratio on copper accumulation, photosynthetic activity and growth of Cu2+-treated Salix viminalis L. ‘Cannabina’. Photosynthetica 50:353. https://doi.org/10.1007/s11099-012-0040-8
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1–2):248–254
Brooks RR (1998) Plants that hyperaccumulate heavy metals. Their role in phytoremediation, microbiology, archaeology, mineral exploration and phytomining. CAB International, Wallingford, p 380
Chance B, Maehly AC (1955) Assay of catalases and peroxidases. Methods Enzymol 2:764–775
Clemens S (2001) Molecular mechanisms of plant metal tolerance and homeostasis. Planta 212:475–486
Cohen MD, Kargacin B, Klein CB, Costa M (1993) Mechanism of chromium carcinogenicity and toxicity. Crit Rev Toxicol 23:255–281
Dhindsa RS, Dhindsa PP, Thorpe TA (1980) Leaf senescence correlated with increased levels of membrane permeability and lipid-peroxidation and decreased levels of superoxide dismutase and catalase. J Exp Bot 32:93–101
Forte J, Mutiti S (2017) Phytoremediation potential of Helianthus annuus and Hydrangea paniculata in copper and lead-contaminated soil. Water Air Soil Pollut 228:1–11
Gadd GM (2007) Geomycology: biogeochemical transformations of rocks, minerals, metals and radionuclides by fungi, bioweathering and bioremediation. Mycol Res 111:3–49
Ghorbanpour M, Hadian J (2015) Multi-walled carbon nanotubes stimulate callus induction, secondary metabolites biosynthesis and antioxidant capacity in medicinal plant Satureja khuzestanica grown in vitro. Carbon 94:749–759
Ghorbanpour M, Hatami H (2015) Changes in growth, antioxidant defense system and major essential oils constituents of Pelargonium graveolens plant exposed to nano-scale silver and thidiazuron. Ind J Plant Physiol. 20(2):116–123
Giannopolitis CN, Ries SK (1977) Superoxide dismutases occurrence in higher plants. Plant Physiol 59:309–314
Hatami M, Hosseini SM, Ghorbanpour M, Kariman K (2019) Physiological and antioxidative responses to GO/PANI nanocomposite in intact and demucilaged seeds and young seedlings of Salvia mirzayanii. Chemosphere 233:920–935
Houben D, Sonnet P (2010) Leaching and phytoavailability of zinc and cadmium in a contaminated soil treated with zero-valent iron. In: Proceedings of the 19th world congress of soil science, soil solutions for a changing world, pp 1–6
Jabeen R, Ahmad A, Iqbal M (2009) Phytoremediation of heavy metals: physiological and molecular mechanisms. Bot Rev 75:339–364
Janas KM, Zieli A, Ska-Tomaszewska J, Rybaczek D, Maszewski J, Posmyk MM, Amarowicz R, Kosińska A (2010) The impact of copper ions on growth, lipid peroxidation, and phenolic compound accumulation and localization in lentil (Lens culinaris Medic.) seedlings. J Plant Physiol 167:270–276
Juwarkar AA, Singh SK, Mudhoo A (2010) A comprehensive overview of elements in bioremediation. Rev Environ Sci Biotechnol 9:215–288
Kumar NP, Dushenkov V, Motto H, Raskin I (1995) Phytoextraction: the use of plants to remove heavy metals from soils. Environ Sci Technol 29:1232–1238
Ladislas S, El-Mufleh A, Gerente C, Chazarenc F, Andres Y, Bechet B (2012) Potential of aquatic macrophytes as bioindicators of heavy metal pollution in urban stormwater runoff. Water Air Soil Pollut 223:877–888
Liang D, Wang B, Song S, Wang J, Wang L, Ren X, Zhao X (2019) Analysis of genetic effects on a complete diallel cross test of Pinus koraiensis. Euphytica 215(5):92
Liu JH, Inoue H, Moriguchi T (2008) Salt stress-mediated changes in free polyamine titers and expression of genes responsible for polyamine biosynthesis of apple in vitro shoots. Environ Exp Bot 62:28–35
Macnair MR, Tilstone GH, Smith SE (2000) The genetics of metal tolerance and accumulation in higher plants. In: Terry N, Banuelos G (eds) Phytoremediation of contaminated soil and water. CRC Press, Boca Raton, pp 235–250
Maiti S, Ghosh N, Mandal C, Das K, Dey N, Adak MK (2012) Responses of the maize plant to chromium stress with reference to antioxidation activity. Braz J Plant Physiol 24(3):203–212
Malik JA, Kumar S, Thakur P, Sharma S, Kaur N, Kaur R, Pathania D, Bhandhari K, Kaushal N, Singh K, Srivastava A (2011) Promotion of growth in mungbean (Phaseolus aureus Roxb.) by selenium is associated with stimulation of carbohydrate metabolism. Biol Trace Elem Res 143(1):530–539
Malik JA, Goel S, Kaur N, Sharma S, Singh I, Nayyar H (2012) Selenium antagonises the toxic effects of arsenic on mungbean (Phaseolus aureus Roxb.) plants by restricting its uptake and enhancing the antioxidative and detoxification mechanisms. Environ Exp Bot 77:242–248
Marschner H (1995) Mineral nutrition of higher plants, 2nd edn. Academic, London
Mehta SK, Gaur JP (1999) Heavy metal induced proline accumulation and its role in ameliorating metal toxicity in Chlorella vulgaris. New Physiol 143:253–259
Mohammadi H, Hatami M, Feghezadeh K, Ghorbanpour M (2018) Mitigating effect of nano-zerovalent iron, iron sulfate and EDTA against oxidative stress induced by chromium in Helianthus annuus L. Acta Physiol Plant 40:69
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22(5):867
Nasiri J, Gholami A, Panahpour E (2013) Removal of cadmium from soil resources using stabilized zero-valent iron nanoparticles. J Civ Eng Urban 3:338–341
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol 49:249–279
Obrouchova NV, Bystrova EI, Ivanov VB, Anupova O, Seregin I (1998) Root growth responses to lead in young maize seedlings. Plant Soil 200:55–61
Padmavathiamma PK, Li LY (2007) Phytoremediation technology: hyperaccumulation metals in plants. Water Air Soil Pollut 184:105–126
Panda SK, Choudhury S (2005) Chromium stress in plants. Braz J Plant Physiol 17:95–192
Piechalak A, Tomaszewska B, Barałkiewicz D, Malecka A (2002) Accumulation and detoxification of lead in legumes. Phytochemistry 60:153–162
Poguberović SS, Krčmar DM, Maletić SP, Kónya Z, Pilipović DD, Kerkez DV, Rončević SD (2016) Removal of As (III) and Cr(VI) from aqueous solutions using “green” zero-valent iron nanoparticles produced by oak, mulberry and cherry leaf extracts. Ecol Eng 90:42–49
Prasad MNV, Strzaka K (2002) Physiology and biochemistry of metal toxicity and tolerance in plants. Plant Sci 161:881–889
Qu X, Alvarez PJ, Li Q (2013) Applications of nanotechnology in water and wastewater treatment. Water Res 47:3931–3946
Radojevic M, Bashkin VN (1999) Practical environmental analysis. Royal Society of Chemistry, Cambridge
Sandmann G, Böger P (1980) Copper-mediated lipid peroxidation processes in photosynthetic membranes. Plant Physiol 66:797–800
Shanker KA, Cervantes C, Loza-Taversa H, Avudainayagam S (2005) Chromium toxicity in plants. Environ Int 31:739–753
Shi J, Abid AD, Kennedy IM, Hristova KR, Silk WK (2011) To duckweeds (Landoltia punctata), nanoparticulate copper oxide is more inhibitory than the soluble copper in the bulk solution. Environ Pollut 159:1277–1282
Singh J, Lee BK (2016) Influence of nano-TiO2 particles on the bioaccumulation of Cd in soybean plants (Glycine max): a possible mechanism for the removal of Cd from the contaminated soil. J Environ Manag 170:88–96
Singh HP, Mahajan P, Kaur S, Batish DR, Kohli RK (2013) Chromium toxicity and tolerance in plants. Environ Chem Lett 11(3):229–254
Song J, Zhang H, Duan C, Cui X (2018) Exogenous application of succinic acid enhances tolerance of Larix olgensis seedling to lead stress. J For Res 29(6):1497–1505
Sun RL, Zhou QX, Jin CX (2006) Cadmium accumulation in relation to organic acids in leaves of Solanum nigrum L. as a newly found cadmium hyperaccumulator. Plant Soil 285:125–134
Sundaramoorthy P, Alagappan C, Kaliyaperumal SG, Pachikkaran U, Logalashmanan B (2010) Chromium stress in paddy: (i) nutrient status of paddy under chromium stress; (ii) phytoremediation of chromium by aquatic and terrestrial weeds. CR Biol 333:597–607
Tafazoli M, Hojjati SM, Biparva P, Kooch Y, Lamersdorf N (2017) Reduction of soil heavy metal bioavailability by nanoparticles and cellulosic wastes improved the biomass of tree seedlings. J Plant Nutr Soil Sci 180:683–693
Tian H, Ghorbanpour M, Kariman K (2018) Manganese oxide nanoparticle-induced changes in growth, redox reactions and elicitation of antioxidant metabolites in deadly nightshade (Atropa belladonna L.). Ind Crops Prod 126:403–414
Tripathi DK, Singh VP, Prasad SM, Chauhan DK, Dubey NK, Rai AK (2015) Silicon-mediated alleviation of Cr(VI) toxicity in wheat seedlings as evidenced by chlorophyll florescence, laser induced breakdown spectroscopy and anatomical changes. Ecotoxicol Environ Saf 113:133–144
Tucker M (1974) A modified heating block foe plant tissue digestion 1. Commun Soil Sci Plant Anal 5:539–546
Vamerali T, Bandiera M, Mosca G (2010) Field crops for phytoremediation of metal-contaminated land. A review. Environ Chem Lett 8:1–17
Van Assche F, Clijsters H (1990) Effect of metal on enzyme activity in plants. Plant Cell Environ 13:195–206
Verma S, Dubey R (2003) Lead toxicity induces lipid peroxidation and alters the activities of antioxidant enzymes in growing rice plants. Plant Sci 164:645–655
Vilardi G, Verdone N, Di Palma L (2017) The influence of nitrate on the reduction of hexavalent chromium by zero-valent iron nanoparticles in polluted wastewater. Desalin Water Treat 86:252–258
Wang C, Luo H, Zhang Z, Wu Y, Zhang J, Chen S (2014) Removal of As(III) and As(V) from aqueous solutions using nanoscale zero valent iron-reduced graphite oxide modified composites. J Hazard Mater 268:124–131
Watanabe T, Murata Y, Nakamura T, Sakai Y, Osaki M (2009) Effect of zero-valent iron application on cadmium uptake in rice plants grown in cadmium-contaminated soils. J Plant Nutr 32:1164–1172
Zayed A, Lytle CM, Jin-Hong Q, Terry N, Qian JH (1998) Chromium accumulation, translocation and chemical speciation in vegetable crops. Planta 206:293–299
Zenk M (1996) Heavy metal deteoxification. Curr Opin Plant Biol 3:211–216
Zhuang P, Yang Q, Wang H, Shu W (2007) Phytoextraction of heavy metals by eight plant species in the field. Water Air Soil Pollut 184:235–242
Zu YQ, Li Y, Chen JJ, Chen HY, Qin L, Schvartz C (2005) Hyperaccumulation of Pb, Zn and Cd in herbaceous plants grown on lead–zinc mining area in Yunnan, China. Environ Int 31:755–762
Acknowledgements
This study was supported by Deputy of Research and Technology of Azarbaijan Shahid Madani University (95/D/897), Tabriz, Iran.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that he/she has no conflict of interest.
Rights and permissions
About this article
Cite this article
Mohammadi, H., Amani-Ghadim, A.R., Matin, A.A. et al. Fe0 nanoparticles improve physiological and antioxidative attributes of sunflower (Helianthus annuus) plants grown in soil spiked with hexavalent chromium. 3 Biotech 10, 19 (2020). https://doi.org/10.1007/s13205-019-2002-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-019-2002-3