Abstract
As a major root-knot nematode (RKN), Meloidogyne incognita causes serious losses in the yield of sweet potato (Ipomoea batatas L.). To successfully colonize the host plant, RKNs elicit changes of dramatic physiological and morphological features in the plants. The expression of several genes is regulated as the nematode establishes its feeding site. Therefore, in this study, we analyzed the proteomes in the fibrous roots of sweet potato plants by an infection of RKN to understand the effect of the infection on the plant root regions. This study revealed differences in proteomes of the RKN-resistant sweet potato cultivar Juhwangmi and RKN-sensitive cultivar Yulmi. During plant growth, Juhwangmi plants were shown to be more resistant to M. incognita than Yulmi plants. No M. incognita egg formation was observed in Juhwangmi plants, whereas 587 egg masses were formed in Yulmi plants. Differentially expressed 64 spots were confirmed by proteomic analysis using 2-D gel electrophoresis with three spots up-regulated in the two cultivars during RKN infection. Of these 64 protein spots, 20 were identified as belonging to such different functional categories as the defense response, cell structure, and energy metabolism. This study provides insight into the molecular and biochemical mechanics of the defense response and metabolism of sweet potato plant during nematode invasion. We anticipate that this study will also provide a molecular basis for useful crop breeding and the development of nematode-tolerant plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant-parasitic nematodes (PPNs) cause an estimated average crop yield loss of 10.7% in life-sustaining crops and 14% in economically important crop plants (Palomares-Rius and Kikuchi 2013). These losses are usually estimated to exceed 100 billion US dollars. Among PPNs, root-knot nematodes (RKNs; Meloidogyne spp.) are sessile internal parasites. The most economically significant species are M. hapla in cool climates and M. arenaria, M. javanica, and M. incognita. These are considered important RKNs in the major crop plants and can be easily found by the galls or knots in the root regions (Bird 1996; Caillaud et al. 2008). These RKNs usually cause dramatic changes of physiological and morphological features in the plants. In the RKN-infected plan cells, expressions of some genes are repressed by RKNs to establish feeding sites, whereas expression of nematode genes was induced during infection (Williamson and Gleason 2003). They also showed the immune response to nematodes (Goverse and Smart 2014).
Sweet potato (Ipomoea batatas L.) is one of the important food crop plants, especially in parts of Asia and Africa. It serves as a nutrient source for humans, providing energy, fiber, and antioxidants, including carotenoids and anthocyanin, and can be used to produce industrial raw materials for animal feed, starch, and alcohol (Diaz et al. 2014; Grace et al. 2014). The major damage agents of sweet potato are fungal and viral diseases (Clark and Moyer 1998; Kreuze 2002). The PPNs can also decrease its productivity up to 10.2–11.4% (Kistner et al. 1993; Palomares-Rius and Kikuchi 2013). The main PPNs affecting sweet potato are RKNs: M. incognita Chitwood, M. javanica Chitwood, M. arenaria Chitwood and M. hapla Chitwood (Kistner et al. 1993; Agu 2004). Sweet potato is a highly suitable host especially for the southern RKN, thereby M. incognita causes severe damage to the storage roots and occurs worldwide in the tropics (Bridge and Starr 2010).
The molecular genetics and physiological mechanisms of resistance of sweet potato to RKNs are poorly understood. Previous studies indicate a large number of RKN resistance-related genes in sweet potato, suggesting that RKN resistance might be inherited in the form of multiple molecular genetic factors (Jones and Dukes 1980). Cervantes-Flores et al. (2002a, b) evaluated five sweet potato cultivars with respect to resistance to different RKN species. They found that the resistance response of each cultivar differed depending on the nematode species; therefore, different genes may be involved in the sweet potato resistance to RKNs.
The aim of this research was to study the isolation and molecular analysis of RKN resistance proteins of sweet potato during M. incognita infestation using proteomic analysis. The study analyzes the resistance and proteomes of RKN-sensitive and -resistant cultivars during an infection with RKN M. incognita Chitwood 1949, to determine the role of RKN-resistant molecular mechanisms in sweet potato plants. The results presented herein enhance the understanding of plant defense mechanisms against RKN infestation in RKN-sensitive and -resistant sweet potato cultivars.
Materials and methods
Plant materials
Two sweet potato (Ipomoea batatas L. Lam) cultivars used in this study, the most RKN-sensitive cultivar Yulmi and the most RKN-resistant cultivar Juhwangmi, were obtained from Bioenergy Crop Research Center, National Crop Research Institute (RDA, Muan, Jeonnam, Korea).
Plant treatment with M. incognita
Sweet potato plants were cultivated and treated with M. incognita according to a method of Lee et al. (2012). The Yulmi and Juhwangmi cultivar cuttings (approximately 10 cm long) with three leaves attached were transplanted into plastic pots (24 cm width, 18 cm length, and 12 cm height) filled with sandy loam soil infested with M. incognita at a density of 1154 ± 176 s-stage juveniles per 300 g of soil. M. incognita infested soil was collected from natural occurrence greenhouse in experimental farm of Gyeongsang National University. As a control, the soil was steam-sterilized at 115 °C and 1.5 atmospheric pressure for 20 min. For treatments under growth chamber conditions, the sweet potato cuttings were grown in a growth chamber conditions (16 h photoperiod, 30/22 °C day/night temperature, and 70% relative humidity) with light (intensity: 200 µmol s−1 m−2). The plants were harvested 50 days after planting and washed in tap water, and plant fresh weights and RKN egg masses were then determined. The plants were cultivated in ten replicates.
Analysis of plant RKN resistance
The egg masses formed by M. incognita in the fibrous roots of the sweet potato plants were dyed with a Phloxin B solution and counted (Viaene et al. 2012). The design of the experiment was completely randomized, with ten replicates.
Two-dimensional electrophoresis
Total protein was isolated from the fibrous roots of each sweet potato plant using a modified phenol-based method (Hajduch et al. 2005). Two-dimensional SDS-PAGE (2-DE) and isoelectric focusing (IEF) and were performed as described previously (Lee et al. 2012). Total protein extract was separated on Bio-Rad 17 cm immobilized pH gradient gel (IPG) strips (pH 5–8). After IEF, the IPG strips were equilibrated according to the manufacturer’s protocol (Bio-Rad, Hercules, CA, USA). SDS-PAGE was performed using PROTEAN II xi Cell (Bio-Rad). The 2-DE gels were stained with colloidal Coomassie brilliant blue (CBB). At least ten independent proteins were isolated from different infected fibrous root samples and used in 2-DE analysis. After electrophoresis, gel images were analyzed using a PDQuest software (Version 7.2.0; Bio-Rad) and GS-800 Calibrated Imaging Densitometer (Bio-Rad).
MALDI-TOF/TOF MS analysis
The spots were excised from the CBB-stained gels and subjected to reduction, alkylation, and in-gel digestion, as described previously (Kwon et al. 2016). The analyses were carried out using an ABI 4800 Plus TOF–TOF Mass Spectrometer (Applied Biosystems, Framingham, MA, USA). Spectral data (MS and MS/MS) were unpacked using the NCBI (https://www.ncbi.nlm.nih.gov/), Protein Pilot V.3.0 and UniProt database (version 20131104; 30,938,908 sequences), at 100 ppm mass tolerance. Database search criteria with the MS/MS spectra were as follows: a single missing peak, carbamidomethylation of cysteines, and oxidation of methionines.
Gene expression analysis
Quantitative real-time PCR analysis was investigated using a Bio-Rad CFX96 thermal cycler (Bio-Rad, USA) with EvaGreen fluorescent dye according to the manufacturer’s instructions. The transcriptional expression levels were analyzed by quantitative RT-PCR using gene-specific primers (Table 1).
Results
Differential RKN resistance of the two sweet potato cultivars
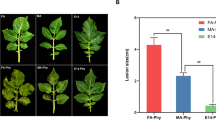
According to the reports of Bioenergy Crop Research Center, Yulmi is the most RKN-sensitive sweet potato cultivar, whereas Juhwangmi is the most RKN-resistant cultivar (Choi et al. 2006). Therefore, we first tested and compared the resistance of Juhwangmi and Yulmi cultivars to M. incognita. When the plants were grown in the growth chambers in the soil infested with M. incognita, the shoot and root growth of Yulmi plants were inhibited by 38.7 and 34.8%, respectively, compared with uninfested conditions, whereas the shoot and root growth of Juhwangmi plants were not inhibited by RKN treatment (Fig. 1a, b). M. incognita formed 587 infested egg masses in Yulmi plants, while no egg formation was observed in Juhwangmi plants (Fig. 1c, d). Therefore, consistent with previous reports, our results demonstrated that, in comparison with the Yulmi cultivar, the Juhwangmi cultivar was highly resistant to M. incognita under growth chamber conditions.
The effect of the root-knot nematode M. incognita on the growth of Yulmi and Juhwangmi sweet potato plants conducted in growth chambers. a Growth inhibition of Yulmi and Juhwangmi cultivars by M. incognita. The plants were photographed 50 days after planting the cuttings in steam-sterilized sand (control) and non-sterilized sand (treatment) containing 1154 ± 176 M. incognita second-stage juveniles per 300 g of soil. b Fresh weight of shoots and roots. The fresh weights (g) were determined 50 days after the cuttings had been planted in steam-sterilized sand and non-sterilized sand. c Egg masses formed by M. incognita. Egg masses were photographed 50 days after the cuttings had been planted in non-sterilized sand containing M. incognita. d The quantification of egg masses formed by M. incognita. Egg masses were counted 50 days after the cuttings had been planted in non-sterilized sand containing M. incognita. Egg masses were not formed in the sweet potato cultivars cultured in sterilized soil. Data represent mean ± SD of ten replicates. The experimental results are the average of ten independent plants and four different biological replications
2-DE analysis of the sweet potato fibrous root proteomes in response to RKNs
Total protein isolated from the fibrous roots of Yulmi and Juhwangmi cultivars was electrophoresed and compared by 2-DE (Fig. 2). The pIs ranges of the spots are pH 5–8, and the molecular masses of most spots are 14.4–116.0 kDa. To evaluate expressed protein patterns, the spots in all replicate gels were quantitatively compared using PDQuest software. Although the general protein spot patterns of fibrous root remained largely unchanged in the two cultivars, 64 spots showed significant differences in expression in Yulmi and/or Juhwangmi plants under control and M. incognita infection conditions. Of these 64 protein spots, the intensity of 48 protein spots in Yulmi samples (red arrows; spots 1, 2, 3, 5, 6, 8, 9–20, 25, 26, 28–44, 46, 47, and 49–57) was specifically increased upon exposure to RKNs compared with control conditions; the intensity of four spots (green arrows; spots 27, 48, 58, and 59) decreased during RKN infection (Fig. 2 and Table 1). The intensity of five spots specific for Juhwangmi (black arrows; spots 4, 7, 21, 22, and 25) also increased after RKN exposure, compared with control conditions. Interestingly, three spots (sky blue arrows; spots 23, 24, and 64) were up-regulated in both cultivars during M. incognita infection.
High-resolution 2-DE of total protein extracted from fibrous roots of the RKN-sensitive Yulmi and RKN-resistant Juhwangmi sweet potato cultivars. The proteins were first separated on IPG strips (pH 5–8), followed by SDS-PAGE on 11.5% polyacrylamide gels. Gels were stained with colloidal CBB. Spot numbers indicate proteins that were differentially expressed in Yulmi or Juhwangmi plants. Red arrows indicate Yulmi-specific spots up-regulated during RKN infection; green arrows indicate Yulmi-specific spots up-regulated during RKN infection; black arrows indicate Juhwangmi-specific spots up-regulated during RKN infection; sky blue arrows indicate spots up-regulated in both cultivars during RKN infection. 10 gels per a biological replicate are assessed
Identification of proteins involved in the response of the sweet potato fibrous roots to RKN infection and their functional categorization
The excised protein spots were analyzed by MALDI-TOF/TOF MS. Unfortunately, 44 out of the 64 spots remained unidentified, with 20 protein spots identified (Table 1). Twelve protein spots were identified in Yulmi samples, including cell division cycle protein (spot 1), heat shock protein 70 (HSP70; spots 2, 3, 5, and 6), alpha-tubulin (spot 10), ascorbate peroxidase (APX; spot 25), sporamin A precursor (spot 27), glycine-rich RNA-binding protein 2 (spot 46), lysosomal alpha-mannosidase (spot 48), mitochondrial ATP synthase (spot 51), and actin 4 (spot 54). Two HSP70 proteins (spots 4 and 7) were identified in Juhwangmi samples, whereas GTP-binding protein Ran 3 (spot 23) was identified in both cultivars. Five nematode proteins were also identified, including enolase (spots 12 and 53), glutathione S-transferase (GST; spot 28), and peroxiredoxins (Prxs; spots 30 and 33).
Using AgriGO software (Du et al. 2010), identified 15 sweet potato proteins were analyzed to Gene Ontology (GO) terms for description and annotation of their predictive biological functions. In the biological process category, GO overrepresentation analysis showed that GO terms “cellular process”, “metabolic process”, and “response to stimulus” in the domain “biological process” were significantly overrepresented among identified sweet potato proteins (Fig. 3 and Table 1). Other categories, such as “cellular component organization”, “biological regulation”, “multicellular organismal process”, “multi-organism process” and “developmental process” were also represented among proteins sets, suggesting involvement of the corresponding biological functions in the general response of sweet potato to nematode infection.
Quantitative analysis of identified proteins involved in the response of the sweet potato fibrous roots to RKNs
To investigate the molecular change that occurs under RKN-infected conditions in the fibrous roots of sweet potato, the identified sweet potato 15 proteins were quantitatively analyzed (Fig. 4). The expression patterns of three protein spots, such as spot 1, spot 5, and spot 25, were increased in Yulmi under RKN infection compared with control conditions by 5.9, 5.4 and 2.6 folds, respectively, whereas spot 23 were increased in Juhwangmi by RKN infection compared with control conditions by 1.9-fold (Fig. 4a). However, 27 and 48 protein spots showed decreased expression patterns during RKN treatment in Yulmi only. The other protein spots showed induced expression patterns during RKN infection in Yulmi (spots 2, 3, 6, 10, 46, 51 and 54) or Juhwangmi (spot 4) (Fig. 4b). The protein spots were not detected in both cultivars during control conditions.
Comparison of the expression levels of the identified sweet potato proteins from the fibrous roots of Yulmi and Juhwangmi cultivars in response to RKN infection. a Changes of relative spot intensity of six identified proteins under RKN treatments. b Spot intensities of induced eight identified proteins under RKN treatments in Yulmi or Juhwangmi. Data presented represent the average of five replicates. Statistical significance of differences between the control and treatment groups were determined by one-way ANOVA with LSD post hoc test (P < 0.05 and P < 0.01)
We also attempted to determine whether RKN infection results in a change of nematode proteins in fibrous roots of Yulmi, thus identified nematode five proteins were quantitatively analyzed (Fig. 5). The relative quantities of these RKN proteins were increased only during M. incognita infection in the fibrous root of Yulmi.
Comparison of the expression levels of nematode proteins identified in the fibrous roots of Yulmi cultivar plants infected with RKN. Data presented represent the average of five replicates. Statistical significance of differences between the control and treatment groups were determined by one-way ANOVA with LSD post hoc test (P < 0.05 and P < 0.01)
Correlation between protein and transcript abundance in the sweet potato fibrous root during RKN treatment
To determine whether the expression levels of identified proteins correlated with the abundance of their transcriptional expression levels, we investigated quantitative real-time PCR analysis using gene-specific primers for several genes encoding proteins in the sweet potato fibrous root during RKN treatment (Fig. 6). Our results showed that consistent with proteomic data, transcript levels of HSP 70 (spot 2), APX (spot 25), and Actin (spot 54) up-regulated in fibrous roots of Yulmi during RKN infections.
Quantitative real-time PCR of genes encoding selected proteins that were responded in the fibrous roots of Yulmi and Juhwangmi cultivars in response to RKN infection. Alpha-tubulin served as an internal control. Data shown are the mean ± SE of three independent samples. Bars labeled with the same letter are not significantly different (P < 0.05) according to Duncan’s multiple range test
Discussion
Despite considerable efforts invested in the investigation of the physiological responses of sweet potato to RKN infection, the biochemical and molecular mechanisms involved remain incompletely understood. In the present study, we evaluated the differences in fibrous root proteomic profiles of RKN-resistant and -sensitive sweet potato cultivars. The expression of numerous proteins was altered in the fibrous roots of each cultivar when M. incognita infected and fed on these sweet potato organs (Fig. 2). M. incognita not only triggers a defense response in the sweet potato root tissue but also redesigns the morphological features on the root region to form a gall and converts sweet potato cells into giant cells (GCs) for feeding (Fig. 1).
Differentially up-regulated proteins involved in the cell cycle and structure-related metabolism were detected in the fibrous roots of Yulmi plants (Fig. 3 and Table 1). Regulation of the cell cycle is of pivotal importance to plant growth and development, also during RKN infection (Inze and De Veylder 2006). The proteomic results in this study show that the response of the cell division cycle protein correlates with increased plant cell division that appears at the infection site and is caused by M. incognita infection and feeding (Fig. 1 and Table 1). Cells selected by M. incognita for feeding become multinucleate GCs. Engler et al. (1999) reported cell cycle activation in GCs. Ibrahim et al. (2011) also reported that the expression levels of cyclin in GCs are increased compared with other cyclin-dependent kinases by Meloidogyne infection. Our proteomic result exhibit changed expression of proteins involved in the cellular and cytoskeleton structure. We found that the expression of alpha-tubulin and actin proteins is increased in the fibrous roots of Yulmi sweet potato plants after M. incognita infection (Fig. 3 and Table 1). Therefore, we suggest that the up-regulation of the cell division cycle proteins, alpha-tubulin, and actin in RKN-infected sweet potato fibrous roots coincides with the cellular division and rearrangements of the cytoskeleton that appear during GC generation.
When a nematode invades a plant root, it must control or repress the plant defense response to successfully establish a constant feeding site (Bird 1996; Williamson and Gleason 2003). Our proteomic analysis data revealed changes in the protein expressions associated with response of the defense mechanism. Stress-responsive proteins induced in Yulmi or Juhwangmi plants after RKN infection. The number of RKN-induced proteins involved in defense responses was higher in Yulmi fibrous roots than in Juhwangmi fibrous roots (Fig. 3 and Table 1). Yulmi-specific RKN-responsive proteins included those encoding putative HSP70 proteins (spots 2, 3, 5, and 6), APX (spot 25), and sporamin A proteins (spot 27). Juhwangmi-specific RKN-responsive proteins were two HSP70 proteins (spots 4 and 7). It is known that HSPs are molecular chaperones responsible for protein folding, degradation, and translocation during cellular processes, and stabilizing cell membranes (Wang et al. 2004). In plants, expression analyses of spinach and Arabidopsis Hsp70 genes revealed that many Hsp70 s are responded to stress conditions, such as drought, cold and heat (Sung et al. 2001). However, the cellular functions of Hsp70 during nematode infestation are not fully understood. Interestingly, accumulation of some nematode HSP proteins was also reported during nematode infection (Him et al. 2009). Therefore, we suggest that HSPs likely play a defensive role in the interaction of plant and RKN, affecting the plant’s defense response and/or acting as protective molecules, e.g., chaperones. Sporamins, encoded by a multigene family, are the major storage glycoproteins of the sweet potato storage roots. They perform important functions, e.g., as serine protease inhibitors with a trypsin-inhibiting activity (Yeh et al. 1997). Proteinase inhibitors constitute important plant defense strategies and act as antifeedants against nematodes (Böckenhoff and Grundler 1994). Cai et al. (2003) reported that the trypsin-inhibiting activity is a significant factor that hampers the generation of cyst nematodes in the sporamin expressing hairy roots in the sugar beet. The expression of sporamin proteins reduced in Yulmi plant roots during RKN infection detected in this study adds to the growing body of evidence corroborating the biological function of these sporamins in nematode resistance. APXs are intracellular enzymes, with high affinity for ascorbate, an electron donor in the hydrogen peroxide (H2O2)-reducing reaction (Chen and Asada 1989). Recent studies have focused on the changes in gene expression and activity of APXs subjected to various stress conditions including pathogen infection in plants (Shigeoka et al. 2002). Simonetti et al. (2010) reported changes in APX activity in wheat roots in response to a nematode (Heterodera avenae) attack. Cytosolic APX isozymes were induced in the roots of both lines in response to nematode infection. In this study, APX protein was up-regulated in the RKN-infected Yulmi cultivar plants. Therefore, our results suggested that in the sweet potato fibrous roots, infected with RKN, a rise in APX accumulation appears, probably a ROS-regulating response triggered by the increasing presence of H2O2 and similar to that activated under abiotic stress conditions (Table 2).
The establishment of GCs, together with hypertrophy and hyperplasia of the root cortex cells that lead to the root-knots formation, is manifested by extensive expressional changes, and could be discerned in the differential protein expressions of in the roots of the nematode-sensitive Yulmi cultivar. A coordinated expression of several identified proteins occurs to orchestrate the manipulation and regulation of fundamental features of development in the plant cells during compatible host–nematode interactions (Jammes et al. 2005). The notion that regulation of the plant–nematode interaction contributes to nematode-induced stress-responsive proteins was confirmed by data from the fibrous roots of RKN-susceptible Yulmi plants (Table 1 and Fig. 4). GST (spot 28) and Prxs (spots 30 and 33) were highly expressed in these roots. In GCs at the feeding site, the endophytic parasitic nematodes must adapt to the host defense system and environment conditions. Melillo et al. (2006) reported that oxidative burst appears in a compatible interaction between M. incognita and tomato, in plant cells surrounding the migrating nematode, and in recently differentiated feeding sites. By comparison, at later stages of infection, in mature feeding cells, H2O2 concentrations are much lower, possibly as a result of an active regulation of plant defenses during nematode infection. Many ROS scavenging enzymes have been detected in RKNs and are thought to protect the parasite from ROS damages (Molinari and Miacola 1997). GST and Prxs are known to stress-related defense proteins, and detoxify ROS in response to a variety of stress conditions (Dubreuil et al. 2007). To overcome harmful effects of the oxidative burst, RKNs have acquired ROS removing or scavenging defense system including Prxs and GSTs (Molinari and Miacola 1997). Therefore, previous findings combined with our results suggest that significance of antioxidant mechanisms during plant–RKN interaction.
Conclusions
In summary, we identified changes in the abundances of important functional proteins in RKN-sensitive and -resistant sweet potato cultivars during nematode parasitism. Some of these host proteins participate in GC development at the feeding cell, required by M. incognita and for formation of gall structure. Our studies provide new insights into interaction with host–parasite in sweet potato, a major root crop plant. In the future, some of these proteins may be used to control PPN infection through molecular genetic engineering in plants to silence or overexpress the genes that promote or suppress formation of gall and GC structure.
Author contribution statement
YHK, JJL, SWL, DWL, JSC conceived and designed the experiments. JH, JCW, YHJ, KJN and JJL performed the experiments. YHK, JJL, SWL, JCJ and DWL analyzed the data. JH, JCW, JWY, HWL and SCP contributed analysis/materials/reagents tools. YHK and JJL wrote the paper.
References
Agu CM (2004) Growth and yield of sweet potato as affected by Meloidogyne incognita. Trop Sci 44:89–91
Bird DM (1996) Manipulation of host gene expression by root-knot nematodes. J Parasitol 82:881–888
Böckenhoff A, Grundler FMW (1994) Studies on the nutrient uptake of the beet cyst nematode H. schachtii by in situ microinjection of fluorescent probes into the feeding structures in Arabidopsis thaliana. Parasitology 109:249–254
Bridge J, Starr JL (2010) Plant nematodes of agricultural importance a color handbook. Academic Press, San Diego, pp 77–78
Cai D, Thurau T, Tian Y, Lange T, Yeh KW, Jung C (2003) Sporamin-mediated resistance to beet cyst nematodes (Heterodera schachtii Schm.) is dependent on trypsin inhibitory activity in sugar beet (Beta vulgaris L.) hairy roots. Plant Mol Biol 51:839–849
Caillaud MC, Dubreuil G, Quentin M, Barbeoch LP, Lecomte P, Engler J, Abad P, Rosso MN, Favery B (2008) Root-knot nematodes manipulate plant cell functions during a compatible interaction. J Plant Physiol 165:104–113
Cervantes-Flores JC, Yencho GC, Davis EL (2002a) Efficient evaluation of resistance to three root-knot nematode species in selected sweet potato cultivars. Hort Sci 37:390–392
Cervantes-Flores JC, Yencho GC, Davis EL (2002b) Host reactions of sweet potato genotypes to root-knot nematodes and variation in virulence of Meloidogyne incognita populations. Hort Sci 37:1112–1116
Chen GX, Asada K (1989) Ascorbate peroxidase in tea leaves: occurrence of two isoenzymes and the differences in their enzymatic and molecular properties. Plant Cell Physiol 30:987–998
Choi DR, Lee JK, Park BY, Chung MN (2006) Occutrrence of root-knot nematodes in sweet potato fields and resistance screening of sweet potato cultivars. Kor J Appl Entomol 45:211–216
Clark CA, Moyer JW (1998) Compendium of sweet potato diseases. APS Press, Saint Paul
Diaz JT, Chinn MS, Truong VD (2014) Simultaneous saccharification and fermentation of industrial sweet potatoes for ethanol production and anthocyanins extraction. Ind Crop Prod 62:53–60
Du Z, Zhou X, Ling Y, Zhang Z, Su Z (2010) AgriGO: a GO analysis toolkit for the agricultural community. Nucleic Acids Res 38:W64–W70
Dubreuil G, Magliano M, Deleury E, Abad P, Rosso MN (2007) Transcriptome analysis of root-knot nematode functions induced in the early stages of parasitism. New Phytol 176:426–436
Engler JA, Vleesschauwer VD, Burssens S, Celenza JLJ, Inzé D, Montagu MV, Engler G, Gheysen G (1999) Molecular Markers and cell cycle inhibitors show the importance of cell cycle progression in nematode-induced galls and syncytia. Plant Cell 11:793–807
Goverse A, Smart G (2014) The activation and suppression of plant innate immunity by parasitic nematodes. Annu Rev Phytopathol 52:243–265
Grace MH, Yousef GG, Gustafson SJ, Truong VD, Yencho GC, Lila MA (2014) Phytochemical changes in phenolics, anthocyanins, ascorbic acid, and carotenoids associated with sweet potato storage and impacts on bioactive properties. Food Chem 145:717–724
Hajduch M, Ganapathy A, Stein JW, Thelen JJ (2005) A systematic proteomic study of seed filling in soybean. Establishment of high-resolution two-dimensional reference maps, expression profiles, and an interactive proteome database. Plant Physiol 137:1397–1419
Him NA, Gillan V, Emes RD, Maitland K, Devaney E (2009) Hsp-90 and the biology of nematodes. BMC Evol Biol 9:254
Ibrahim HMM, Hosseini P, Alkharouf NW, Hussein EHA, Gamal El-Din AEKY, Aly MA, Matthews BF (2011) Analysis of gene expression in soybean (Glycine max) roots in response to the root knot nematode Meloidogyne incognita using microarrays and KEGG pathways. BMC Genom 12:220
Inze D, De Veylder L (2006) Cell cycle regulation in plant development. Ann Rev Gen 40:77–105
Jammes F, Lecomte P, De Almeida-Engler J, Bitton F, Martin-Magniette ML, Renou JP, Abad P, Favery B (2005) Genome-wide expression profiling of the host response to root-knot nematode infection in Arabidopsis. Plant J 44:447–458
Jones A, Dukes PD (1980) Heritabilities of sweet potato resistances to root-knot caused by Meloidogyne incognita and M. javanica. J Am Soc Hortic Sci 105:154–156
Kistner MH, Daiber KC, Bester C (1993) The effect of root-knot nematodes (Meloidogyne spp.) and dry land conditions on the production of sweet potato. JS Afr Soc Hortic Sci 3:108–110
Kreuze J (2002) Molecular studies on the sweet potato virus disease and its two causal agents. In: Acta Universitatis Agriculturae Sueciae Agraria 335. Department of Plant Biol, Sveriges lantbrukuniversitet, Uppsala, Sweden
Kwon YS, Lee DY, Rakwal R, Baek SB, Lee JH, Kwak YS, Seo JS, Chung WS, Bae DW, Kim SG (2016) Proteomic analyses of the interaction between the plant-growth promoting rhizobacterium Paenibacillus polymyxa E681 and Arabidopsis thaliana. Proteomics 16:122–135
Lee JJ, Park KW, Kwak YS, Ahn JY, Jung YH, Lee BH, Jeong JC, Lee HS, Kwak SS (2012) Comparative proteomic study between tuberous roots of light orange- and purple-fleshed sweet potato cultivars. Plant Sci 193–194:120–129
Melillo MT, Leonetti P, Bongiovanni M, Castagnone-Sereno P, Bleve-Zacheo T (2006) Modulation of reactive oxygen species activities and H2O2 accumulation during compatible and incompatible tomato–root-knot nematode interactions. New Phytol 170:501–512
Molinari S, Miacola C (1997) Antioxidant enzymes in phytoparasitic nematodes. J Nematol 29:153–159
Palomares-Rius JE, Kikuchi T (2013) Omics fields of study related to plant-parasitic nematodes. J Integ Omic 3:1–10
Shigeoka S, Ishikawa T, Tamoi M, Miyagawa Y, Takeda T, Yabuta Y, Yoshimura K (2002) Regulation and function of ascorbate peroxidase isoenzymes. J Exp Bot 372:1305–1319
Simonetti E, Alba E, Montes MJ, Delibes A, Lopez-Brana I (2010) Analysis of ascorbate peroxidase genes expressed in resistant and susceptible wheat lines infected by the cereal cyst nematode, Heterodera avenae. Plant Cell Rep 29:1169–1178
Sung DY, Vierling E, Guy CL (2001) Comprehensive expression profile analysis of the Arabidopsis Hsp70 gene family. Plant Physiol 126:789–800
Viaene N, Smol N, Bert W (2012) General techniques in nematology. Academia Press, Gent. Belgium. Pp58-59
Wang W, Vinocur B, Shoseyov O, Altman A (2004) Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trend Plant Sci 9:244–252
Williamson VM, Gleason CA (2003) Plant–nematode interactions. Curr Opin Plant Biol 6:327–333
Yeh KW, Chen JC, Lin MI, Chen YM, Lin CY (1997) Functional activity of sporamin from sweet potato (Ipomoea batatas Lam.): a tuber storage protein with trypsin inhibitory activity. Plant Mol Biol 33:565–570
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (NRF-2015R1C1A1A02036323), and KRIBB Research Initiative Program (KGM5281711).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by M. Stobiecki.
Rights and permissions
About this article
Cite this article
Ha, J., Won, J.C., Jung, Y.H. et al. Comparative proteomic analysis of the response of fibrous roots of nematode-resistant and -sensitive sweet potato cultivars to root-knot nematode Meloidogyne incognita . Acta Physiol Plant 39, 262 (2017). https://doi.org/10.1007/s11738-017-2560-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-017-2560-0