Abstract
In this pot experiment, cucumbers (Cucumis sativus L.) were grown in a model soil contaminated by three different concentrations of cadmium (40, 160, and 320 mg.kg−1) with different accompanied anions (Cl−, SO4 2−). In all variants, the most Cd (90 %) was accumulated in the roots, but higher content in the case of Cl−. The distribution of Cd in various cucumber organs was as follows: root > stem > leaf > fruits. However, in variants with higher doses of Cd with SO4 2−, the ratio was changed as follows: root > leaf > stem > fruits. In all variants, least of Cd (max. 1 %) was found in fruits. Variants with the highest Cd doses were significantly different by comparison with all other variants, but higher content was in the case of Cl− anion. Stimulation effect on the biomass production and growth of aerial parts and roots of plants in all variants with Cd was observed. Toxicity symptoms, mainly in the presence of leaf chlorosis and yellowing, were more visible in the variants with Cl−, in comparison with SO4 2−. The amounts of phenol compounds in leaves rose almost in all variants. Only the variants with higher Cd content with SO4 2− showed slight reduction. One possible explanation of reduced content may be their bounding on Cd. The content of salicylic acid was reduced in all variants with Cd treatment. However, it is difficult to conclude their role in plant defence responses to heavy metal, because their actual defence mechanism is still unclear. However, from these results, we can suggest that the accompanying anion and the form in which Cd exists may have an impact on the involvement of various antioxidant systems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Developing and expanding human activities and industrial processes have now resulted in a rapid rise of pollution in groundwater and soil, especially by heavy metals (HM), such as lead (Pb) or cadmium (Cd). Their subsequent removal from the environment is always a difficult task, which complicates their high mobility and persistence in the environment (Tao et al. 2013). High toxicity and widespread of these metals in the environment are a high risk to all living organisms, including humans (Pál et al. 2006).
Toxic effect of Cd on plants is usually accompanied by various symptoms of toxicity, usually in the form of growth inhibition and a decline in biomass production or pigment content (Bavi and Kholdebarin 2011). Plants used very often in defence again stress conditions their own antioxidant enzyme systems. Start of these cascades reactions is associated with the production of some stress signal molecules (Yadav and Singh 2013). In the case of HM, there have been described, e.g., salicylic acid (SA) (Szalai et al. 2013), jasmonic acid, and ethylene (Maksymiec et al. 2007). The synthesis of other antioxidants, e.g., glutathione, ascorbate, and phenolic metabolites, is also stimulated (Wang et al. 2008). The production of the above-mentioned compounds then represents a cellular response of plants against the toxicity of Cd (Popova et al. 2008). Cucumis sativus, cucumber, is an economically important vegetable planted almost worldwide (Boualem et al. 2014). Lin et al. (2012) have described it as fairly sensitive plants to stress conditions. Toxic effect of Cd could then inhibit not only its growth but also the overall agricultural yield.
One of the known and important signaling molecules is SA. It is a simple phenolic compound produced by plants under many different stress factors and also features in the multiple antioxidant defence mechanisms (Saruhan et al. 2011; Kovács et al. 2014). It is also published that this molecule could be a new plant regulator (Hayat et al. 2007). SA also has the ability to alleviate the inhibition of growth, which is the primary manifestation of Cd toxicity. This conclusion was confirmed in the past for various plant species, such as barley (Metwally et al. 2003), soybean (Drazic and Mihailovic 2005), maize (Krantev et al. 2008), rice (Chao et al. 2009), hemp (Shi et al. 2009), pea (Popova et al. 2009), wheat (Moussa and El-Gamal 2010), flax (Belkhadi et al. 2010), mustard (Ahmad et al. 2011), or cucumber seedlings (Çanakci and Karaboga 2013). However, the real reason of SA-induced resistance, despite all the findings are still not fully understood (Li et al. 2013).
Other substances that can interact with metals are phenolic compounds (PC). Their defence action resides in the ability to bind these metals, due to their intra- and inter-specific competition capacity, and thereby inactivating them (Gill and Tuteja 2010; Llugany et al. 2013). It was also described their increased accumulation under various stress conditions, where they can also represent as the so-called biomarkers of metal exposure (Białońska et al. 2006; Sivaci and Elmas 2012; Dutta and Maharia 2012).
The effect of accompanying anions on the HM content was observed in our previous studies (Tuma et al. 2008; 2010; 2014), and the biggest differences between all variants were shown by SO4 2− and Cl− anions. From this reason, we investigate in the present study the effect of SO4 2− and Cl− anions on the uptake and the subsequent translocation of Cd from roots to shoots by cucumber (Cucumis sativus L.), and the ability of plants to accumulate Cd in different plant organs. We examined the influence of different accompanying anions and Cd stress on SA and certain protective compounds, such as PC, during Cd stress. Problems of this combination are relatively poor studied and described, especially in connection with the mechanisms of absorption and tolerance of plants. Our aim was to reveal the possible connection between SA content and Cd tolerance and between SA and PC during Cd stress. We focused on the dependence between these characteristics and the content of Cd.
Materials and methods
The research was carried out like a pot experiment in greenhouse of Botanical garden of medicinal plants in Hradec Kralove (Eastern Czech region, Czech Republic, at an altitude of 278 m.a.s.l.). The photoperiod was proportional to the climatic conditions of this place (50°10′34″N; 15°50′19″E); relative humidity was set on 60 % and average temperature 26 °C (day)/17 °C (night).
Cucumber plants (Cucumis sativus L. var. Charlotte) were grown in plastic pots (7.5 L), four plants in each container, which was filled with 7.5 kg of alluvial soil, clay loamy in a texture. The agrochemical characteristics of the soil medium were as follows: pH/KCl—7.20; content of available P 30.0 µg.L−1, Cu 1880.0 µg.L−1, Al 20100.0 µg.L−1, Ca 1985.0 mg.L−1, Mg 42.3 mg.L−1, Fe 71.5 mg.L−1, Mn 20.3 mg.L−1, and K 76.0 mg.L−1 (in the Mehlich III. leach). The total HM content in aqua regia was Cd 0.5; Zn 143.0, Pb 34.0, As 6.0, Hg 0.1, and Cr 43.00 mg.kg−1. Seven variants were chosen, each in four replications. The first was a control, with no added Cd. The others were with artificially added Cd of 40, 160, and 320 mg.kg−1; always with the same anion (Cl−, SO4 2−). In the text and tables, variants are labelled as control, Cd40S, Cd40Cl, Cd160S, Cd160Cl, Cd320S, and Cd320Cl. The specific amount of chemicals and labels of all variants is shown in Table 1. The weighed amount of chemicals was dissolved in 300 mL of distilled water, and the solutions were evenly applied to the soil in the experimental pots to the equally developed plants 35 days after seeding.
The growth of cucumber plants was determined using fresh weight per plant. Chlorophyll fluorescence was measured using chlorophyll fluorometer OS1p (Opti-Sciences, USA), 42 days after Cd contamination. The whole fully developed plants including fruits were harvested in 1 day, 49 days after Cd contamination. Roots were washed three times with distilled water and dried on filter paper. They were also macroscopically observed and documented for the symptoms of toxicity. Each plant was divided into the root, stem, leaves, and fruits part. Parts were homogenized into smaller pieces, dried to constant weight (ca 48 h) at 65 °C (Venticell 707; Ilabo, Borsovska, Kyjov, CZ), and processed using a blender to a fine powder. Mineralization of the dried and ground samples was carried out by means of what is referred to as the “dry combustion method” in the combustion muffle furnace (CALOR SN 305; MIWY, Lipnik n. Becvou, CZ). Samples by weight 1–50 g were in a quartz (platinum) crucible placed in the furnace. There is set in the burning temperature program according to the character of the samples. The samples were slowly burn (maximum temperature 450 °C) to the white ash. The samples were then dissolved in 10 % HNO3. Concentrations of Cd and K were determined using device AAS SOLAAR M5 (Thermo Electron Spectroscopy Ltd., Solaar House, Cambridge, UK). The sample is then fogging the flame acetylene air, and the absorbance is measured at a wavelength belonging to specified analyte. Cd is 228.8 nm. The appropriate concentration is read from the calibration curve of the device software. Estimates of measurement uncertainty based on the calculation of the experimental measurement data are reference material for Cd 20 %.
The software Statistica 10.0 (StatSoft Inc., Tulsa, OK) was used to analyse the data. All measurements were replicated four times. Data from each part of the plants were analysed separately. The analysis of variance (P < 0.05) and Tukey’s test was used to determine significant differences between variants and for the yield graphs.
Phenolic compounds (PC) assay
0.5 g of dry fine powder was mixed with 10 mL of 75 % HPLC grade methanol solution (Sigma-Aldrich, St. Louis, MO, USA). Extraction of samples was performed by Multi Reax shaker (Heidolph, Schwabach, Germany) for 24 h. Samples were centrifuged for 10 min in the frequency of 1024 rpm by MPW 223a centrifuge (MPW, Warsaw, Poland). Supernatant was removed by Hypodermic syringe CHIRANA Luer (CHIRANA T. Injecta, Stará Turá, Slovak Republic) and filtrated by Nalgene 25 mm Syringe Filters 0.45 µm (Thermo Fisher Scientific, Waltham, MA, USA). 1 mL of each filtrated was diluted in 10 mL of distilled water. 1 mL of 2 N Folin-Ciocalteu’s phenol reagent (Sigma-Aldrich, St. Louis, MO, USA) was added to samples, after 5 min followed by 10 mL of 7 % sodium carbonate (Sigma-Aldrich, St. Louis, MO, USA). Volumetric flasks were filled up by distilled water, mixed and left for 90 min.
The absorbance was read at the wavelength of 765 nm using UV/VIS Spectrophotometer Lambda 25 (Perkin Elmer, Waltham, MA, USA). The concentration of samples was calculated from a calibration line, where gallic acid in various concentrations was used.
Salicylic acid (SA) assay by HPLC
0.5 g of dry fine powder was mixed with 5 mL of 75 % HPLC grade methanol solution (Sigma-Aldrich, St. Louis, MO, USA). Extraction of samples was performed by Multi Reax shaker (Heidolph, Schwabach, Germany) for 24 h. Samples were centrifuged for 15 min in the frequency of 3000 rpm by MPW 223a centrifuge (MPW, Warsaw, Poland). 1.5 mL of supernatants were evaporated under nitrogen purity 4.0 (SIAD) on evaporator NDK 200-2 (Hangzhou Miu Instruments Co., Ltd.), ca 4 h at 30 °C. Prior analysis, the solid rests were dissolved in methanol using Lab Dancer minishaker (IKA) and centrifuged.
The chromatographic analysis was performed by HPLC–DAD system Infinity 1260 (Agilent Technologies, Santa Clara, CA, USA). Separation was performed on chromatographic column Kinetex C18, 5 µm (150 × 4.6 mm ID) and isocratic elution was used. Mobile phase composed of a mixture of acetonitrile/3.35 % phosphoric acid (40/60, v/v) at flow rate 1.5 mL min−1. SA was detected in UV region at 235 nm. Each sample was analysed in two repetitions. The content of SA was calculated using calibration curve method.
Results and discussion
A stimulation effect on the biomass production and growth of aerial parts and roots of plants in all variants with Cd was observed (Fig. 1). Positive effect of low levels of Cd on plant growth previously described Shamsi et al. (2007). All Cd treatments increased plants average fresh weights (FW), compared to control variant on average 30 %. There is no difference between variants with Cl− anion. However, in variants with SO4 2−, FW decreased with higher doses of Cd. The significantly highest values from these variants had the variant Cd40S, in which plants average FW increased, on average 50 % compared to the control variant.
Obvious symptoms of Cd toxicity were monitored in all variants with Cd treatment. These were reflected mainly in the form of leaf chlorosis and yellowing. Leaf chlorosis of and loss of pigments by Cd treatment is documented in different plant species (Gao et al. 2011), even in cucumber (Yu et al. 2013). Much more numerous it was in variants with Cl− accompanying anion if we compare it with SO4 2− anion. Representative leaves of all variants with visible differences between accompanying anions are shown in Supplement 1. The most visible symptoms and serious damage had the variant Cd320Cl. Data are consistent with López-Chuken et al. (2010) who demonstrated that chloride (Cl−) has been related to an increased phytoavailability of Cd in the soil.
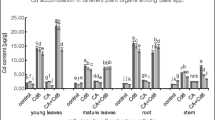
Most of Cd was translocated by the roots in all variants (Fig. 2), namely, there was more than 90 % of the total metal content. Similar translocation was observed, for example, by Lin et al. (2012). With the increasing Cd concentration, the Cd content in plants rose, and the toxic effects were also intensified. It was obvious for both accompanying anion. The influence of metal content in the soil on the metal concentration in plants observed Lin et al. (2012). On the other hand, the increased concentration of Cd can result in the reduction of absorption rate of the roots and conversely increase the persistence of this element on the root surface (Jia et al. 2008). In our experiment, Cl− anion resulted in a higher Cd content in roots in comparison with SO4 2− anion. It was obvious in all Cd concentrations, but at the highest concentration, the difference was more evident and noticeable. Makovnikova et al. (2006) demonstrated that Cl− probably tends Cd to move from the solid soil, thus increases its bioavailability. According to Lin et al. (2012), the law of Cd distribution in cucumber seedlings is: root > leaf > stem. However, this statement was in our experiment confirmed only in variants with higher doses of Cd accompanied by SO4 2− anion (Fig. 2). Especially variants Cd160S and Cd320S had in leaves on average by 4 % more of the total amount of Cd compared with other variants. In other variants, including the control, the law of Cd distribution was in the order of roots > stems > leaves > fruits. At least Cd was translocated into the fruits, around max 1 % of the total content of this HM in the plant. Both variants with the highest doses of Cd were significantly different in comparison with all other variants. The highest content was found in variant Cd320Cl, namely, 26.93 mg.kg−1, which is on average by 110 % more of the total amount of Cd compared with the same variant with anion SO4 2− (Cd320S).
Content of cadmium (mg.kg−1) in cucumber plants grown in three different concentrations of Cd accompanied with Cl− or SO4 2− anions. 1 roots, 2 stems, 3 leaves, 4 fruits. Error bars represent mean ± SE (n = 4). Values within columns, followed by the same letter(s), are not significantly different according to Tukey’s test (P < 0.05)
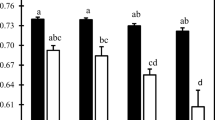
The content of SA (Fig. 3a) was reduced in all variants with Cd treatment. By contrast, Pál et al. (2006) demonstrated that exposure to HM increased plant endogenous SA content. Kovács et al. (2014) described an induced SA synthesis after exposure to Cd in wheat, but no direct connection between the initial SA level and tolerance against Cd. Li et al. (2013) also observed increased tolerance to oxidative damage caused by Cd, mainly due to an increased number of antioxidant enzymes due to SA. In our experiment, variants Cd40S and Cd160S had lower amount of SA comparing with the same variants with Cl− anion. Different behave was in the variant Cd320S, which had much more SA than the variant Cd320Cl and significantly higher content of SA than all other variants with Cd treatment. Despite the fact that during stress reactions, SA was described as a signal molecule which is involved in defence reactions. Although from our and other contradictory results (Li et al. 2013), it is difficult to conclude the role of SA in defence responses to HM and the actual defence mechanism of SA in different environmental stresses is still unclear.
Effect of cadmium supplementation on the content of salicylic acid (µg.g−1) (A) and phenolic compounds (mg 100 g−1) (B) in cucumber leaves. Error bars represent mean ± SE (n = 4). Values within columns, followed by the same letter(s), are not significantly different according to Tukey’s test (P < 0.05)
An increased content of Cd in these variants compared to stem led to a significant reduction of PC amounts in leaves (Fig. 3b). Other variant with Cd treatment showed a higher content of PC compared with the control variant. Sivaci and Elmas (2012) showed that plants may accumulate PC under the various stress conditions. By Gill and Tuteja (2010), PC is part of secondary metabolites that, together with others, are part of the plant defence machinery. Their antioxidant activity and metal-binding capacity are also documented (Llugany et al. 2013). This can be a reason, why the content was reduced in these variants of our experiment. There is not too much free PC, because they are bounded on Cd. In this case, it can be one of the defence systems which this plants use against the HM stress. They can play a role as antioxidants and we can use them like biomarkers of metal exposure. With this agree Michalak (2006) and Białońska et al. (2006). Contrarily, even higher Cd content in leaves of Cd320Cl variant did not lead to the same massive reduction of PC content. From this, we can suggest that the accompanying anion and the form in which Cd exists may have an impact on the involvement of various antioxidant systems.
A similar effect of increasing participation of defensive mechanisms is obvious from the fluorescence values (Fig. 4). All variants with Cd treatment showed higher values of minimal and maximal fluorescence against the control variant. Similar effect of low Cd treatment (5–15 μmol L−1) on increasing chlorophyll fluorescence parameters was observed (Li et al. 2015). According to Sofo et al. (2010), in photosystem, it is formed a very high amount of energy due to Cd toxicity and plants usually prevent against it by increasing maximal chlorophyll fluorescence. The variants Cd160S and Cd320S had in the leaves on average by 23 % higher intensity of F 0 and F m compared to the same Cd doses with different anions (Cl−). Higher Cd content in the leaves in these variants, decreasing effect on PC and higher intensity of chlorophyll fluorescence, can mean an involving of different defensive mechanisms in plant depending on the form of Cd.
Most of K+ was translocated in the fruits by all variants (Fig. 5). There are no big differences between control and treatment variants. The variants with SO4 2− anion accumulated in stems more K+ than the same variants with Cl− anion. Cl variants did not differ from the control variant. According to Wyszkowski and Wyszkowska (2009), the contamination effect of soil with Cd on the macroelement content depends on plant species and the organ. In our case, the form in which Cd is available may have an effect on K+ content in cucumber stems. Big difference in K+ content showed all treatment variants in leaves compared to the control. All of them had bigger values. This same increase in the K+ content under the Cd stress found several authors, for example, in in vitro plantlets (Gonçalves et al. 2009b), in above-ground parts of spring barley (Wyszkowski and Wyszkowska 2006), or in carrot plants (Auda and Ali 2010). On the contrary, a significant fall in the concentration of K+ under the Cd stress for different plant species is well documented—beans and peas (Obata and Umebayashi 1997), wheat (Veselov et al. 2003), oat (Ciećko et al. 2005), maize (Kurtyka et al. 2008), mungbeans (Wahid et al. 2008), and potatoes (Gonçalves et al. 2009a). The diverse effect of Cd on various plants may originate from the fact that Cd can damage cellular plasma membrane in the roots of sensitive plants, which may result in a reduced water and elements uptake (Wyszkowski and Wyszkowska 2009). Here, it can be a connection with Cd. In general, the above-ground parts, hence leaves, are more susceptible to the toxicity of Cd. Conspicuous leaf chlorosis then suggest that Cd metabolism takes place mainly in these parts. Yet, we must not forget the fact that different plants can have developed different tolerance mechanisms and their response to the HM can vary significantly (Benabid and Ghorab 2013). The increase of K+ content in treated leaves can mean an involvement of this element in defence mechanism against Cd stress. It has been observed its involvement especially in photosynthetic rate, plant growth, and yield (Umar and Moinuddin 2002). Uptake of Cd2+ can also be alleviated by appropriate concentration of K+ (Zhao et al. 2004). On the other hand, even low concentrations of Cd can positively affect the increment in uptake opportunities of some cations. Gonçalves et al. (2009b) see the main reason in hyperpolarization of the plasma membranes, which results in increasing in the transmembrane potential.
Content of potassium (mg.kg−1) in cucumber plants grown in three different concentrations of Cd accompanied with Cl− or SO4 2− anions. 1 roots, 2 stems, 3 leaves, 4 fruits. Error bars represent mean ± SE (n = 4). Values within columns, followed by the same letter(s), are not significantly different according to Tukey’s test (P < 0.05)
Different influences on Cd translocation by anions Cl− and SO4 2− can be caused by the different chemical structures and effects inside the plants. The presence of Cl− ions in plant tissues is relatively important. This element not only stabilizes the membrane potential, but also regulates the activity of many important enzymes (White and Broadley 2001). In relation to HM binding, Cl on Cd is an interesting effect. This combination dramatically increases the penetration of metal through the leaf membranes, primarily due to increases diffusivity of Cd (Ozkutlu et al. 2007). Similarly, the metabolic pathways associated with the element sulphur have been described in association with defence mechanisms against the toxicity of Cd (Küpper and Kochian 2010). Phytochelatines have a vital role in defensive mechanisms against HM, where thiol groups are described as the place of Cd binding. This fact was observed, especially in young leaves (Küpper et al. 2004), roots and seeds (Vogel-Mikuš et al. 2010). The difference between these described Cd salts is also evident from the detailed translocation. Koren et al. (2013) observed a difference in the accumulation of Cd in mesophyll. It was preferably stored in vacuoles (in the case of CdCl2), respectively, in apoplast (in the case of CdSO4). While in symplast increasing concentrations of Cd had stimulatory effect on the number of O-ligands, in apoplast ligands based on sulphur prevailed. Authors also showed that in veins of plants treated with CdSO4 and CdCl2, there are no distinct differences in chemical state of Cd, so the mechanisms of uptake, transport, and translocation at the root level probably govern the level of Cd accumulation in shoots. In our study, we observed similar differences of plant behaviour depending on Cd salt, especially in PC content and chlorophyll fluorescence. From this, we can suggest that the accompanying anion and the form in which Cd exists may have an impact on the involvement of various antioxidant systems. The observation that chemical form of added Cd to the nutrient solution influences the level of Cd accumulation is novel, so another study of localization and chemical state of Cd is needed.
Conclusions
Stimulation effect on the biomass production and growth of aerial parts and roots of plants in all variants with Cd was observed. The highest values from these variants had the variant Cd40S, in which plants average FW increased, compared to the control variant on average 50 %. Cd toxicity symptoms were reflected mainly in the leaf chlorosis and yellowing. More visible symptoms were in the variants with accompanying Cl− anion, in comparison with SO4 2− anion. The most visible symptoms and serious damage had the variant Cd320Cl. Cl− has been related to an increased phytoavailability of Cd in the soil. Most Cd was translocated in the roots by all variants, namely, there were more than 90 % of the total metal content. With the increasing Cd concentration, the Cd content in plants rose and the toxic effects were also intensified. In our experiment, Cl− anion resulted to a higher Cd content in roots in comparison with SO4 2− anion. It was obvious in all Cd concentrations, but at the highest concentration, the difference was the most evident and noticeable. Distribution of Cd in various cucumber organs was as follow: root > stem > leaf > fruits. However, in variants with higher doses of Cd with SO4 2−, the ratio was changed as follows: root > leaf > stem > fruits. This increased content of Cd in these variants compared to the stem lead to a significant reduction of PC content in leaves but increased chlorophyll fluorescence. Other variant with Cd treatment showed higher content of PC compared to the control variant. This can be a reason, why in these variants their content was reduced. There is not too much free PC, because they are bounded on Cd. In this case, it can be one of the defence systems which plant use against the HM stress. Contrarily, even higher Cd content in leaves of Cd320Cl variant did not lead to the same massive reduction of PC content. In all variants, at least of Cd (max 1 %) was found in fruits. Variants with the highest Cd doses were significantly different in comparison with all other variants, but higher content was in the case of Cl− anion. The content of SA was reduced in all variants with Cd treatment. However, it is difficult to conclude their role in plant defence responses to heavy metal, because their actual defence mechanism is still unclear. However, from these results, we can suggest that the accompanying anion and the form in which Cd exists may have an impact on the involvement of various antioxidant systems.
Author contribution statement
J. Tuma designed an experiment and contributed to the discussion; J. Simek conducted research; V. Dohnal was involved in the determination of salicylic acid level; K. Musil was involved in the determination of phenolic compounds level; J. Simek and Z. Ducaiova contributed to the manuscript language preparation and carried out statistical analysis. All the authors have read and approved the final manuscript. Writing was led by J. Simek.
References
Ahmad P, Nabi G, Ashraf M (2011) Cadmium-induced oxidative damage in mustard [Brassica juncea (L.) Czern. and Coss.] plants can be alleviated by salicylic acid. South Afr J Bot 77:36–44. doi:10.1016/j.sajb.2010.05.003
Auda MA, Ali ES (2010) Cadmium and zinc toxicity effects on growth and mineral nutrients of carrot (Daucus carota). Pak J Bot Pak 42:341–351
Bavi K, Kholdebarin B (2011) Effect of cadmium on growth, protein content and peroxidase activity in pea plants. Pak J Bot 43:1467–1470
Belkhadi A, Hediji H, Abbes Z et al (2010) Effects of exogenous salicylic acid pre-treatment on cadmium toxicity and leaf lipid content in Linum usitatissimum L. Ecotoxicol Environ Saf 73:1004–1011. doi:10.1016/j.ecoenv.2010.03.009
Benabid H, Ghorab MF (2013) Study of the translocation and distribution of cadmium into bean plants (Phaseolus vulgaris) using labelled Cd-109. World J Nano Sci Eng 3:108–111. doi:10.4236/wjnse.2013.33015
Białońska D, Zobel AM, Kuraś M et al (2006) Phenolic compounds and cell structure in bilberry leaves affected by emissions from a Zn–Pb smelter. Water Air Soil Pollut 181:123–133. doi:10.1007/s11270-006-9284-x
Boualem A, Fleurier S, Troadec C et al (2014) Development of a Cucumis sativus TILLinG platform for forward and reverse genetics. PLoS One 9:e97963. doi:10.1371/journal.pone.0097963
Çanakci S, Karaboga Z (2013) Some physiological and biochemical responses to cadmium in salicylic acid applied cucumber (Cucumis sativus L.) seedlings. Pak J Bot 45:1963–1968
Chao Y-Y, Chen C-Y, Huang W-D, Kao CH (2009) Salicylic acid-mediated hydrogen peroxide accumulation and protection against Cd toxicity in rice leaves. Plant Soil 329:327–337. doi:10.1007/s11104-009-0161-4
Ciećko Z, Kalembasa S, Wyszkowski M, Rolka E (2005) The magnesium content in plants on soil contaminated with cadmium. Pol J Environ Stud 14:365–370
Drazic G, Mihailovic N (2005) Modification of cadmium toxicity in soybean seedlings by salicylic acid. Plant Sci 168:511–517. doi:10.1016/j.plantsci.2004.09.019
Dutta RK, Maharia RS (2012) Antioxidant responses of some common medicinal plants grown in copper mining areas. Food Chem 131:259–265. doi:10.1016/j.foodchem.2011.08.075
Gao C, Wang Y, Xiao D-S et al (2011) Comparison of cadmium-induced iron-deficiency responses and genuine iron-deficiency responses in Malus xiaojinensis. Plant Sci 181:269–274. doi:10.1016/j.plantsci.2011.05.014
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem PPB Société Fr Physiol Végétale 48:909–930. doi:10.1016/j.plaphy.2010.08.016
Gonçalves JF, Antes FG, Maldaner J et al (2009a) Cadmium and mineral nutrient accumulation in potato plantlets grown under cadmium stress in two different experimental culture conditions. Plant Physiol Biochem 47:814–821. doi:10.1016/j.plaphy.2009.04.002
Gonçalves JF, Nicoloso FT, Becker AG et al (2009b) Photosynthetic pigments content, δ-aminolevulinic acid dehydratase and acid phosphatase activities and mineral nutrients concentration in cadmium-exposed Cucumis sativus L. Biologia (Bratisl) 64:310–318. doi:10.2478/s11756-009-0034-6
Hayat S, Ali B, Ahmad A (2007) Salicylic acid: biosynthesis, metabolism and physiological role in plants. In: Hayat S, Ahmad A (eds) Salicylic acid: a plant hormone. Springer, Netherlands, pp 1–14
Jia Y, Zhang Y, Li H (2008) Translocation and allocation of cadmium in cucumber and rape seedlings. Chin J Ecol 27:117–121
Koren Š, Arčon I, Kump P et al (2013) Influence of CdCl2 and CdSO4 supplementation on Cd distribution and ligand environment in leaves of the Cd hyperaccumulator Noccaea (Thlaspi) praecox. Plant Soil 370:125–148. doi:10.1007/s11104-013-1617-0
Kovács V, Gondor OK, Szalai G et al (2014) Synthesis and role of salicylic acid in wheat varieties with different levels of cadmium tolerance. J Hazard Mater 280:12–19. doi:10.1016/j.jhazmat.2014.07.048
Krantev A, Yordanova R, Janda T et al (2008) Treatment with salicylic acid decreases the effect of cadmium on photosynthesis in maize plants. J Plant Physiol 165:920–931. doi:10.1016/j.jplph.2006.11.014
Küpper H, Kochian LV (2010) Transcriptional regulation of metal transport genes and mineral nutrition during acclimatization to cadmium and zinc in the Cd/Zn hyperaccumulator, Thlaspi caerulescens (Ganges population). New Phytol 185:114–129. doi:10.1111/j.1469-8137.2009.03051.x
Küpper H, Mijovilovich A, Meyer-Klaucke W, Kroneck PMH (2004) Tissue- and age-dependent differences in the complexation of cadmium and zinc in the cadmium/zinc hyperaccumulator Thlaspi caerulescens (Ganges ecotype) revealed by X-ray absorption spectroscopy. Plant Physiol 134:748–757. doi:10.1104/pp.103.032953
Kurtyka R, Malkowski E, Kita A, Karcz W (2008) Effect of calcium and cadmium on growth and accumulation of cadmium, calcium, potassium and sodium in maize seedlings. Pol J Environ Stud 1:51–56
Li XM, Ma LJ, Bu N et al (2013) Effects of salicylic acid pre-treatment on cadmium and/or UV-B stress in soybean seedlings. Biol Plant 58:195–199. doi:10.1007/s10535-013-0375-4
Li S, Yang W, Yang T et al (2015) Effects of Cadmium Stress on Leaf Chlorophyll Fluorescence and Photosynthesis of Elsholtzia argyi–A Cadmium Accumulating Plant. Int J Phytoremediation 17:85–92. doi:10.1080/15226514.2013.828020
Lin L, Liao M, Mei L (2012) A review of cucumber under cadmium stress. 2012 Asia Pacific conference on environmental science and technology (APEST 2012), Kuala Lumpur, Malaysia. Adv Biomed Eng 6:443–447
Llugany M, Tolrà R, Martín SR et al (2013) Cadmium-induced changes in glutathione and phenolics of Thlaspi and Noccaea species differing in Cd accumulation. J Plant Nutr Soil Sci 176:851–858. doi:10.1002/jpln.201300096
López-Chuken UJ, Young SD, Sánchez-González MN (2010) The use of chloro-complexation to enhance cadmium uptake by Zea mays and Brassica juncea: testing a “free ion activity model” and implications for phytoremediation. Int J Phytoremediation 12:680–696. doi:10.1080/15226510903353161
Makovnikova J, Barancikova G, Dlapa P, Dercova K (2006) Anorganické kontaminanty V pôdnom ekosystéme. Chem Listy 100:424–432
Maksymiec W, Wójcik M, Krupa Z (2007) Variation in oxidative stress and photochemical activity in Arabidopsis thaliana leaves subjected to cadmium and excess copper in the presence or absence of jasmonate and ascorbate. Chemosphere 66:421–427. doi:10.1016/j.chemosphere.2006.06.025
Metwally A, Finkemeier I, Georgi M, Dietz K-J (2003) Salicylic acid alleviates the cadmium toxicity in barley seedlings. Plant Physiol 132:272–281. doi:10.1104/pp.102.018457
Michalak A (2006) Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Pol J Environ Stud 15:523–530
Moussa HR, El-Gamal SM (2010) Effect of salicylic acid pretreatment on cadmium toxicity in wheat. Biol Plant 54:315–320. doi:10.1007/s10535-010-0054-7
Obata H, Umebayashi M (1997) Effects of cadmium on mineral nutrient concentrations in plants differing in tolerance for cadmium. J Plant Nutr 20:97–105. doi:10.1080/01904169709365236
Ozkutlu F, Ozturk L, Erdem H et al (2007) Leaf-applied sodium chloride promotes cadmium accumulation in durum wheat grain. Plant Soil 290:323–331. doi:10.1007/s11104-006-9164-6
Pál M, Horváth E, Janda T et al (2006) Physiological changes and defense mechanisms induced by cadmium stress in maize. J Plant Nutr Soil Sci 169:239–246. doi:10.1002/jpln.200520573
Popova L, Maslenkova L, Yordanova R et al (2008) Salicylic acid protects photosynthesis against cadmium toxicity I pea plants. Gen Appl Plant Physiol 34:133–148
Popova LP, Maslenkova LT, Yordanova RY et al (2009) Exogenous treatment with salicylic acid attenuates cadmium toxicity in pea seedlings. Plant Physiol Biochem PPB Société Fr Physiol Végétale 47:224–231. doi:10.1016/j.plaphy.2008.11.007
Saruhan N, Saglam A, Kadioglu A (2011) Salicylic acid pretreatment induces drought tolerance and delays leaf rolling by inducing antioxidant systems in maize genotypes. Acta Physiol Plant 34:97–106. doi:10.1007/s11738-011-0808-7
Shamsi IH, Wei K, Jilani G, Zhang G (2007) Interactions of cadmium and aluminum toxicity in their effect on growth and physiological parameters in soybean. J Zhejiang Univ Sci B 8:181–188. doi:10.1631/jzus.2007.B0181
Shi GR, Cai QS, Liu QQ, Wu L (2009) Salicylic acid-mediated alleviation of cadmium toxicity in hemp plants in relation to cadmium uptake, photosynthesis, and antioxidant enzymes. Acta Physiol Plant 31:969–977. doi:10.1007/s11738-009-0312-5
Sivaci A, Elmas E (2012) The combined effects of cadmium and salinity on some pigments and total phenolic compounds of Myriophyllum heterophyllum Michx. and Potamogeton crispus L. Afr J Agric Res 26:3813–3818
Sofo A, Dichio B, Montanaro G, Xiloyannis C (2010) Photosynthetic performance and light response of two olive cultivars under different water and light regimes. Photosynthetica 47:602–608. doi:10.1007/s11099-009-0086-4
Szalai G, Krantev A, Yordanova R et al (2013) Influence of salicylic acid on phytochelatin synthesis in Zea mays during Cd stress. Turk J Bot 37:708–714
Tao S, Sun L, Ma C et al (2013) Reducing basal salicylic acid enhances Arabidopsis tolerance to lead or cadmium. Plant Soil 372:309–318. doi:10.1007/s11104-013-1749-2
Tuma J, Skalicky M, Tumova L et al (2008) The translocation of zinc in Avena Sativa L. depending on fertilisation with zinc and mobile anions. Cereal Res Commun 36:1083–1086
Tuma J, Skalicky M, Tumova L, Safrankova M (2010) Translocation of nickel in Avena sativa: the effect of accompanying mobile anions. Fresenius Environ Bull 19:2974–2980
Tuma J, Skalicky M, Tumova L, Flidr J (2014) Influence of cadmium dose and form on the yield of oat (Avena Sativa L.) and the metal distribution in the plant. J Elem 19:795–809. doi:10.5601/jelem.2014.19.3.448
Umar S, Moinuddin V (2002) Genotypic differences in yield and quality of groundnut as affected by potassium nutrition under erratic rainfall conditions. J Plant Nutr 25:1549–1562. doi:10.1081/PLN-120005407
Veselov D, Kudoyarova D, Symonyan M, Veselov S (2003) Effect of cadmium on ion uptake, transpiration and cytokinin content in wheat seedling. Bulg J Plant Physiol 29:353–359
Vogel-Mikuš K, Arčon I, Kodre A (2010) Complexation of cadmium in seeds and vegetative tissues of the cadmium hyperaccumulator Thlaspi praecox as studied by X-ray absorption spectroscopy. Plant Soil 331:439–451. doi:10.1007/s11104-009-0264-y
Wahid A, Ghani A, Javed F (2008) Effect of cadmium on photosynthesis, nutrition and growth of mungbean. Agron Sustain Dev 28:273–280. doi:10.1051/agro:2008010
Wang Z, Zhang Y, Huang Z, Huang L (2008) Antioxidative response of metal-accumulator and non-accumulator plants under cadmium stress. Plant Soil 310:137–149. doi:10.1007/s11104-008-9641-1
White PJ, Broadley MR (2001) Chloride in Soils and its Uptake and Movement within the Plant: a Review. Ann Bot 88:967–988. doi:10.1006/anbo.2001.1540
Wyszkowski M, Wyszkowska J (2006) The content of macroelements in spring barley (Hordeum vulgare L.) and theirs relations with above-ground parts mass of plants and the enzymatic activity of heavy metal contaminated soil. Pol J Environ Stud 15:212–221
Wyszkowski M, Wyszkowska J (2009) The effect of soil contamination with cadmium on the growth and chemical composition of spring barley (Hordeum vulgare L.) and its relationship with the enzymatic activity of soil. Parlar Scientific Publications, pp 1046–1053
Yadav K, Singh NB (2013) Effects of benzoic acid and cadmium toxicity on wheat seedlings. Chil J Agric Res 73:168–174. doi:10.4067/S0718-58392013000200013
Yu L, Gao R, Shi Q et al (2013) Exogenous application of sodium nitroprusside alleviated cadmium induced chlorosis, photosynthesis inhibition and oxidative stress in cucumber. Pak J Bot 45:813–819
Zhao Z-Q, Zhu Y-G, Li H-Y et al (2004) Effects of forms and rates of potassium fertilizers on cadmium uptake by two cultivars of spring wheat (Triticum aestivum L.). Environ Int 29:973–978. doi:10.1016/S0160-4120(03)00081-3
Acknowledgments
This study was financially supported by Particular Research Program, University of Hradec Kralove, No. 2108/2013 and by ESF under Project Number CZ.1.07./2.3.00/30.0052.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by N.A. Anjum.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Simek, J., Tuma, J., Dohnal, V. et al. Salicylic acid and phenolic compounds under cadmium stress in cucumber plants (Cucumis sativus L.). Acta Physiol Plant 38, 172 (2016). https://doi.org/10.1007/s11738-016-2192-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-016-2192-9