Abstract
The changes in the antioxidant enzymes activity, total protein and proline content and their correlations with freezing tolerance (FT) (expressed as LT50) were investigated at 11 different olive cultivars at cold-acclimation (CA, in February) and non-acclimation (NA, in August) stages. Leaf samples were collected from each cultivar and were divided into two groups. The first group was immediately frozen in liquid nitrogen for further biochemical analysis. The second ones was subjected to different freezing temperatures (−5, −10, −15 and −20 °C) for 10 h, in order to determine their FT. The unfrozen control samples were kept at 4 °C. The results showed that Fishomi, Mission and Shengeh were the most freezing tolerant among other cultivars. In contrast, Zard, Manzanilla and Amigdalolia were the most sensitive ones. The cold acclimation enhanced the activities of superoxide dismutase (SOD), peroxidase (POD), ascorbate peroxidase (APX), catalase (CAT), polyphenol oxidase (PPO) and total protein content. However, proline content and phenylalanine ammonia-lyase (PAL) activity did not change or even decreased slightly at CA stage, compare to those samples at NA stage. It was found that LT50 to be closely correlated to POD, CAT, and PPO activity at CA and NA stages. Overall, higher leaf POD, CAT, and PPO activity could be used as important selection criteria in screening tolerant olive cultivars for cold zone climatic.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Freezing temperature is one of the most important environmental factors limiting the productivity and distribution of plants (Levitt 1980). The ability of many temperate plants to increase their FT when exposed to low temperatures is known as cold acclimation (CA) (Levitt 1980). CA is a complex process involving a number of physiological and biochemical changes (Wisniewski et al. 2003), including the induction of genes encoding changes in lipid and plant cell wall compositions, increased levels of sugars, activation of antioxidative mechanisms, etc. (Guy 1990; Thomashow 1999). It has been reported that the major target for freezing injury is cell membranes (Levitt 1980). This could increase the level of reactive oxygen species (ROS) and thereafter result in severe oxidative injury, which give rise to lipid peroxidation, membrane deterioration, protein degradation and metabolic function disruption (Lin et al. 2005).
To alleviate or protect from low temperature-induced oxidative injury, plants have evolved mechanisms to scavenge these ROS by non-enzymatic and enzymatic antioxidant systems, such as superoxide dismutase (SOD), peroxidase (POD), ascorbate peroxidase (APX) and catalase (CAT) (Wise 1995; Lee and Lee 2000). SOD is a metallo-enzyme that scavenges the toxic superoxide radicals and catalyzes the conversion of two superoxide anions into oxygen and H2O2 (Miyake and Yakota 2000). Then, POD and CAT convert H2O2 into H2O and O2, whereas APX decomposes H2O2 by oxidation of co-substrates such as phenolic compounds and/or antioxidants (Sudhakar et al. 2001). Furthermore, polyphenol oxidase (PPO) and phenylalanine ammonia-lyase (PAL) activities are also important in the response of plants against freezing stress, because they can help to avoid serious oxidative damage induced by freezing (Ortega-García and Peragón 2009). Indeed, tolerance of plant towards adverse environmental conditions is correlated to increasing capacity to scavenge or detoxify ROS (Guo et al. 2006).
Olive is one of many tropical and subtropical crops which are often grown close to climatic limits of their cold-tolerance (Bartolozzi et al. 1999). The optimum temperature for growth in olive tree is 20–30 °C. The majority of olive cultivars cannot survive below −12 °C (Gomez del Campo and Barranco 2005) and are damaged by frost below −7 °C (Palliotti and Bongi 1996). However, the increasing demand for olive oil has extended the cultivation of olive tree into regions with colder climates than those of original Mediterranean basin. Moreover, the better oil can be yielded from olive trees growing in the colder climates (Palliotti and Bongi 1996). To find a way for the trees to survive and be productive in the colder climate is a goal of economic value. Selection and use of cold-hardy cultivars are the most effective ways of avoiding freezing damage.
Previous studies also showed that there is a correlation between some physiological and biochemical characteristics such as stomatal density (Roselli et al. 1989), stomatal size (Roselli and Venora 1990), phenolic compounds (Roselli et al. 1992), differential thermal analysis (Martin et al. 1993), ionic leakage (Barranco et al. 2005; Azzarello et al. 2009), fractal analysis (Azzarello et al. 2009), impedance spectroscopy (Azzarello et al. 2009), soluble sugars (Bartolozzi and Fontanazza 1999; Bartolozzi et al. 2001; Gulen et al. 2009) and total soluble proteins (Eris et al. 2007; Cansev et al. 2009) with frost-tolerant in olive genotypes. Currently, interesting results to quantify cold tolerance have come from the study of some antioxidant enzymes (Solecka and Kacperska 2003; Cansev et al. 2009; Ortega-García and Peragón 2009). A significant increase in the activity of CAT, APX and nicotinamide adenine dinucleotide phosphate (NADPH) oxidase as well as in dehydrin protein levels in cold-acclimatized olive trees was reported (Cansev et al. 2009). In addition, recently was documented that PPO and PAL can be considered elements for determining the recovery capacity and resistance to freezing temperatures of different olive varieties (Ortega-García and Peragón 2009). Furthermore, Luo et al. (2007) found that FT in Populus suaveolens cuttings to be closely correlated to the levels of SOD, POD, CAT and APX during freezing acclimation stage. Overall, our knowledge on the biochemical response of the olive tree to freezing stress is not still well known. The understanding of the role and behavior of antioxidant protective system, protein and proline contents in the response of the olive tree to freezing stress can help prevent the injury caused by this abiotic stress. The present study was evaluation of the changes in the defensive enzymes activities (SOD, POD, APX, CAT, PPO and PAL), total protein and proline content and, their correlations with freezing hardiness (expressed as LT50) at 11 different olive cultivars during both CA and NA stages.
Materials and methods
Plant material
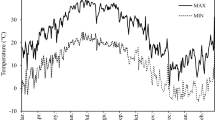
Fully expanded, uniformly sized leave from 2-year old shoots of 11 local and introduced olive (Olea europaea L.) cultivars, Arbequina, Coratina, Fishomi, Zard, Mari, Roughani, Mission, Amigdalolia, Shengeh, Leccino and Manzanilla, were collected from 40-year-old trees from the Roudbar olive Research Institute, Iran, at CA and NA stages. CA leaves were field-collected in winter (in February), while NA leaves were collected from the same individual olive trees in summer (in August). The average temperature was 8.5 °C (range 2.8–14.2 °C) and 25.7 °C (range 22.8–30.6 °C) in February and August, respectively. Mean of minimum and maximum daily temperatures during experimental recorded in Roudbar region (from March 2011 to February 2012) was recorded in Roudbar region as presented in Fig. 1.
Leaf samples were collected randomly from three trees of each cultivar, packed on ice and taken to the laboratory. The leaves were divided into two groups, either frozen immediately in liquid nitrogen and kept at −80 °C until further biochemical analysis or wrapped (ten leaves per temperature in each replication) in aluminum foil along with moistened paper and were placed into a programmable test chamber (KATO, Japan) to determine FT.
Determination of FT
Ion leakage was used to assess FT in olive cultivars during CA and NA stages, by exposing whole and healthy leaves (ten leaves in each replication per each temperature) to different freezing temperatures. The programmable chamber temperature was decreased stepwise at 1.5 °C/h to −5 °C and thereafter at 5 °C/h until −20 °C. Then, whole leaf samples were exposed to freezing temperatures (−5, −10, −15 and −20 °C) for 10 h. The unfrozen control treatment consisted of samples placed directly at 4 °C in the dark for 24 h (unfrozen samples). Relative humidity (RH) inside the test chamber was kept at 45–50 % and darkness condition was simulated. After exposing whole leaf samples to each freezing temperature, recovery was performed by rising the temperature at the same rate until 4 °C for slow thawing. Then, the leaves were cut into equal pieces (one disc with 10 mm in diameter from each leaf) using a metal cork borer (10 mm) with sharp edges. Ion leakage of leaf discs was measured as described by Deshmukh et al. (1991) with some modification. Leaf discs (ten pieces in each replicate), were placed in the test-tube containing 10 mL of distilled water, and kept at 45 °C for 30 min in a water bath. The initial conductivity of the solution was measured using Mi 306 EC/TDS meter conductivity meter (“Milwaukee Instruments”, Hungary). The tubes were then kept in a boiling water bath for 10 min, and their conductivity was measured once again after cooling to room temperature. Percentage of ion leakage for each treatment was converted to percentage of injury as:
where % IL (t) and % IL (c) are measurements of percentage of IL from the respective freeze-treatment temperature and the unfrozen control, respectively (Lim et al. 1998). Finally, LT50, a measure of FT, was derived for 11 olive cultivars by determining the freeze test temperature at which 50 % injury (midpoint of maximum and minimum percentage of injury) occurred, as explained in Lim et al. (1998).
Enzymes assay
Olive leaves (0.5 g) were ground with pestle and mortar in liquid nitrogen. The hard leaf tissue of olives was strongly ground into a fine powder by the pestle with high pressure and speed. Then, fine powder transferred into precooled tubes and 1 mL of 50 mM potassium phosphate buffer (pH 7.0), containing 1 mM EDTA and 4 % PVPP was subsequently added to tubes. The homogenate was centrifuged at 15,000g for 15 min at 4 °C. The supernatant was used to measure the activities of SOD, POD, APX, CAT, and PPO and to determine total protein content. All assays were done at 25 °C using a spectrophotometer (T80, PG Instrument., UK).
SOD (EC 1.15.1.1) activity was determined by measuring its ability to inhibit the photoreduction of nitro blue tetrazolium (NBT) according to the methods of Beauchamp and Fridovich (1971). The reaction mixture contained 50 mM phosphate buffer (pH 7.0), 200 mM methionine, 1.125 mM NBT, 1.5 mM EDTA, 75 µM riboflavin, and 0–50 µL of the enzyme extract. Riboflavin was added as the last component. Reaction was carried out in test-tubes at 25 °C under illumination supplied by two fluorescent lamps (20 W). The reaction was initiated by switching on the light and allowed to run for 15 min, and light switching off stopped the reaction. The tubes were then immediately covered with aluminum foil in order to stop the reaction, and then absorbance of the mixture was then read at 560 nm. Identical tubes with complete reaction mixture containing no enzyme extract and developing maximum color served as a control. A non-illuminated complete reaction mixture with no color development served as a blank. Under experimental conditions, the initial rate of reaction, as measured by the difference in the increase of absorbance at 560 nm in the presence and absence of leaf extract was proportional to the amount of enzyme. One unit SOD activity was defined as the amount of enzyme required to inhibit 50 % of the rate of NBT reduction measured at 560 nm. The SOD activity of the extract was expressed as activity unit/g fresh weight (U/g FW).
POD (EC 1.11.1.7) activity in leaves was assayed by the oxidation of guaiacol in the presence of H2O2. The increase in absorbance was recorded at 470 nm (Chance and Maehly. 1955). The reaction mixture contained 100 µL of crude enzyme extract, 500 µL of 5 mM H2O2, 500 µL of 28 mM guaiacol, and 1,900 µL of 50 mM potassium phosphate buffer (pH 7.0). POD activity of the extract was expressed as activity U/g FW min.
CAT (EC 1.11.1.6) activity was assayed according the method of Beers and Sizer (Beers and Sizer 1952). The decomposition of H2O2 was monitored by the decrease in absorbance at 240 nm. The assay mixture contained 2.6 mL of 50 mM potassium phosphate buffer (pH 7.0), 400 µL of 15 mM H2O2, and 40 µL of enzyme extract. The CAT activity of the extract was expressed as activity U/g FW min.
APX (EC 1.11.1.11) activity was measured according to Nakano and Asada (1980). The reaction mixture contained 50 mM (pH 7.0) potassium phosphate buffer (pH 7.0), 0.1 mM EDTA, 0.25 mM ascorbate, 1.0 mM H2O2, and 100 µL of the enzymes extract. H2O2-dependent oxidation of ascorbate was followed by a decrease in the absorbance at 290 nm. The APX activity of the extract was expressed as activity U/g FW min.
PPO (EC 1.10. 3. 1) activity was assayed with 4-methylcatechol as a substrate as described in (Luh and Phithakpol 1972) with some modifications. The assay of the enzyme activity was performed using 2 mL of 0.1 mM sodium phosphate buffer (pH 6.8), 0.5 mL of 100 mM 4-methylcatechol, and 0.5 mL of the enzyme solution. The increase in absorbance at 420 nm was recorded. The PPO activity was expressed as activity U/100 g FW min.
PAL activity was assayed according to the method of Assis et al. (2001), with slight modifications. One hundred microliter of enzyme extract was incubated with 2.4 mL of borate buffer 50 mM, pH 8.8 and 0.5 ml of l-phenylalanine (20 mM) for 60 min at 37 °C. The reaction was stopped with 1 mL HCl (1 M). PAL activity was determined by the production of cinnamate, which was measured at 290 nm absorbance. The control mixture was stopped by adding 1 ml HCl immediately after mixing the crude enzyme preparation with l-phenylalanine. Specific enzyme activity was defined as µmoles cinnamic acid/h/FW.
Proline and protein content
Proline content was determined spectrophotometrically by adopting the ninhydrin method of Bates et al. (1973). Three hundred mg of fresh leaf samples were homogenized in 3 % sulfosalicylic acid and the homogenate was centrifuged at 12,000g for 10 min. Two microliter of the supernatant was reacted with 2 mL acid ninhydrin (Acid-ninhydrin was prepared by warming 1.25 g ninhydrin in 30 mL glacial acetic acid and 20 mL·6 M phosphoric acid, with agitation, until dissolved) and 2 mL of glacial acetic acid in a test tube for 60 min at 100 °C, and the reaction terminated in an ice bath. The reaction mixture was extracted with 4 mL toluene, mixed vigorously with a test tube stirrer for 15–20 s. Free proline was quantified spectrophotometrically at 520 nm using l-proline as a standard, and its content was expressed as μmol/g FW.
Protein content was also determined according to the method of Bradford (1976) using BSA as a standard.
Statistical analysis
The experiment was performed in a randomized complete block design in factorial arrangement. The values are mean values ± SE of three replicates. Statistical analysis was carried out using SAS software (Version 9.1, SAS Instituted, Cary, NC, USA). Analysis of variance between treatment means was carried out with using LSD test at p ≤ 0.05. Regression and correlations between LT50 and biochemical traits were also analyzed using Sigmaplot (version 11). The graphics were made using Excel and Sigmaplot softwares.
Results
Seasonal pattern of FT
FT was indicated as LT50 of 11 olive cultivars by plotting all the conductivity data against temperature (Fig. 2). The results showed that FT of all studied cultivars increased significantly during CA stage (winter, mean of LT50 was −11.89 °C), in compared to NA stage (summer, mean of LT50 was −6.97 °C). At CA stage, the most FT was found with Fishomi (LT50 −13.4 °C), Mission (LT50 −13.2 °C) and Shengeh (LT50 −13 °C) cultivars, respectively, whereas Zard (LT50 −9.8 °C), Manzanilla (LT50 −10.6 °C) and Amigdalolia (LT50 −10.8 °C) were found to be the most sensitive cultivars respectively (Fig. 2). Leccino, Arbequina, Coratina, Roghani and Mari have shown moderate tolerance. Similarly, during the NA stage, Fishomi (LT50 −8.2 °C), Mission (LT50 −8.1 °C) and Shengeh (LT50 −8 °C) were the most freezing resistant, respectively, whereas Amigdalolia (LT50 −5.9 °C), Zard (LT50 −6 °C), and Manzanilla (LT50 −6.4 °C) were the most sensitive cultivars, respectively.
Changes in LT50 of 11 olive cultivars (Fis Fishomi, Mis Mission, She Shengeh, Lec Leccino, Cor Coratina, Arb Arbequina, Rog Roughani, Man Manzanilla, Ami Amigdalolia, Mar Mari, Zar Zard) during cold (CA) and non (NA) Acclimation. Vertical bars indicate standard errors from means (n = 3). Values with different letters are significantly different, as determined by the LSD test (p ≤ 0.05)
Antioxidant enzymes activity
The enzymatic activities of SOD, POD, APX, CAT, PPO and PAL in leaves of the 11 olive cultivars were measured under CA and NA stages.
The SOD activity of all studied cultivars, except for Mari and Amigdalolia, were significantly increased at the CA stage compare to NA stage (Fig. 3a). In addition, SOD activity was different among cultivars in both CA and NA stages. The highest SOD activity was assessed in cvs. Leccino (229.23 U/g FW), Mission (223.59 U/g FW) and Arbequina (204.61 U/g FW), at CA stage, whereas the highest SOD activity was assayed in cvs. Mari (226.01 U/g FW), Leccino (213.85 U/g FW) and Amigdalolia (203.38 U/g FW) at NA stage. In contrast, the lowest SOD activity was assessed in cvs. Shengeh (163.58 U/g FW), Zard (166.66 U/g FW) and Mari (167.18 U/g FW) at CA stage, whereas the lowest SOD activity was observed in cvs. Arbequina (120.27 U/g FW), Fishomi (120.6 U/g FW) and Shengeh (122.97 U/g FW) at NA stage.
Changes in antioxidant enzyme activities; SOD (a) and APX (b) of 11 olive cultivars (Fis Fishomi, Mis Mission, She Shengeh, Lec Leccino, Cor Coratina, Arb Arbequina, Rog Roughani, Man Manzanilla, Ami Amigdalolia, Mar Mari, Zar Zard) during winter (CA stage) and summer (NA stage). Vertical bars indicate standard errors from means (n = 3). Values with different letters are significantly different, as determined by the LSD test (p ≤ 0.05)
There was no linear regression between SOD activity and LT50 at both of CA and NA stages. Furthermore, there was no significant (ns) correlation coefficient between LT50 and SOD activity under CA (r = −0.489ns) and NA (r = 0.229ns) stages.
The APX activity of all studied cultivars, except for Zard was significantly higher at CA stage than NA stage (Fig. 3b). The highest APX activity was found in Mari (599.87 U/g FW min), Amigdalolia (597.74 U/g FW min) and Roughani (597.18 U/g FW min) in CA stage, whereas the lowest APX activity was assessed in Arbequina (262.11 U/g FW min), Manzanilla (306.66 U/g FW min) and Mission (352.97 U/g FW min) at NA stage. There was no linear regression between the APX activity and LT50 at both of CA and NA stages. Furthermore, there was no significant correlation coefficient between LT50 and SOD activity at both CA (r = 0.220ns) and NA (r = −0.010ns) stages.
There was no significant difference in POD activity between the CA and NA stages (Fig. 4a). However, POD activity was different among cultivars at both CA and NA stages (Fig. 4a). At CA stage sampling, the highest POD activity was found in the freezing-tolerant cvs. Fishomi (1.6 U/g FW min) and Mission (1.5 U/g FW min), whereas the lowest POD activity was observed in the freezing-sensitive cvs Zard (0.38 U/g FW min) and Amigdalolia (0.39 U/g FW min). Similarly, the freezing-tolerant cvs Mission (1.61 U/g FW min) and Fishomi (1.2 U/g FW min) had the highest POD activity, whereas the freezing-sensitive cvs i.e. Amigdalolia (0.35 U/g FW min) and Zard (0.38 U/g FW min) had the highest POD activity at the NA stage sampling.
Changes in POD activity (a) and the relationship of LT50 with POD activity (b); CAT activity (c) and the relationship of LT50 with CAT activity (d); PPO (e) and the relationship of LT50 with PPO activity (f) of 11 olive cultivars (Fis Fishomi, Mis Mission, She Shengeh, Lec Leccino, Cor Coratina, Arb Arbequina, Rog Roughani, Man Manzanilla, Ami Amigdalolia, Mar Mari, Zar Zard) during winter (CA stage) and summer (NA stage). Vertical bars indicate standard errors from means (n = 3). Values with different letters are significantly different, as determined by the LSD test (p ≤ 0.05)
There was a linear regression between POD activity and LT50 at both of CA and NA stages. In addition, a significant negative correlation was found between the LT50 and POD at both of CA (r = −0.752**) and NA (r = −0.816**) stages (Fig. 4b).
The results showed, CAT activity all of olive cultivars significantly increased during the CA stage than NA stage (Fig. 4c). Significant differences in CAT activity were also found among cultivars at both of CA and NA stages (Fig. 4c). The highest CAT activity during CA stage was found in the freezing-tolerant cvs Fishomi (70.11 U/g FW min), Mission (65.01 U/g FW min) and Leccino (60.11 U/g FW min), whereas the lowest CAT activity was found in the freezing-sensitive cvs Manzanilla (37.49 U/g FW min), Coratina (39.79 U/g FW min) and Amigdalolia (40 U/g FW min). Similarly to CA stage, the highest and the lowest CAT activity was found in Fishomi (52.43 U/g FW min) and Manzanilla (30.71 U/g FW min), during the NA stage, respectively.
There was a linear regression between CAT activity with LT50 at both of CA and NA stages (Fig. 4d). Furthermore, a significant negative correlation was also found between the LT50 and the CAT activity at both of CA (r = −0.78**) and NA (r = −0.68*) stages (Fig. 4d).
The PPO activity of the leaf tissue of all studied olive cultivars except for Shengeh and Roughani was significantly higher at CA stage compare to NA stage (Fig. 4e). In addition, PPO activity was different among cultivars at both CA and NA stages (Fig. 4e). The highest and the lowest PPO activity was found in the freezing-tolerant cv Mission (0.96 U/100 g FW min) and in the freezing-sensitive cv Zard (0.27 U/100 g FW min) at CA stage, respectively. Also, at NA stage, cultivars such as Fishomi, Shengeh, and Coratina showed higher PPO activity than those of Zard, Amigdalolia, Manzanilla, Roughani and Mari cultivars. At NA stage, the highest and the lowest PPO activity was detected in Mission (0.86 U/100 g FW min) and Amigdalolia (0.14 U/100 g FW min), respectively.
There was linear regression between PPO activity with LT50 at both of CA and NA stages, (Fig. 4f). In addition, a significant negative correlation was also found between LT50 with PPO activity at both of CA (r = −0.810**) and NA (r = −0.878**) stages.
According to the results, PAL activity of almost all studied cultivars, except for Shengeh and Zard, were significantly increased at the NA stage, which were higher compare to CA stage (Fig. 5). During the CA stage, the highest and the lowest PAL activity were observed in Zard (29.8 U/g FW) and Fishomi (18.00 U/g FW), respectively. At the NA stage, Roughani, Leccini and Amigdalolia cultivars had the highest PAL activity, while, Shengeh, Arbiqeina and Manzanilla cultivars had the lowest activity.
Changes in PAL activity of 11 olive cultivars (Fis Fishomi, Mis Mission, She Shengeh, Lec Leccino, Cor Coratina, Arb Arbequina, Rog Roughani, Man Manzanilla, Ami Amigdalolia, Mar Mari, Zar Zard) during winter (CA stage) and summer (NA stage). Vertical bars indicate standard errors from means (n = 3). Values with different letters are significantly different, as determined by the LSD test (p ≤ 0.05)
There was no linear regression between LT50 with PAL activity both CA and NA stages. Furthermore, there was no significant correlation coefficient between LT50 and PAL activity at both CA (r = 0.493ns) and NA (r = 0.162ns) stages.
Total protein content
According to the Fig. 6a, total protein content of all cultivars significantly increased during the CA stage than NA stages. Furthermore, total protein content was different among cultivars at both CA and NA stages (Fig. 6a). During CA stage, the highest and the lowest total protein content were observed in Mission (73.32 mg/g FW) and Shengeh (32.48 mg/g FW) cultivars, respectively. In NA stage, Leccino (47.65 mg/g FW) showed the highest total protein content and shengeh (28.76 mg/g FW) demonstrated the lowest total protein content in the NA stage. There was no linear regression between total protein content and LT50 at both CA and NA stages. In addition, there was no significant correlation between protein content and LT50 at both CA (r = −0.232ns) and NA (r = 0.018ns) stages.
Changes in leaf protein content (a) and proline content (b) of 11 olive cultivar (Fis Fishomi, Mis Mission, She Shengeh, Lec Leccino, Cor Coratina, Arb Arbequina, Rog Roughani, Man Manzanilla, Ami Amigdalolia, Mar Mari, Zar Zard) during winter (CA stage) and summer (NA stage). Vertical bars indicate standard errors from means (n = 3). Values with different letters are significantly different, as determined by the LSD test (p ≤ 0.05)
Proline content
In contrast to protein, leaf proline content of olive cultivars did not change or decreased slightly at CA stage compared to those at NA stage (Fig. 6b). Zard (2.60 µmol/g FW) and Manzanilla (1.9 µmol/g FW) cultivars had the highest and the lowest proline content at NA stage respectively (Fig. 6b). However, there was no significant difference in proline content at the CA stage among the cultivars.
There was no linear regression between proline content and LT50 at both of CA and NA stages. In addition, there was no significant correlation between the LT50 and the proline content at both of CA (r = 0.376ns) and NA (r = −0.383ns) stages.
Discussion
Olive cultivars may differ in freezing tolerance due to the differential survival of their specific organs and tissues (Bartolozzi and Fontanazza 1999). Therefore, selecting freezing tolerant cultivars and recognition the mechanism of FT could greatly progress freezing resistance for olive plants (Bartolozzi and Fontanazza 1999). In this study, we determined FT of 11 olive cultivars as indicated by LT50 during winter and summer. FT of all studied cultivars considerably increased during winter (CA stage), in comparison with non-acclimation (NA) stage during summer. The results also showed that Fishomi, Mission and Shengeh cultivars can be considered as the most freezing-tolerant among the other studied cultivars (Fig. 2). In contrast, Zard, Amigdalolia and Manzanilla can were considered as the most freezing-sensitive cultivars. The values of LT50 obtained in the present work are, somewhat similar to those reported in the literatures. Asl Moshtaghi et al. (2009) also reported that cvs. Zard (LT50 −8.7 °C) and Roghani (LT50 −8 °C) were more sensitive than Dehghan (LT50 −12.3 °C). Furthermore, Manzanilla was reported as a frost-sensitive cultivar in previous studies (Barranco et al. 2005; Cansev et al. 2009). In this study, we found also that Leccino, Coratina and Arbequina have freezing resistance values close to that of freezing-tolerant cultivars (Fig. 2). In previous studies (Bartolozzi and Fontanazza 1999; Mancuso 2000; Gomez del Campo and Barranco 2005) Leccino, Coratina and Arbequina cultivars were showed a moderate to high resistance values close to that of cultivars tolerant. These results suggest that during CA, freezing-tolerant cultivars may develop superior defense responses to alleviate ROS toxicity and have lower ion leakage and LT50 when compared with cold-sensitive cultivars. Therefore, the cultivars difference LT50 may be associated with their genetic background and capacity of metabolic defense responses (such as antioxidants, membrane lipids, and fatty acid constituents) during CA (Cyril et al. 2002; Zhang and Ervin 2008). The scavenging enzymes are a key protein fraction in the acquisition of FT in plants (Lee and Chen 1992). The current study also support the hypothesis that scavenging enzymes play an important role in protecting olive plants against freezing stress.
SOD is a metallo-enzyme that scavenges the toxic superoxide radicals and catalyzes the conversion of two superoxide anions into oxygen and H2O2 (Miyake and Yakota 2000). SOD activity significantly increased in olive cultivars during winter except, in Mari and Amigdalolia cultivars (Fig. 3a). Increase in SOD activity during cold acclimation has also been reported at pine and spruce (Tandy et al. 1989; Tao and Jin 1992). In our study, the highest SOD activity was detected in Leccino, Mission and Arbequina cultivars, in contrast, the lowest ones was assayed in Shengeh, Zard and Mari cultivars at CA stage. However, a similar increase was not seen in olive cultivars during NA stage. In addition, correlation coefficient between LT50 with SOD activity under CA and NA was not statistically significant. This is in agreement with previous study that showed SOD activity is not directly related to FT (McKersie et al. 1999).
APX is one of the key enzymes of the ascorbate glutathione cycle and decomposes H2O2 by oxidation of co-substrates such as phenolic compounds and/or antioxidants (Sudhakar et al. 2001). APX activity increased significantly in olive cultivars at the CA stage, except for Zard (Fig. 3b). The highest APX activity was found in Mari, Amigdalolia and Zard cultivars during CA stage; in contrast, Manzanilla and Mission showed the lowest activity. No significant difference was found between LT50 and APX activity under CA and NA stages. The cultivars such as Zard, Amigdalolia and Mari, showed the lowest FT, while the higher APX activity was found in the freezing-tolerant cultivars i.e. Shengeh and Mission. The result suggests that, although it may contribute to the detoxification of ROS, the activity of the APX enzyme is not a direct key factor in the FT of olive cultivars. Cansev et al. (2009), also suggested that, in cold-tolerance of olive genotypes, APX activity may not be correlated with the degree of cold hardiness. In contrast, Lin et al. (2005) and Luo et al. (2007) reported a significant correlation between APX activity and frost tolerance of hardened leaves in poplar tree.
POD is another antioxidant enzyme that converts H2O2 into H2O and O2 (Sudhakar et al. 2001). Under CA and NA conditions, POD activity in the freezing-tolerant Fishomi and Mission cultivars was the highest, whereas in the freezing-sensitive cultivars such as Zard, Amigdalolia and Manzanilla its activity was the lowest (Fig. 4a). The results also showed a significant negative correlation between the LT50 and POD under both CA and NA stages (Fig. 4b). The higher POD activity in freezing-tolerant cultivars reflects the better ROS-scavenging capacity that resulted to the lower damage to lipids of the plasma membrane under freezing stress conditions. In accordance with this study, Lin et al. (2005) also reported that the rise of POD activities during freezing acclimation was closely correlated to the decrease LT50 of Prunus tomentosa cuttings.
CAT enzyme found mainly in peroxisomes and converts H2O2 into H2O and O2 (Sudhakar et al. 2001). CAT activity increased significantly in all studied olive cultivars during cold acclimation, which suggests a more efficient scavenging of H2O2 that may result in a better protection against peroxidation (Cansev et al. 2009). The highest CAT activity was assayed in the freezing-tolerant Fishomi and Mission cultivars, during both CA and NA condition, whereas the lowest activity was observed in the cold-sensitive cultivars such as Manzanilla, Amigdalolia and Zard cultivars (Fig. 4c). Furthermore, a quite high correlation was also found between the LT50 and the CAT activity in both CA and NA stage (Fig. 4d). The higher CAT activity at freezing-tolerant olive cultivars suggests the more effective H2O2 removal, which might be produced by an enhanced activity of SOD under freezing-stress conditions. These results are in agreement with previous study of Cansev et al. (2009), reported that CAT activity is directly correlated with the degree of cold-hardiness in olive leaf tissue. Furthermore, Luo et al. (2007) found that the LT50 in P. suaveolens cuttings to be closely correlated to the levels of CAT during freezing acclimation.
PPOs catalyze the oxidation of O-diphenols to O-diquinones, as well as the hydroxylation of monophenols at enzymatic browning reactions (Mayer 2006). PPOs are also important in the response of plants against freezing stress and they can help avoid serious oxidative damage induced by freezing (Ortega-García and Peragón 2009). In the present study, PPO activity in the leaf tissues of all cultivars except Shengeh and Manzanilla cultivars increased significantly at CA stage (Fig. 4e). The highest and the lowest PPO activity were found in the freezing-tolerant cv. Mission and in the freezing-sensitive cv. Zard at both of CA and NA stages, respectively. A negative correlation was found between LT50 and PPO activity at both CA and NA stages (Fig. 4f). These finding suggests that PPO might be considered as an elements to determine the recovery capacity and resistance to freezing temperatures of different olive cultivars (Ortega-García and Peragón 2009).
The response of PAL activity may be part of a recovery process of the olive leaf against cold stress (Ortega-García and Peragón 2009). In present study, the highest and the lowest PAL activity was found in Zard and Fishomi cultivars during CA stage, respectively (Fig. 5). However, there was no significant correlation between LT50 and PAL activity both CA and NA stage. In agreement with this study, Ortega-García and Peragón (2009) demonstrated that when the cold stress is low or medium, the high PAL activity may be a mechanism for plant recovery but when the cold stress is high, the elevated level PPO activity can help avoid serious oxidative damage induced by freezing stress as we find about PPO activity in this study.
The induction of protein synthesis during cold acclimation has been suggested to be causally related to the induction of FT in plants (Chen and Li 1982). An accumulation of soluble and specific proteins may help to avoid the alteration of the permeability of the cell membranes, caused by dehydration during the formation of extracellular ice (Levitt 1980; Guy 1990). In the present work, total protein content in the leaf tissues of all cultivars increased considerably during CA stage in comparison with NA stage (Fig. 6a). But, there is no significant correlation between the LT50 and the total protein content at both during CA and NA stages. However, Fishomi and Mission which have high level FT had the highest total protein content at CA stage, but Shengeh, which has high FT also exhibited the lowest total protein content during CA and NA stages. The results obtained in this study are contradictory to pervious report for olive cultivars (Cansev et al. 2009). Cansev et al. (2009) reported that the higher total protein content was associated with the greater cold-hardiness of the olive cultivars.
Proline has been suggested to play a multiple role in plant stress tolerance. Proline may inhibit membrane lipid peroxidation in plant tissues by acting as an antioxidant to neutralize the chilling-induced free radicals (Xin and Li 1993). In this study, the accumulation of leaf proline content during NA stage was higher than CA stage. The highest and lowest proline content at NA stage was found in Manzanilla and Zard, respectively. However, no significant difference was found for proline content among olive cultivars at CA stage (Fig. 6b). No correlation was also found between the LT50 and the proline content at both CA and NA stages. In contrast with this result, a positive correlation between the accumulation of proline and improved cold tolerance was reported in maize (Zhou et al. 2002).
Conclusions
Based on observation in this study, Fishomi, Mission and Shengeh cultivars were found to have the highest FT among the 11 studied olive cultivars. In contrast, Zard, Manzanilla, Amigdalolia and Mari cultivars were more sensitive, while Leccino, Coratina, Arbequina and Roughani cultivars had values close to FT cultivars. The results also showed that CA enhanced the activities of SOD, POD, APX, CAT, PPO enzymes and total protein content. However, proline content and PAL activity of olive cultivars did not change or even decreased slightly in CA stage compared with those in NA stage. LT50 in olive cultivars was found to be correlated to the levels of POD, CAT, and PPO activity during both summer (NA) and winter (CA). Therefore, substantial activity of POD, CAT, and PPO could be used as selection criteria for screening tolerant olive tree for cold zone climates.
Author contribution
A Hashempour carried out the experiment, wrote this manuscript and helped in the design. M Ghasemnezhad provided all the technical support during the laboratory work, helped in the design and manuscript preparation. R Fotouhi Ghazvin and MM Sohani helped in design of the experiment and manuscript preparation. All of the authors have read and approved the final manuscript.
References
Asl Moshtaghi E, Shahsavar AR, Taslimpour MR (2009) Ionic leakage as indicators of cold hardiness in Olive (Olea europaea L.). World Appl Sci J 7:1308–1310
Assis JS, Maldonado R, Munoz T, Escribano MI, Merodio C (2001) Effect of high carbon dioxide concentration on PAL activity and phenolic contents in ripening cherimoya fruit. Postharvest Biol Technol 23:33–39
Azzarello E, Mugnai S, Pandolfi C, Masi E, Marone E, Mancuso S (2009) Comparing image (fractal analysis) and electrochemical (impedance spectroscopy and electrolyte leakage) techniques for the assessment of the freezing tolerance in olive. Trees 23:159–167
Barranco D, Ruiz N, Campo MG (2005) Frost tolerance of eight olive cultivars. Hortic Sci 40:558–560
Bartolozzi F, Fontanazza G (1999) Assessment of frost tolerance in olive (Olea europaea L.). Sci Hortic 81:309–319
Bartolozzi F, Rocchi P, Camerini F, Fontanazza G (1999) Changes of biochemical parameters in olive (Olea europaea L.) leaves during an entire vegetative season, and their correlation with frost resistance. Acta Hortic 474:435–440
Bartolozzi F, Mencuccini M, Fontanazza G (2001) Enhancement of frost tolerance in olive shoots in vitro by cold acclimation and sucrose increase in the culture medium. Plant Cell Tissue Organ Cult 67:299–302
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207
Beauchamp C, Fridovich J (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287
Beers PF, Sizer IW (1952) A spectrophotometric assay measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem 195:133–138
Bradford MM (1976) A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–252
Cansev A, Gulen H, Eris A (2009) Cold-hardiness of olive (Olea europaea L.) cultivars in cold-acclimated and non-acclimated stages: seasonal alteration of antioxidative enzymes and dehydrin-like proteins. J Agric Sci 147:51–61
Chance B, Maehly SK (1955) Assay of catalase and peroxidase. Methods Enzymol 2:764–775
Chen HH, Li PH (1982) Potato cold acclimation. In: Li PH, Sakai A (eds) Plant cold hardiness and freezing stress, vol 2. Academic Press, New York, pp 5–22
Cyril J, Powell GL, Duncan RR, Baird WV (2002) Changes in membrane polar lipid fatty acids of seashore paspalum in response to low temperature exposure. Crop Sci 42:2031–2037
Deshmukh PS, Sairam RK, Shukla DK (1991) Measurement of ion leakage as a screening technique for drought resistance in wheat genotypes. Indian J Plant Physiol 34:89–91
Eris A, Gulen H, Barut E, Cansev A (2007) Annual patterns of total soluble sugars and proteins related to cold-hardiness in olive (Olea europaea L. ‘Gemlik’). J Hortic Sci Biotechnol 82:597–604
Gomez del Campo M, Barranco D (2005) Field evaluation of frost tolerance in 10 olive cultivars. Plant Gen Res 3:385–390
Gulen H, Cansev A, Eris A (2009) Cold hardiness of olive (Olea europaea L.) cultivars in cold-acclimated and non-acclimated stages: seasonal alteration of soluble sugars and phospholipids. J Agric Sci 147:459–467
Guo FX, Zhang MX, Chen Y, Zhang WH, Xu SJ, Wang JH, An LZ (2006) Relation of several antioxidant enzymes to rapid freezing resistance in suspension cultures cells from Alpine Chorispor abungeana. Cryobiology 52:241–250
Guy CL (1990) Cold acclimation and freezing stress tolerance: role of protein metabolism. Annu Rev Plant Physiol Plant Mol Biol 41:187–223
Lee SP, Chen THH (1992) Molecular biology of plant cold hardiness development. In: Li PH, Christersson L (eds) Advances in plant cold hardiness. CRC Press, Boca Raton, pp 1–30
Lee DH, Lee CB (2000) Chilling stress-induced changes of antioxidant enzymes in the leaves of cucumber: in gel enzyme activity assays. Plant Sci 159:75–85
Levitt J (1980) Responses of plants to environmental stresses. Academic Press, New York
Lim CC, Arora R, Townsend EC (1998) Comparing Gompertz and Richards functions to estimate freezing injury in jury in Rhododendron using electrolyte leakage. J Amer Soc Hortic Sci 123:246–252
Lin SZ, Zhang ZY, Liu WF, Lin YZ, Zhang Q, Zhu BQ (2005) Role of glucose-6-phosphate dehydrogenase in freezing—induced freezing resistance of Populus suaveolens. J Plant Physiol Mol Biol 35:34–40
Luh BS, Phithakpol B (1972) Characteristics of polyphenoloxidase related to browning in cling peaches. J Food Sci 37:264–268
Luo L, Lin SZ, Zheng HQ, Lei Y, Zhang Q, Zhang ZY (2007) The role of antioxidant system in freezing acclimation-induced freezing resistance of Populus suaveolens cuttings. For Stud China 9:107–113
Mancuso S (2000) Electrical resistance changes during exposure to low temperature measure chilling and freezing tolerance in olive tree (Olea europaea L.) plants. Plant Cell Environ 23:291–299
Martin GC, Denney JO, Ketchie DO, Osgood JW, Connel JH, Sibbet GS, Kammereck R, Krueger WH, Nour GA (1993) Freeze damage and cold hardiness in olive: findings from the 1990 freeze. Calif Agric 47:1–12
Mayer AM (2006) Polyphenol oxidases in plants and fungi: going places? Rev Phytochem 67:2318–2331
McKersie BD, Bowley SR, Jones KS (1999) Winter survival of transgenic alfalfa overexpressing superoxide dismutase. Plant Physiol 119:839–847
Miyake C, Yakota A (2000) Determination of the rate of photoreduction of O2 in the water-water cycle in water melon leaves and enhancement of the rate by the limitation of photosynthesis. Plant Cell Physiol 4:335–343
Nakano Y, Asada K (1980) Spinach chloroplasts scavenge hydrogen peroxide on illumination. Plant Cell Physiol 21:1295–1307
Ortega-García F, Peragón J (2009) The response of phenylalanine ammonia-lyase, polyphenol oxidase and phenols to cold stress in the olive tree (Olea europaea L. cv. Picual). J Sci Food Agric 89:1565–1573
Palliotti A, Bongi G (1996) Freezing injury in the olive leaf and effects of mefluidide treatment. J Hortic Sci 71:57–63
Roselli G, Venora G (1990) Relationship between stomatal size and winter hardiness in the olive. Acta Hortic 286:89–92
Roselli G, Benelli G, Morelli D (1989) Relationship between stomatal density and winter hardiness in olive (Olea europaea L.). J Hortic Sci 64:199–203
Roselli G, La Porta N, Morelli D (1992) Valutazioni del germoplasma di olivo per la tolleranza a stress da freddo. Anti Convegno Germoplasma Frutticolo 9:107–112
Solecka D, Kacperska A (2003) Phenylpropanoid deficiency affects the course of plant acclimation to cold. Physiol Plant 119:253–262
Sudhakar C, Lakshmi A, Giridarakumar S (2001) Changes in the antioxidant enzyme efficacy in two high yielding genotypes of mulberry (Morus alba L.) under NaCl salinity. Plant Sci 161:613–619
Tandy NE, Giolio RTD, Richardson CJ (1989) Assay and electrophoresis of superoxide dismutase from red spruce (Picea rubens Sarg.), Lobloly pine (Pinu staedaL.) and Scots pine (Pinus sylvestrisL.). Plant Physiol l90:742–748
Tao DL, Jin YH (1992) Organic free radicals and free-radical scavengers in overwintering conifer needles. Sci Silvae Sin 28:194–197
Thomashow MF (1999) Plant cold acclimation: freezing tolerance genes and regulatory mechanisms. Annu Rev Plant Physiol Plant Mol Biol 50:571–599
Wise RR (1995) Chilling-enhanced photooxidation: the production, action and study of reactive oxygen species produced during chilling in the light. Photosynth Res 45:79–97
Wisniewski M, Carole B, Gusta LV (2003) An overview of cold hardiness in woody plants: seeing the forest through the trees. Hortic sci 38:952–959
Xin Z, Li P (1993) Relationship between proline and abscisic acid in the induction of chilling tolerance in maize suspension-cultured cells. Plant Physiol 103:607–613
Zhang X, Ervin EH (2008) Metabolic defense responses of bermudagrass during acclimation to freezing stress—a review. Acta Hortic 783:181–194
Zhou BY, Li YB, Chen JZ, Ji ZL, Hu ZQ (2002) Effects of low temperature stress and ABA on flower formation and endogenous hormone of litchi. Acta Hortic Sin 29:577–578
Acknowledgments
The authors wish to express sincere gratitude to the the staffs of Olive Research Station of Roudbar, especialy Dr. Ramezani malkroudi for providing plant materials.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by L. Bavaresco.
Rights and permissions
About this article
Cite this article
Hashempour, A., Ghasemnezhad, M., Fotouhi Ghazvini, R. et al. Olive (Olea europaea L.) freezing tolerance related to antioxidant enzymes activity during cold acclimation and non acclimation. Acta Physiol Plant 36, 3231–3241 (2014). https://doi.org/10.1007/s11738-014-1689-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-014-1689-3