Abstract
Efficient Agrobacterium tumefaciens mediated T-DNA delivery and subsequent shoot organogenesis has been achieved from Bacopa monnieri. Various factors influenced T-DNA delivery as evident from transient GUS assay. The transient GUS expression was significantly higher (97.7 %) in explants that were pre-cultured before bacterial infection on medium supplemented with 100 μM acetosyringone. Incorporation of acetosyringone into the co-cultivation medium also enhanced transient GUS activity. Explant injury with carborundum paper, co-cultivation period of 2 days and a bacterial density of 0.4 OD600 showed higher transient GUS expression. Following co-cultivation, shoot organogenesis was achieved from leaf segments on basal Murashige and Skoog medium containing 58 mM sucrose. Supplementation of antibiotics (cefotaxime or carbenicillin) at > 250 μg/ml into the medium significantly promoted shoot organogenesis from leaf explants (71.5 % in control and > 83.0 % on medium containing 500 μg/ml of carbenicillin or cefotaxime). Stable transformation of regenerated shoots was confirmed on the basis of GUS activity and PCR amplification of DNA fragments specific to reporter gene (uidA) and selection marker gene (nptII). The expression level of nptII gene in independent transgenic lines was studied using quantitative real time-PCR. Stable transformed shoots after rooting were successfully established in the pots.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bacopa monnieri (L.) Wettst. (Scrophulariaceae); common name brahmi’ or ‘jala-brahmi’ is an amphibious plant of tropics. It is normally found growing on the banks of rivers and lakes. It is traditionally used in India as an important constituent of Ayurvedic formulations and reported to possess anticancer (Elangovan et al. 1995), antioxidant (Tripathi et al. 1996) and cardiotonic (Mathur et al. 2002) properties. It is also used to counteract the effects of anxiety, mental stress and neurosis (Singh et al. 1979). Saponins (bacosides) extracted from plant have been reported to posses memory enhancing properties (Pal and Sarin 1992). The discovery of the memory-enhancing property of the bacosides has led to increased collection of raw material for the extraction of active compounds resulting in decline of natural populations (Tiwari et al. 2001).
Since plant is reported to possess many important active ingredients, attributing to various medicinal properties, there is an opportunity to undertake genetic transformation aiming at engineering of this plant to produce novel metabolites or for up-regulation of the existing compounds. Genetic transformation technology is thought to be one of the most suitable approaches to enhance production of secondary metabolites (Canter et al. 2005). Though genetic transformation of a number of important plant species has been reported, there are limited reports of such efforts in case of medicinal plants (Gomez-Galera et al. 2007). Therefore, there is an urgent need to optimize various factors for efficient genetic transformation of this important herb.
Although, shoot organogenesis from different explants have been reported in B. monnieri (Tiwari et al. 1998, 2006; Banarjee and Srivastava 2008; Ceasar et al. 2010), yet there is only a preliminary report on the development of genetic transformation available in literature (Nisha et al. 2003). Further, it has been reported that plants with higher secondary metabolite contents are recalcitrant to organogenesis following genetic transformation (Wei et al. 2006). Keeping in view the medicinal importance of B. monnieri, there is an urgent need to develop an efficient genetic transformation protocol so that an effective programme focussing on metabolic engineering for the production of high value compounds can be taken up. Further, development of efficient regeneration system is a prerequisite for the production of transgenic plants (Aggarwal et al. 2011). Therefore, the present study was aimed to investigate the factors affecting efficient Agrobacterium tumefaciens mediated genetic transformation and subsequent shoot organogenesis from transformed tissues of B. monnieri. The expression level of nptII gene in stable transgenic lines was studied using quantitative real time – PCR (qRT-PCR).
Materials and methods
Plant material, chemicals, glassware
Elite plants of B. monnieri (L.) Wettst. were collected from Tau Devi Lal Herbal Park, Chhachhrauli, Yamunanager (Haryana), India and maintained in earthen pots under green house conditions. All routine chemicals were purchased from HiMedia Laboratories (Mumbai, India), growth regulators and antibiotics were purchased from Sigma Chemical Co. (MO, USA). Unless otherwise mentioned, all experiments were conducted in 300 ml glass culture bottles (Kasablanka, Mumbai, India) containing 50 ml of medium. The pH of medium was adjusted to 5.8 before autoclaving at 121 °C for 20 min. Cultures were established using nodal explants following the procedure mentioned earlier (Kumar et al. 2010) and incubated at 25±1 °C under cool white fluorescent lamps (Philips India Ltd, Mumbai, India) with the light intensity of 42 μmolm−2s−1 inside the culture vessel in 16–h light/8–h dark cycle. The actively growing shoot cultures were maintained on Murashige and Skoog medium (Murashige and Skoog 1962), containing 58 mM sucrose and gelled with 0.7 % (w/v) agar (MS medium) supplemented with 2.5 μM benzyladenine (BA). The fully expanded leaves (14–20 days old) from these microshoots were used as explants for transformation and shoot regeneration experiments.
Determination of kanamycin sensitivity
Tolerance limit (sensitivity) of the leaf explants for kanamycin was determined by culturing leaf segments on shoot induction medium (SIM, Table 1) supplemented with different concentrations of kanamycin (0 to 80 μg/ml). All antibiotic solutions were filter sterilized and added to the medium after autoclaving when the temperature has come down to 40 °C.
Binary vector and Agrobacterium strain
The binary vector pBI121 carrying reporter gene uidA (β-glucuronidase) (GUS) and selection marker gene neomycin phosphotransferase II (nptII) was used for genetic transformation. These genes were expressed under the control of CaMV 35S and nos promoters respectively (Jefferson et al. 1987). The plasmid was introduced into A. tumefaciens disarmed strain LBA4404 (Hoekema et al. 1983) by the freeze–thaw method (Holsters et al. 1978). The presence of pBI121 plasmid was confirmed in the antibiotic resistant bacterial colonies by PCR using nptII gene specific primers. The transformed A. tumefaciens strain was maintained at 28 °C on yeast extract peptone (YEP) agar medium (10 g/1 bacto peptone, 10 g/l yeast extract, 05 g/l NaCl and 15 g/l agar, pH-7.0) containing 15 μg/ml rifampicin and 50 μg/ml kanamycin and used for the genetic transformation experiments.
Co-cultivation and infection
A single bacterial colony was inoculated in 10 ml YEP broth supplemented with 50 μg/ml kanamycin and 15 μg/ml rifampicin and grown overnight at 28 °C on a gyratory shaker (250 rpm). From the overnight grown culture, 0.5 ml bacterial suspension was inoculated into fresh 50 ml of YEP medium and grown for 24 h. Bacterial cells were pelleted by centrifugation (4000 × g, 5 min) and resuspended in YEP medium supplemented with 100 μM acetosyringone (unless otherwise mentioned) to attain the desired OD600. Leaf explants that were cultured on pre-culture medium (PCM, Table 1) for 0–5 days were injured following different procedures namely, pricking with hypodermic needle, cutting with surgical blade, rubbing with carborundum paper. These were then infected with above mentioned suspension of A. tumefaciens for different time periods (10 ml, 0–30 min) in Petri plates. Following infection, tissues were blotted on sterile blotting paper to remove the excess of bacterial cells. Infected tissues were cultured for 1–5 days on antibiotic-free co-cultivation medium (CCM, Table 1). Culture plates were sealed with cling film and incubated under different photoperiods viz. continuous light, 16-h light/8-h dark or continues dark.
Selection and regeneration of transformed shoots
Following co-cultivation, leaf explants were washed (4–5 times) with sterile distilled water containing 500 μg/ml carbenicillin, blotted on sterile filter paper and transferred on to selection medium supplemented with 50 μg/ml kanamycin and 500 μg/ml carbenicillin (selection-cum-shoot induction medium: SM; Table 1). The effect of two antibiotics, namely, cefotaxime and carbenicillin (0–500 μg/ml) was tested on shoot organogenesis from leaf explants. The effect of varying concentration of BA (0.0–12.5 μM) and 2, 4-dichlorophenoxyacetic acid (2, 4-D, 0.0–12.5 μM) and sucrose concentrations (58, 116, 174 and 232 mM) in basal MS medium was tested for shoot organogenesis from leaf explants.
Histochemical GUS assay
GUS assay was carried out using regenerated kanamycin resistant shoots (for scoring stable expression) and freshly infected explants after 2 days of incubation on selection medium (for scoring transient expression) following the method of Jefferson et al. (1987). Tissues were incubated at 37 °C in 100 mM sodium phosphate buffer (pH 7.0), containing 1 mM 5-bromo-4-chloro-3-indolyl-β-D-glucuronic acid (X-Gluc), 0.5 mM potassium ferrocyanide and 0.1 % (v/v) Triton X-100 for 12 h. Following incubation, tissues were cleared to remove chlorophyll by washing several times with 70 % ethanol and then slowly increased to absolute ethanol. The tissues showing blue colour after the removal of chlorophyll were scored.
Molecular analysis
Genomic DNA was extracted from the shoot tissues following CTAB method (Doyle and Doyle 1990). PCR amplification of DNA fragments specific to nptll and uidA genes was carried out from the leaves of GUS positive putative transgenic shoots and untransformed shoots. PCR reaction mixture consisted of 20 ng of genomic DNA, 1.0 U of Taq DNA polymerase (Larova, Teltow, Germany), 100 μmol dNTPs mixture, 2.0 μl reaction buffer (10X), 10 nmol each primer and sterile Milli-Q water (Millipore, India) was added to make up the final volume to 20 μl. Amplification conditions were as follows: initial denaturation 94 °C for 5 min followed by 31 cycles of 94°C for 1 min, 58°C for 45 sec and 72°C for 1.5 min a with final extension at 72°C for 5 min. A fragment of about 1,500 bp specific to uidA gene was amplified using primer pair (forward primer 5-GGTGGGAAAGCGCGTTACAAG-3 and reverse primer 5-GTTTACGCGTTGCTTCCGCCA-3) and a fragment of about 720 bp gene specific to nptII was amplified using primer pair (forward primer 5-GAGGCTATTCGGCTATGACTC-3 and reverse primer 5-ATCGGGAGAGGCGATACCGTA-3). Plasmid DNA of pBI121 was used as a positive control and leaves from untransformed shoots of B. monnieri were the source of DNA for negative control.
PCR amplification of 16S rRNA locus specific fragment of about 1,500 bp was carried out using DNA extracted from putative transformed and untransformed shoots using primer pair 5-AGAGTTTGATCCTGGCTCAG-3 and 5- ACGGGCGGTGTGTTC-3 specific to bacterial 16S rRNA. Bacterial genomic DNA was used as positive control. Amplification conditions were same as mentioned above. The amplified products were separated on a 1.0 % (w/v) agarose gel and viewed using UV transilluminator (BioRad, CA, USA) following ethidium bromide staining.
RT-PCR analysis of nptII gene
Total RNA was isolated from young putative transgenic shoots through monophasic lysis reagent Trizol (Invitrogen, USA). The RNA was further treated with DNase I (Invitrogen, USA) to avoid PCR amplification from genomic DNA. The first strand cDNA synthesis was carried out using DNase-treated total RNA (2.5 μg) with the help of cDNA synthesis kit (MBI, Fermentas, USA) in a reaction volume of 20 μl according to the manufacturer’s protocol. The first strand cDNA was immediately subjected to PCR amplification with nptII gene-specific primers independently as mentioned above.
Gene expression analysis using quantitative real time–PCR (qRT–PCR)
Quantitative real time-polymerase chain reaction (qRT-PCR) analysis of nptll gene was carried out using the Real Master Mix SYBR ROX Master Mix (5 prime, GmbH, Hamburg) on Realplex 4 real-time PCR system (Eppendorf AG, Hamburg) to determine critical thresholds (Ct) values, using gene-specific primers (forward primer 5-GAATGAACTGCAGGACGAG-3 and reverse primer 5- ATACTTTCTCGGCAGGAGCA -3). For quantification of gene expression in transgenic lines, actin-9 (forward primer 5-CTATTCTCCGCTTTGGACTTGGCA-3 and reverse primer 5- AGGACCTCAGGACAACGGAAACG -3) as described previously by Volkov et al. (2003) was used as an endogenous control. Conditions for the qRT-PCR reactions were as follows: 95 °C for 2 min, 40 ycles of 95 °C for 15 s, 55 °C for 15 s, and 68 °C for 20 s. Gene expression was quantified using the comparative method Ct:2−ΔΔCt method.
Statistical analysis
Unless otherwise mentioned all experiments were conducted in triplicates and repeated three times. The data were analyzed by analysis of variance and the means were compared using Duncan’s multiple-range test (P<0.05). All the analyses were performed using Graphpad Prism software (GraphPad V 4.03).
Results and discussions
In the present investigation, factors influencing genetic transformation and subsequent shoot organogenesis of selected elite clone of B. monnieri were studied.
Genetic transformation
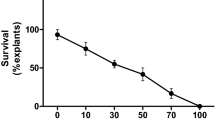
Experimental results investigating the sensitivity of leaf explants to antibiotics showed that presence of kanamycin in the medium caused considerable toxicity to explants and all the explants died on media containing > 50 μg/ml kanamycin (Fig. 1). Kanamycin also inhibited shoot organogenic potential of explants as compared to those cultured on kanamycin-free medium (70 % explants showed shoot organogenesis). Therefore, kanamycin (50 μg/ml) was used in the medium for selection of transformed tissues. These results are in line with the earlier reports on some other medicinal plants like Withania somnifera (Pandey et al. 2010), Centella asiatica (Krishnan et al. 2008) including B. monnieri (Nisha et al. 2003). The carbenicillin concentration was kept at 500 μg/ml for the elimination of residual bacteria from the cultures. This concentration of carbenicillin was not only effective for the complete elimination of A. tumefaciens but also promoted shoot organogenic potential of explants (Table 2).
In the present study, A. tumefaciens strain LBA4404 derivative from strain Ach 5 (Hoekema et al. 1983) harbouring binary vector pBI121 was used. This strain was successfully used for transformation of many plants like Withania somnifera, Mentha arvensis, and Morus alba (Pandey et al. 2010; Aggarwal and Kanwar 2007; Kumar et al. 2008). Moreover, it has also been reported that elimination of strain LBA4404 from plant tissues is relatively easy (Maheswaran et al. 1992).
Various factors namely pre-culture, bacterial density, mode of injury, incubation conditions, pH of co-cultivation medium, phenolics etc., influenced transformation efficiency of leaf explants (Table 3). Culture medium and incubation conditions of explants, prior to A. tumefaciens infection have been reported to influence T-DNA delivery in some plant species (Aggarwal et al. 2011; Padmanabhan and Sahi 2009). In the present study, leaves pre-cultured on PCM medium (Table 1) containing 50 μM acetosyringone for 3 days and incubated under 16-h light/8-h dark cycle showed maximum transient GUS activity (97.7 %; Table 3). The higher GUS activity is probably due to presence of vir genes inducing compounds such as acetosyringone in the medium (Stachel et al. 1985). Earlier, pre-culturing of explants on a particular medium prior to infection with A. tumefaciens has been reported to enhance transformation efficiency (Kumar et al. 2008; Padmanabhan and Sahi 2009).
Supplementation of 100 μM acetosyringone in the co-cultivation medium significantly increased transient GUS activity from 62.2 % (on medium lacking acetosyringone) to 68.8 % (Table 3). Earlier, acetosyringone has been reported to enhance Agrobacterium mediated genetic transformation efficiency in Centella asiatica (Krishnan et al. 2008) and Eucalyptus tereticornis (Aggarwal et al. 2011).
Method of injury to the tissue prior to bacterial infection was also observed to play an important role in improving the efficiency of A. tumefaciens mediated T-DNA delivery. Injury of leaf tissue with carborundun paper significantly enhanced transient GUS activity from 54.4 % (in intact explants) to 76.6 % explants (Table 3). Wounding of tissue before infection could allow bacterial penetration deep into the tissue facilitating the accessibility of plant cells to Agrobacterium on one hand and induction of vir genes as a result of phenolics secretion on other which could be main reasons for enhanced bacterial efficiency for T-DNA delivery (Stachel et al. 1985). Method of injury to plant tissue before co-cultivation has also been shown to influence transformation frequency in Vitis vinifera (Dutt et al. 2007) and Vigna radiate (Sonia et al. 2007).
The pH of medium during co-cultivation also influenced efficiency of T-DNA delivery. Significantly higher frequency of explants showed transient GUS activity when co-cultured on medium having pH of 5.2 (68.8 %) as compared to explants cultured on medium having pH of 5.8 (48.8 %) (Table 3). Earlier, lower pH during co-cultivation was reported to be beneficial for A. tumefaciens mediated transformation across the species (Godwin et al. 1991).
The density of bacterial suspension used for infection of explant also influenced transient GUS activity (Table 3). Maximum transient GUS activity was obtained in explants that were infected with bacterial suspension of OD600 0.4–0.6. At higher bacterial density, decreased transient GUS activity is possibly resulting from damage caused to tissue due to over growth of bacteria leading to tissue necrosis. This could result from increased production of toxic compounds due to bacterial overgrowth as reported earlier (Sonia et al. 2007).
A co-cultivation period following A. tumefaciens infection influenced the expression of transient GUS activity (Table 3). A maximum of 59.9 % explants showed transient GUS activity when these were co-cultivated for 2 days. In this study, co–cultivation period of more than 4 days caused excessive bacterial growth leading to tissue necrosis. It is established that co-cultivation of explants with A. tumefaciens for an appropriate duration is required for efficient T-DNA delivery. However prolonged co-cultivation was reported to result in death of explants (James et al. 1993).
In the present investigation, effect of three different photoperiods on transient GUS expression was studied (Table 3). Incubation of infected explants under complete darkness during co-cultivation resulted in higher transient GUS activity (78.8 % explants). Earlier, photoperiod during co-cultivation was also reported to influence transient expression of reporter gene in Dianathus caryophyllus and E. tereticornis (Aggarwal et al. 2011; Zuker et al. 1999). In this study a transformation efficiency of 65.7 % has been obtained in the rooted shoots that are ready for the transfer to soil.
Shoot organogenesis
Shoot organogenic potential of leaf explants after co-cultivation was assessed on MS medium variously supplemented with BA and 2, 4-D is summarized in Table 4. Callus formation was recorded in all explants cultured on media supplemented with plant growth regulators. Maximum explants (71.5 %) showed shoot organogenesis on plant growth regulator free (PGR free) MS medium (Fig. 2a). Shoot organogenic potential of leaf explants varied in presence of PGRs (Table 4). Effect of BA and 2,4-D on shoot organogenesis has been reported in many plant species including B. monnieri (Aggarwal et al. 2010; Kumar et al. 2002; Tiwari et al. 2001).
Genetic transformation of B.monnieri. a Shoot organogenesis from leaf explants of B. monnieri on MS medium b Regeneration of transgenic shoots from leaf explants of B. monnieri on selection medium supplemented with kanamycin after infection with Agrobacterium tumefaciens c Multiplication of transformed shoots on MS medium supplemented with kanamycin d Transformed acclimatized B. monnieri plants
Agrobacterium mediated genetic transformation procedures utilize antibiotics for the elimination of bacteria from cultures after co-cultivation. In the present study, incorporation of cefotaxime and carbenicillin was found to be beneficial for the shoot organogenesis in terms of both percent explants resulting shoot organogenesis and the number of shoots differentiated per explant (Table 2). Maximum shoot organogenesis was observed in explants cultured on medium containing 500 μg/ml carbenicillin (84.2 %) followed by medium containing 500 μg/ml cefotaxime (83.0 %), whereas explants cultured on antibiotic-free basal MS medium (control) resulted in shoot organogenesis in significantly less number of explants (71.5 %). Beneficial effect of antibiotics on adventitious shoot formation was also reported in many plant species inluding B. monnieri (Aggarwal et al. 2010; Hammerschlag et al. 1997; Tiwari et al. 2006). Carbenicillin is known to possess auxin like structural features (Robert et al. 1989) and cefotaxime has been shown to interfere with ethylene biosynthesis (Pius et al. 1993). The growth regulatory activity of these antibiotics has also been attributed to their interference with the metabolism of PGRs (Padilla and Burgos 2010).
In plant, cell and tissue cultures, sucrose is generally used as carbon source. Sucrose concentrations have been shown to affect the growth and morphogenesis of shoots (Gurel and Gulsen 1998; Kumar et al. 1999). In the present study, shoot organogenic potential decreased with increase in sucrose concentration from 58 mM to 232 mM. Maximum shoot organogenesis was observed when 58 mM sucrose (normal concentration used in tissue culture) was used in the medium (Table 5). These results are in line with previous reports on influence of sucrose on shoot organogenesis (Kumar et al. 1999; Naik et al. 2010). Sucrose dependent developmental regulation of morphogenesis has also been reported in liverworts (Mehra 1972) and Chlorophytum borivilianum (Kumar et al. 2010).
Although, kanamycin resistance shoots (Fig. 2 b & c) was indicative of expression of the nptII gene, yet GUS activity was also examined to confirm the expression of these newly incorporated genes. Kanamycin resistant shoots showed positive GUS activity indicating stable incorporation of these genes (Fig. 3a). These results were confirmed by PCR amplification of DNA fragments of 720 bp specific to nptII gene (Fig. 3b) and 1,500 bp specific to uidA gene (Fig. 3c) from DNA isolated from putative transgenic shoots. Amplification of DNA fragment specific to 16S rRNA locus of DNA isolated from transgenic shoots was not observed establishing complete elimination of bacteria from these tissues (Fig. 3d). Earlier, such analysis has been successfully used for the detection of bacterial presence in the transformed tissue (Aggarwal et al. 2011).
GUS and molecular analyses of transformants. a Transformed B. monnieri shoots showing stable GUS activity after 6 months of survival under green house conditions b and c Amplification of nptll gene (~720 bp) and uidA gene (~1500 bp) from genomic DNA of transformed tissue respectively Lane-1: Positive control (amplification from pBI121) Lane -2: 1 kb ladder Lane-3:Negative control (Non-transformed tissue) Lane- 4–15: Amplification from DNA of transformed Shoots d Amplification of 16 S rRNA (~1500 bp) fragment using primers specific to bacteria Lane-1: 1 kb ladder Lane-2: Positive control (amplification from bacterial genomic DNA) Lane 3–7:Amplification from DNA of transformed Shoots e RT PCR analysis of nptll gene (~720 bp) from cDNA of transformed tissue Lane-1: Positive control (amplification from pBI121) Lane -2: 1 kb ladder Lane-3:Negative control (Non-transformed tissue) Lane- 4–10: Amplification from cDNA of transformed Shoots
Further, expression of nptII gene was also confirmed at transcription level in the transgenic plants by RT-PCR. The cDNA was synthesized from total RNA isolated from control plants and five independent transgenic lines grown under green house conditions were subjected to PCR as mentioned above. Amplification of DNA fragment from five independent transgenic lines yielded a fragment of 720 bp, specific nptII gene, while amplification was not observed in control samples (Fig. 3e) indicating that transgenic plants expressed the nptII gene. Earlier, RT-PCR analysis was successfully utilized for the detection/integration of transgene in the host genome (Jung et al. 2011; Bakshi et al. 2011). Expression levels of nptII gene was studied in different transgenic lines using qRT-PCR. Different expression levels were observed in different transgenic lines. Line T2 showed maximum expression levels, whereas line T1 showed minimum expression levels of nptII gene (Fig. 4). Differences among transgenic lines in same transformation event are often observed and are likely due to variations in insertion site and events like this can be valuable source for plant genetic improvement (Cai et al. 2012; Hong et al. 2012).
Thus factors for an efficient T-DNA delivery and subsequent plant regeneration from B. monnieri were investigated and transformed plants of B. monnieri were recovered. These transformed plants were successfully established in earthen pots (Fig. 2d) and these showed normal phenotype both under culture and in the pots.
Conclusion
An efficient genetic transformation and subsequent shoot regeneration protocol has been established for B. monnieri using leaf explant. Various factors such as pre-culture, bacterial density, mode of injury, photoperiod, pH during co-cultivation influenced plant regeneration and transient GUS activity. Expression and chromosomal integration of both selection marker gene (nptII) and reporter gene (uidA) genes was confirmed by histochemical, PCR, RT-PCR and qRT-PCR analyses. The present protocol will facilitate the work on metabolic engineering of this high value medicinal plant.
Abbreviations
- GUS:
-
β-Glucuronidase
- nptII :
-
Neomycin phosphotransferase
- RT-PCR:
-
Reverse transcriptase-polymerase chain reaction
- qRT-PCR:
-
Real Time - polymerase chain reaction
- X-Gluc:
-
5-bromo-4-chloro-3-indolyl-β-D-glucuronic acid
References
Aggarwal S, Kanwar K (2007) Comparison of genetic transformation in Morus alba L. via different regeneration systems. Plant Cell Rep 26:177–185
Aggarwal D, Kumar A, Reddy MS (2010) Shoot Organogenesis from elite plants of Eucalyptus tereticornis. Plant Cell Tissue Organ Cult 102:45–52
Aggarwal D, Kumar A, Reddy MS (2011) Agrobacterium tumefaciens mediated genetic transformation of selected elite clone(s) of Eucalyptus tereticornis. Acta Physiol Plant 33:1603–1611
Bakshi S, Sadhukhan A, Mishra S, Sahoo L (2011) Improved Agrobacterium-mediated transformation of cowpea via sonication and vacuum infiltration. Plant Cell Rep 30:2281–2292
Banarjee M, Srivastava S (2008) An improved protocol for in vitro multiplication of Bacopa monnieri (L.). World J Microbiol Biotechnol 24:1355–1359
Cai P, Long H, Deng GB, Pan ZF, Peng ZS, Yu MQ (2012) Molecular cloning, characterization, and expression analysis of genes encoding gibberellin 20-oxidase in Dasypyrum villosum dwarf mutant. Plant Mol Biol Rep. doi:10.1007/s11105-012-0419-5
Canter PH, Thomas H, Ernst E (2005) Bringing medicinal plants into cultivation: opportunities and challenges for biotechnology. Trends Biotechnol 23:180–185
Ceasar SA, Maxwell SL, Prasad KB, Karthigan M, Ignacimuthu S (2010) Highly efficient shoot regeneration of Bacopa monnieri (L.) using a two-stage culture procedure and assessment of genetic integrity of micropropagated plants by RAPD. Acta Physiol Plant 32:443–452
Doyle JJ, Doyle JL (1990) Isolation of plant DNA from fresh tissue. Focus 12:13–15
Dutt M, Li ZT, Dhekney SA, Gray DJ (2007) Transgenic plants from shoot apical meristems of Vitis vinifera L. “Thompson Seedless” via Agrobacterium-mediated transformation. Plant Cell Rep 26:2101–2110
Elangovan V, Govindasamy S, Ramamoorthy N, Balasubramanian K (1995) In vitro studies on the anticancer activity of Bacopa monnieri. Fitoterapia 66:211–215
Godwin I, Todd G, Ford-Lloyd B, Newbury HJ (1991) The effect of acetosyringone and pH on Agrobacterium-mediated transformation vary according to plant species. Plant Cell Rep 9:671–675
Gomez-Galera S, Pelacho AM, Gene A (2007) The genetic manipulation of medicinal and aromatic plants. Plant Cell Rep 26:1689–1715
Gurel S, Gulsen Y (1998) The effects of sucrose agar and pH levels on in vitro shoot production of Almond (Amygdalus communis L.). Turk J Bot 22:363–373
Hammerschlag FA, Zimmerman RH, Yadava UL, Hunsucker S, Gercheva P (1997) Effect of antibiotics and exposure to an acidified medium on the elimination of Agrobacterium tumefaciens from apple leaf explants and on shoot regeneration. J Am Soc Hortic Sci 122:758–763
Hoekema A, Hirsch PR, Schilperoort PJJ, Hooykaas RA (1983) A binary vector strategy based on separation of vir and T-region of the Agrobacterium tumefaciens Ti-plasmid. Nature 303:179–180
Holsters M, De Waele D, Depicker A, Messens E, Van Montagu M, Schell J (1978) Transfection and transformation of Agrobacterium tumefaciens. Mol Gen Genet 163:181–187
Hong YB, Liu SP, Zhu YP, Xie C, Jue DW, Chen M, Kaleri HA, Yang Q (2012) Expression of the MSI-99 m gene in transgenic potato plants confers resistance to Phytophthora infestans and Ralstonia solanacearum. Plant Mol Biol Rep. doi:10.1007/s11105-012-0503-x
James DJ, Uratsu S, Cheng J, Negri P, Viss P, Dandekar AM (1993) Acetosyringone and osmo-protectants like betaine or proline synergistically enhance Agrobacterium-mediated transformation of apple. Plant Cell Rep 12:559–563
Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6:3901–3907
Jung M, Shin SH, Park JM, Lee SN, Lee MY, Ryu KH, Paek KY, Harn CH (2011) Detection of transgene in early developmental stage by GFP monitoring enhances the efficiency of genetic transformation of pepper. Plant Biotechnol Rep 5:157–167
Krishnan VN, Soni KB, Rajmohan K (2008) Agrobacterium tumefaciens mediated genetic transformation in Centella asiatica L. Urban. Curr Biotica 2:1–8
Kumar A, Sood A, Palni LMS, Gupta AK (1999) In vitro propagation of gladiolus hybridus hort.: synergistic effect of heat shock and sucrose on morphogenesis. Plant Cell Tissue Organ Cult 57:105–112
Kumar A, Palni LMS, Sood A, Sharma M, Palni UT, Gupta AK (2002) Heat shock induced somatic embryogenesis in callus cultures of gladiolus in the presence of high sucrose. J Hort Sci Biotechnol 77:3–78
Kumar A, Chakraborty A, Ghanta S, Chattopadhyay S (2008) Agrobacterium-mediated genetic transformation of mint with E. coli glutathione synthetase gene. Plant Cell Tissue Organ Cult 96:117–126
Kumar A, Aggarwal D, Gupta P, Reddy MS (2010) Factors Effecting in vitro propagation and field establishment of Chlorophytum borivillianum. Biol Plant 54:601–606
Maheswaran GM, Welander M, Hutchinson JF, Graham MW, Richards D (1992) Transformation of apple rootstock M26 with Agrobacterium tumefaciens. J Plant Physiol 139:560–568
Mathur S, Gupta MM, Ram M, Sharma S, Kumar S (2002) Herb yield and bacoside A content of field grown Bacopa monnieri accessions. J Herb Spices Med Plants 9:11–18
Mehra PN (1972) Some aspects of differentiation in cryptogams. Res Bull (NS) Punjab University Part III–IV: 221–242
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Naik PM, Manohar SH, Praveen N, Murthy HN (2010) Effects of sucrose and pH levels on in vitro shoot regeneration from leaf explants of Bacopa monnieri and accumulation of bacoside A in regenerated shoots. Plant Cell Tissue Organ Cult 100:235–239
Nisha KK, Seetha K, Rajmohan K, Purushothama GM (2003) Agrobacterium-tumefaciens mediated transformation of Brahmi [Bacopa monnieri (L.)Wettst.], a popular medicinal herb of India. Curr Sci 85:85–89
Padilla IMG, Burgos (2010) Aminoglycoside antibiotics: structure, functions and effects on in vitro plant culture and genetic transformation protocols. Plant Cell Rep 29:1203–1213
Padmanabhan P, Sahi SV (2009) Genetic transformation and regeneration of Sesbania drummondii using cotyledonary nodes. Plant Cell Rep 28:31–40
Pal R, Sarin JPS (1992) Quantitative determination of bacosides by UV spectrophotometry. Indian J Pharm Sci 54:17–18
Pandey V, Misra P, Chaturvedi C, Mishra MK, Trivedi PK, Tuli R (2010) Agrobacterium tumefaciens-mediated transformation of Withania somnifera (L.) Dunal: an important medicinal plant. Plant Cell Rep 29:133–141
Pius J, George L, Eapen S, Rao PS (1993) Enhanced plant regeneration in pearl millet (Pennisetum americanum) by ethylene inhibitors and Cefotaxime. Plant Cell Tissue Organ Cult 32:91–96
Robert ML, Flores MR, Loyola-Vargas VM (1989) Growth promoting activity of certain penicillins on cultivated cells of Bouvardia ternifolia. Phytochem 28:2659–2662
Singh RH, Singh RL, Seni PO (1979) Studies on the anti-anxiety effect of the medha rasayana drug brahmi (Bacopa monniera). Part II experimental studies. J Res Ind Med Yoga Homeopath 14:1–6
Sonia, Saini R, Singh RP, Jaiswal PK (2007) Agrobacterium tumefaciens mediated transfer of Phaseolus vulgaris α-amylase inhibitor-1 gene into mungbean: Vigna radiata (L.) Wilczek using bar as selectable marker. Plant Cell Rep 26:187–198
Stachel SE, Messens E, Van Montagu M, Zambryski P (1985) Identification of signal molecules produced by wounded plant cells that activate T-DNA transfer in Agrobacterium tumefaciens. Nature 318:624–629
Tiwari V, Tiwari NK, Singh BD (1998) Shoot Regeneration and somatic embryogenesis from different explants of Brahmi [Bacopa monnieri (L.) Wettst.]. Plant Cell Rep 17:538–543
Tiwari V, Tiwari NK, Singh DB (2001) Comparative studies of cytokinins on in vitro propagation of Bacopa monnieri. Plant Cell Tissue Organ Cult 66:9–16
Tiwari V, Tewari KN, Singh BD (2006) Shoot bud regeneration from different explants of Bacopa monniera (L.) Wettst. by trimethoprim and bavistin. Plant Cell Rep 25:629–635
Tripathi YB, Chaurasia S, Tripathi E, Upadhaya A, Dubey GP (1996) Bacopa Monniera Linn as an antioxidant: mechanism of action. Ind J Exp Biol 34:521–526
Volkov RA, Panchuk II, Schoffl F (2003) Heat-stress-dependency and developmental modulation of gene expression: the potential of house-keeping genes as internal standards in mRNA expression profiling using real-time RT-PCR. J Exp Bot 54:2343–2349
Wei XP, Gou XP, Yuan T, Russell SD (2006) A highly efficient in vitro plant regeneration system and Agrobacterium-mediated transformation in Plumbago zeylanica. Plant Cell Rep 25:513–521
Zuker A, Ahroni A, Tzfira T, Meir HB, Vainstein A (1999) Wounding by bombardment yields highly yields highly efficient Agrobacterium-mediated transformation of carnation (Dianthus caryophyllus L.). Mol Breeding 5:367–375
Acknowledgments
Authors are thankful to University Grant Commission (UGC), Govt. of India, New Delhi and Thapar University, Patiala for the financial assistance. Thanks are also due to TIFAC-CORE, Thapar University Patiala for the facilities to carry out this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aggarwal, D., Jaiswal, N., Kumar, A. et al. Factors affecting genetic transformation and shoot organogenesis of Bacopa monnieri (L.) Wettst. J. Plant Biochem. Biotechnol. 22, 382–391 (2013). https://doi.org/10.1007/s13562-012-0166-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13562-012-0166-6