Abstract
Eucomis species is a valuable plant with both medicinal and horticultural potential. The current study evaluated the role of plant growth regulator (PGR) on growth, phytochemicals, and antioxidant activity in Eucomis autumnalis subspecies autumnalis. Five cytokinins including topolins and benzyladenine (BA) at 2 µM in combination with varying (0–15 µM) concentrations of naphthalene acetic acid (NAA) were tested. In vitro regenerants were acclimatized in the greenhouse for 4 months. Highest number of shoots (9 shoots/explant) was observed with 15 µM NAA alone or when combined with BA. Acclimatized plants derived from the 15 µM NAA treatment had the highest number of roots, largest leaf area and biggest bulbs. While applied PGRs increased the iridoids and condensed tannins in the in vitro regenerants, total phenolics and flavonoids were higher in the PGR-free treatment. Among the in vitro regenerants, 5 µM NAA and 2 µM BA treatments produced the best antioxidant activity in the DPPH (55 %) and beta-carotene (88 %) test systems, respectively. A remarkable carry-over effect of the PGR was conspicuous in the phytochemical levels and antioxidant activity of the 4-month-old plants. In addition to the optimized micropropagation protocols, the current findings present a promising potential for manipulating the type and concentration of applied PGRs to improve phytochemical production and hence medicinal value in E. autumnalis subspecies autumnalis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The success of micropropagation endeavors is influenced by several intricate physical (e.g., light and temperature) and chemical factors. As a vital chemical component, plant growth regulators (PGRs) regulate various physiological and developmental processes during micropropagation (George et al. 2008). Even though a number of growth stimulating substances are used in micropropagation, cytokinins (CKs) and auxins (acting either individually or in combination) are the most important and popular PGRs (Gaspar et al. 1996; George et al. 2008). Many aspects of cell growth and differentiation as well as organogenesis in micropropagated plants are regulated by an interaction between exogenously applied CKs and auxins (Amoo and Van Staden 2013). Furthermore, interaction of exogenously applied CKs and auxins has been implicated in the upregulation of secondary metabolite content in plants (Dörnenburg and Knorr 1995; Ramachandra Rao and Ravishankar 2002).
There is a continuous effort aimed at identifying new compounds with the ability to stimulate better growth, and alleviate in vitro-induced physiological disorders (Tarkowská et al. 2003). The recent biotechnological advances in the field of phytohormones have significantly facilitated the search for new compounds (Strnad et al. 1997; Tarkowski et al. 2010). Thus, a new group of aromatic CKs commonly referred to as topolins has been identified (Strnad et al. 1997). Topolins have been demonstrated to enhance shoot proliferation, maintain histogenic stability, improve rooting efficiency and alleviate various physiological disorders in micropropagation (Aremu et al. 2012a). Although the positive role of topolins has been reported in a number of micropropagated species, their influence in micropropagated Eucomis autumnalis subspecies autumnalis remains unknown. Furthermore, the effect of combining topolin with auxins in micropropagation remains poorly documented (Aremu et al. 2012a). It is well known that the optimal environmental and chemical conditions for plant growth and development often vary among species and even genotypes. The benefits and need for further research especially to optimize the PGR concentrations for shoot proliferation in Eucomis species have been highlighted (Ault 1995; Taylor and Van Staden 2001). The increasing horticultural and pharmacological value of E. autumnalis subspecies autumnalis which is causing severe strain on wild populations has necessitated the need for more research to ensure conservation (Masondo et al. 2014). As highlighted by these authors, availability of efficient micropropagation protocol is pertinent to fully explore and sustain the economic potential of Eucomis species. Therefore, the current study evaluated the effect of five CKs (topolins forms in comparison to BA) individually and in combination with an auxin on growth, phytochemical content, and antioxidant potential in micropropagated E. autumnalis subspecies autumnalis. Apart from the differences in their response in vitro, both BA and topolins are known to induce contrasting physiological effect on regenerants upon acclimatization. Thus, the carry-over effect of the applied PGRs on acclimatization competence in in vitro-derived E. autumnalis subspecies autumnalis was evaluated.
Materials and methods
Source of plant growth regulators (PGRs)
Benzyladenine (BA) and α-naphthalene acetic acid (NAA) were purchased from Sigma–Aldrich (Steinheim, Germany). The four topolins used were mT (meta-topolin), mTTHP [meta-topolin tetrahydropyran-2-yl or 6-(3-hydroxybenzylamino)-9-tetrahydropyran-2-ylpurine], MemT [meta-methoxytopolin or 6-(3-methoxybenzylamino)purine], and MemTTHP [meta-methoxy 9- tetrahydropyran-2-yl topolin or 2-[6-(3-Methoxybenzylamino)-9-(tetrahydropyran-2-yl)purine]. Details of the preparation of the topolins have been described previously (Doležal et al. 2006, 2007; Szücová et al. 2009).
Explant source, decontamination regime and culture initiation
Explants were obtained from stock plants of E. autumnalis subspecies autumnalis from the University of KwaZulu-Natal (UKZN) Botanical Garden, Pietermaritzburg, South Africa. A voucher specimen (Masondo 2) was identified by Dr. C. Potgieter and deposited in the Bews Herbarium of the UKZN, Pietermaritzburg, South Africa.
Explants were excised from the stock plants and decontaminated as described by Taylor and Van Staden (2001). Decontaminated plant materials were inoculated in culture tubes (100 × 25 mm, 40 ml) containing 10 ml Murashige and Skoog (MS) medium (Murashige and Skoog 1962). The PGR-free medium was supplemented with 30 g/l of sucrose, 0.1 g/l myo-inositol and the pH adjusted to 5.8 with 1 M KOH or HCl as required. The medium was solidified with 3 g/l gelrite (Labretoria, Pretoria, South Africa), then autoclaved at 121 °C and 103 kPa for 20 min. The cultures were incubated in 16/8 h light/dark conditions with a photosynthetic photon flux (PPF) of 40–45 µmol m−2 s−1 at 25 ± 2 °C.
In vitro shoot proliferation using different cytokinins (CKs) and varying concentrations of α-naphthalene acetic acid (NAA)
The effect of five CKs (BA, mT, MemT, mTTHP, and MemTTHP) at three concentrations each (2, 4 and 6 µM) on in vitro shoot proliferation was evaluated (data not shown). Due to the absence of a significant increase in shoot proliferation with an increase in CK concentration, 2 µM CK was used for the current experiment. Using a completely randomized pattern, the experiment was conducted in a 6 × 5 factorial design involving six PGR treatments (CK-free, BA, mT, MemT, mTTHP, and MemTTHP) and five NAA concentrations (0, 2.5, 5, 10 and 15 µM). In a culture jar (110 × 60 mm, 300 ml volume) containing 30 ml MS medium, three sterile leaf explants (1 × 1 cm2) were inoculated with the different CKs and NAA. The control was devoid of any PGRs. Each treatment had 25 explants and the experiment was done twice. Cultures were grown under the same conditions as stated above. After 10 weeks in culture, growth parameters including shoot number, shoot length, root number, and root length were measured.
Acclimatization of in vitro-derived E. autumnalis subspecies autumnalis
For comparison purposes, regenerants from PGR-free, CK as well as the combination of CK with NAA at 2.5 and 15 µM were acclimatized. These regenerants were washed free of gelrite and transferred to 7.5 cm diameter pots containing sand:soil:vermiculite (1:1:1, v/v/v) mixture, treated with 1 % Benlate® (Du Pont de Nemour Int., South Africa). The regenerants had 2 weeks transition in the mist-house with a misting duration of 10 s at 15 min (80–90 % relative humidity), day/night temperature of 30/12 °C, and midday PPF of 30–90 µmol m−2 s−1 under natural photoperiod conditions. For a further 14 weeks, the regenerants were maintained in the greenhouse with a day/night temperature of approximately 30/15 °C, average PPF of 450 µmol m−2 s−1 and 30–40 % relative humidity under natural photoperiod conditions. After 4 months, growth parameters including acclimatization survival (%), leaf number, root number, root length, bulb diameter, and fresh weight were measured. The leaf area was determined using an L1-3100 area meter (Li-Cor Inc., Lincoln, Nebraska, USA).
Phytochemical evaluation of in vitro and greenhouse-acclimatized E. autumnalis subspecies autumnalis plants
Plant materials from the 10-week-old in vitro and 4-month-old acclimatized E. autumnalis subspecies autumnalis were harvested. In vitro regenerants were assayed as whole plants, while the greenhouse grown in vitro-derived plants was separated into aerial (leaves) and underground (bulbs and roots) parts. The plant materials were oven-dried at 50 ± 2 °C for 7 days and milled into powder form. Ground samples were extracted in 50 % methanol (MeOH) at 0.1 g per 10 ml in an ultrasonic sonicator (Julabo GmbH, West Germany) containing ice-cold water for 20 min. The mixture was separated using a Benchtop centrifuge (Hettich Universal, Tuttlingen, Germany) to obtain the supernatant required for the phytochemical quantification. Iridoid content was analyzed as detailed by Bairu et al. (2011) while condensed tannins, flavonoids, and phenolics were as described previously (Aremu et al. 2012b). Iridoid, condensed tannin, flavonoid, and phenolic content were expressed as mg harpagoside equivalents (HE), cyanidin chloride equivalents (CCE), catechin equivalents (CE), and gallic acid equivalents (GAE) per g dry weight (DW), respectively. For each experiment, six replicates were evaluated.
Antioxidant evaluation of in vitro and greenhouse-acclimatized E. autumnalis subspecies autumnalis plants
In vitro (whole plant) and greenhouse (aerial and underground parts) plant materials were oven-dried and extracted using 50 % MeOH. The dried extracts were resuspended in 50 % MeOH at 50 mg/ml for the 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay and 12.5 mg/ml for the beta-carotene/linoleic acid antioxidant model systems. The DPPH free-radical scavenging activity (RSA) of the extract was evaluated as described by Moyo et al. (2010). Ascorbic acid and MeOH were used as positive and negative controls, respectively. Beta-carotene/linoleic acid oxidation inhibitory activity was evaluated as described by Amarowicz et al. (2004) with modifications (Moyo et al. 2010). Butylated hydroxytoluene (BHT) and 50 % MeOH were used as positive and negative controls, respectively.
Data analysis
Experiments were conducted in completely randomized designs. The growth, phytochemical contents, and antioxidant activity data were subjected to analysis of variance (ANOVA) using SPSS software package for Windows (SPSS®, version 16.0 Chicago, USA). Where there was statistical significance (P ≤ 0.05), the mean values were further separated using Duncan’s multiple range test.
Results and discussion
Effect of PGRs on in vitro shoot proliferation and greenhouse growth
The absence of a significant effect on shoot proliferation with increasing concentrations of CK necessitated the need to evaluate the interaction of CKs with auxin (data not shown). The interaction between auxin and CK influences several aspects of cellular differentiation and organogenesis in tissue and organ cultures (Coenen and Lomax 1997; George et al. 2008). Among auxins, NAA is known to easily move across the cell membrane resulting into its rapid accumulation in plant cells (Nordström et al. 2004). Figure 1 depicts the effect of interaction of different CKs with five concentrations of NAA on shoot and root proliferation in E. autumnalis subspecies autumnalis. When compared with the use of MemT, mTTHP and MemTTHP alone, their combination with 5 µM NAA resulted in a higher number of shoots. Although Ault (1995) reported an increase in shoot production with the interaction of BA and NAA for E. autumnalis and E. zambesiaca, similar interaction had no significant effect on the number of shoots produced in E. autumnalis subspecies autumnalis. These contrasting effects of auxin and CK interaction on members of the genus Eucomis may be due to the uniqueness of each plant species and differences in the applied PGR concentrations as well as the endogenous hormone levels.
Effect of combining different cytokinins with naphthalene acetic acid concentrations on a shoot number, b shoot length, c root number and d root length in Eucomis autumnalis subspecies autumnalis after 10 weeks of culture. In each graph, bars represent mean values ± standard error (n = 25) and bars with different letter(s) are significantly different (P ≤ 0.05) based on Duncan’s multiple range test (DMRT). BA 6-Benzyladenine, mT meta-Topolin, mTTHP meta-Topolin tetrahydropyran-2-yl, MemT meta-Methoxytopolin, MemTTHP meta-Methoxytopolin tetrahydropyran-2-yl, NAA α-naphthalene acetic acid. The cytokinins were tested at 2 µM
Although auxins are primarily associated with rooting effects (Gaspar et al. 1996), treatments with NAA (5–15 µM) alone yielded significantly higher numbers of shoots than PGR-free treatments (Fig. 1a). The ability of NAA (alone) to stimulate shoot production in this species indicates the presence of substantial endogenous CK levels which ensured an optimum balance between auxin and CK. Similarly, Cheesman et al. (2010) reported a significant stimulatory effect of indole-3-butyric acid (IBA) and NAA on bulb production in E. zambesiaca. Thus, a detailed endogenous PGR profile of the species is necessary to fully explain these highlighted rare occurrences.
While the 5 µM NAA treatment produced the longest shoots, 2 µM MemTTHP regenerants were the shortest (Fig. 1b). The highest number of roots, approximately 17 roots/explant was obtained in the treatment containing 10 µM NAA alone or in combination with mT (Fig. 1c). From 2.5 to 10 µM NAA, an increase in root number was observed with CK-free, mT and mTTHP treatments. Similar enhanced rooting following application of topolins has been reported for several species (Aremu et al. 2012a) and ascribed to the increases in acropetal transport of CK resulting in less accumulation of non-active CK metabolites that could impede rooting (Podlešáková et al. 2012). On the other hand, combination of BA with NAA had no stimulatory effect on root number compared to the use of BA alone. In fact, BA with lower concentrations of NAA (2.5 and 5 µM) had a significant rooting inhibitory effect than treatment with BA alone. In a similar manner, increasing concentrations of NAA (particularly at 15 µM) had an inhibitory effect on the root length of the regenerants (Fig. 1d). This may be due to an overproduction or accumulation of the metabolic products resulting from the high concentration of the exogenously applied auxin (George et al. 2008).
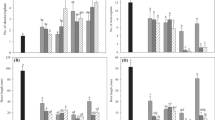
The overall success of micropropagation lies not only in the production of large numbers of in vitro plantlets but also on their survival in field conditions (Hazarika 2006; Pospíšilová et al. 2007). Often, tissue culture regenerants may manifest some structural and physiological changes which make them vulnerable to transplantation shock (Kozai et al. 1997; Amâncio et al. 1999). Even though several intricate factors determine the survival ability of in vitro regenerants, the ‘carry-over’ or ‘residual’ effect of exogenously applied PGRs has been recognized to be fundamental (Werbrouck et al. 1995; Valero-Aracama et al. 2010; Aremu et al. 2012c). Figure 2 represents the 4-month-old acclimatized E. autumnalis subspecies autumnalis derived from cultures containing 15 µM NAA with or without CK. In mT and CK-free treatments, there was an estimated 75–100 % acclimatization success regardless of the concentration of NAA applied (Fig. 3a). Photosynthetic competence which is directly related to the morphology of the leaf is among the crucial factors which affect ex vitro survival (Van Huylenbroeck et al. 2000; Hazarika 2006; Pospíšilová et al. 2007). In this study, the lowest and highest number of leaves was observed in 2.5 µM NAA with mTTHP and MemTTHP treatments, respectively (Fig. 3b). In terms of the leaf length and area (Fig. 3c, d), the most significant effect was obtained with the 15 µM NAA (CK-free) treatment. When compared with the treatment with BA only, the combination with NAA (15 µM) significantly increased the leaf length and area (Fig. 3). It was also evident that the addition of NAA especially at 15 µM improved the root growth in most cases (Fig. 4a, b).
Four-month-old acclimatized Eucomis autumnalis subspecies autumnalis derived from in vitro regenerants supplemented with 15 µM α-naphthalene acetic acid and different cytokinins at 2 µM. BA 6-Benzyladenine, mT meta-Topolin, mTTHP meta-Topolin tetrahydropyran-2-yl, MemT meta-Methoxytopolin, MemTTHP meta-Methoxytopolin tetrahydropyran-2-yl. Scale bar 20 mm
Effect of combining different cytokinins with naphthalene acetic acid concentrations on a frequency of acclimatization survival, b leaf number, c leaf length and d leaf area in 4-month-old acclimatized Eucomis autumnalis subspecies autumnalis. In each graph, bars represent mean values ± standard error (n = 15) and bars with different letter(s) are significantly different (P ≤ 0.05) based on Duncan’s multiple range test (DMRT). BA 6-Benzyladenine, mT meta-Topolin, mTTHP meta-Topolin tetrahydropyran-2-yl, MemT meta-Methoxytopolin, MemTTHP meta-Methoxytopolin tetrahydropyran-2-yl, NAA α-naphthalene acetic acid. All the cytokinins were tested at 2 µM
Effect of combining different cytokinins with naphthalene acetic acid concentrations on a root number, b root length, c bulb diameter and d fresh weight of 4-month-old greenhouse-acclimatized Eucomis autumnalis subspecies autumnalis. In each graph, bars represent mean values ± standard error (n = 15) and bars with different letter(s) are significantly different (P ≤ 0.05) based on Duncan’s multiple range test (DMRT). BA 6-Benzyladenine, mT meta-Topolin, mTTHP meta-Topolin tetrahydropyran-2-yl, MemT meta-Methoxytopolin, MemTTHP meta-Methoxytopolin tetrahydropyran-2-yl, NAA α-naphthalene acetic acid. All the cytokinins were tested at 2 µM
Furthermore, a similar stimulatory ‘carry-over’ effect of NAA was demonstrated in bulb diameter and fresh weight (Fig. 4c, d) whereby plants derived from a treatment containing 15 µM NAA alone had the biggest bulbs and highest fresh weights. While BA with NAA had no superior effect when compared with BA alone, mT and mTTHP regenerants in combination with NAA enhanced the root number and length. The presence and functionality of roots significantly contribute to survival of micropropagated plants (Hazarika 2006). While PGRs such as CK and ethylene are partly associated with rooting, auxins remain the primary signaling PGR (Malá et al. 2009). It was evident that NAA enhances rooting in vitro (Fig. 1), thus allowing for easier establishment and acclimatization upon transferal to the greenhouse, which inevitably explains the enhanced ex vitro growth of NAA-derived E. autumnalis subspecies autumnalis.
Although BA is the most commonly used CK for micropropagation of Eucomis species (Ault 1995; McCartan and Van Staden 1995; Taylor and Van Staden 2001), there is increasing evidence of its negative (carry-over) effects during acclimatization for several micropropagated species (Aremu et al. 2012a). At equimolar CK concentration without NAA, BA-derived plants were similar to topolins and CK-free treatments in most cases (Figs. 3, 4). An exception was the better survival (%) and longer leaf in mT, MemTTHP and CK-free plants when compared with BA treatment (Fig. 3a, c). Generally, the use of topolins had minimal acclimatization benefits when compared with BA treatment in this species. The observed reduced survival and growth (Figs. 3, 4) in MemT-treated plants when compared with CK-free plants suggests potential inhibitory effects of the applied CK on subsequent ex vitro growth and survival.
When compared with mT and mTTHP, application of BA with NAA was less effective for some of the growth parameters of E. autumnalis subspecies autumnalis. For instance, BA with 15 µM NAA treatment had lower survival, smaller leaves (length), reduced root number and smaller bulbs (Figs. 3, 4). However, not all the topolin interactions with NAA were superior to BA, as MemT and MemTTHP with 15 µM NAA treatments were mostly identical to the BA-derived plants. Based on the current findings, it appears as if exogenous application of NAA is more important than CKs (regardless of the types) during micropropagation and acclimatization of E. autumnalis subspecies autumnalis.
Effect of PGRs on phytochemical contents of in vitro regenerants and acclimatized E. autumnalis subspecies autumnalis plants
The importance of the quality and quantity of phytochemicals in micropropagated medicinal plant species has become well recognized globally (Dörnenburg and Knorr 1995). One of the factors known to influence phytochemical levels in plants is the type and concentration of exogenously supplied PGRs (Ramachandra Rao and Ravishankar 2002; Matkowski 2008). The effect of applied PGRs on the concentrations of secondary metabolites in the micropropagated E. autumnalis subspecies autumnalis is presented in Fig. 5. Regenerants derived from 5 µM NAA with mT had the highest (1.886 mg HE/g DW) iridoid content while all the other treatments were generally low (≤1 mg HE/g DW) (Fig. 5a). Although NAA alone had no remarkable influence on iridoid content, its combination (at 2.5 to 10 µM) with MemT significantly increased the level of iridoids in the regenerants. The highest condensed tannin concentration (0.435 mg CCE/g DW) was elicited with 2.5 µM NAA and mTTHP treatment (Fig. 5b). Shoots regenerated from CK (mT, mTTHP) alone or in combination with NAA (2.5, 5 µM) had a significantly increased condensed tannin content in comparison to PGR-free media. These findings suggest a possible synergetic interaction of NAA (2.5–5 µM) with CKs on accumulated iridoids and condensed tannins in regenerated E. autumnalis subspecies autumnalis. As demonstrated in the current study, the observed variations in phytochemical levels from different CK treatments and interaction with auxins have been reported by other researchers (Liu et al. 2007; Coste et al. 2011; Amoo and Van Staden 2013). These diverse effects may have resulted from inherent differences in the structure of the PGRs and how they influence the phytochemical biosynthetic pathways.
Effect of combining different cytokinins with naphthalene acetic acid concentrations on a iridoids, b condensed tannins, c flavonoids and d total phenolics in Eucomis autumnalis subspecies autumnalis after 10 weeks of culture. In each graph, bars represent mean values ± standard error (n = 6) and bars with different letter(s) are significantly different (P ≤ 0.05) based on Duncan’s multiple range test (DMRT). BA 6-Benzyladenine, mT meta-Topolin, mTTHP meta-Topolin tetrahydropyran-2-yl, MemT meta-Methoxytopolin, MemTTHP meta-Methoxytopolin tetrahydropyran-2-yl, NAA α-naphthalene acetic acid. The cytokinins were tested at 2 µM
Addition of NAA had low or no stimulatory effect on the level of flavonoids and total phenolics in the majority of the treatments (Fig. 5c, d). In both cases, PGR-free regenerants accumulated the highest level of flavonoids and total phenolics. These reductions in phytochemical (phenolics in this case) in the presence of PGRs have been documented in some micropropagated plants. For example, CK-free Tectona grandis and Aloe arborescens had a significantly higher concentration of phenolics when compared with BA-treated Tectona grandis (Quiala et al. 2012) and mTTHP- or benzyladenine riboside-treated Aloe arborescens (Amoo et al. 2014). As postulated by the authors, the presence of PGRs (especially at higher concentration) may have exerted inhibitory effects on phenolic biosynthetic pathways.
Although there is an ever increasing number of studies evaluating the role of in vitro culture systems on the production of phytochemicals, information pertaining to the possibility of changes in the chemical content/composition of micropropagated plants after acclimatization is scarce. Nevertheless, such studies allow for elucidation and manipulation of phytochemicals of interest especially at harvest stage (Liu et al. 2004; Nunes et al. 2009; Aremu et al. 2013). Secondary metabolites in the acclimatized E. autumnalis subspecies autumnalis were quantified and compared on the basis of aerial (leaves) and underground (bulbs and roots) parts (Fig. 6). It is noteworthy that the acclimatized plants had several fold more secondary metabolites (with exception to the condensed tannins) when compared with similar treatments from the in vitro regenerants (Figs. 5, 6). In a similar manner, Liu et al. (2004) observed a significantly higher flavonoid content in the tissues of mature greenhouse-grown Artemisia judaica than the in vitro regenerants.
Effect of combining different cytokinins with naphthalene acetic acid concentrations on a, b iridoids, c, d condensed tannins, e, f flavonoids and g, h total phenolics of 4-month-old greenhouse-acclimatized Eucomis autumnalis subspecies autumnalis. In each graph, bar represents mean values ± standard error (n = 6) and bars with different letter(s) are significantly different (P ≤ 0.05) based on Duncan’s multiple range test (DMRT). BA 6-Benzyladenine, mT meta-Topolin, mTTHP meta-Topolin tetrahydropyran-2-yl, MemT meta-Methoxytopolin, MemTTHP meta-Methoxytopolin tetrahydropyran-2-yl, NAA α-naphthalene acetic acid. *Not tested. All the cytokinins were tested at 2 µM
As hypothesized by some researchers (Ahmad et al. 2013; Aremu et al. 2013), age may have been the main contributing factor for these observations. Higher levels of iridoids were observed in the aerial parts compared to the underground parts, with the exception of the BA treatment having higher iridoid contents in the underground parts (Fig. 6a, b). From a conservation perspective, these findings are valuable as it implies that the aerial parts can serve as alternative sources of (bioactive) phytochemicals mainly sourced from the underground parts. Although mTTHP treatment had the highest level of condensed tannins, increasing concentrations of NAA significantly reduced the condensed tannins in the aerial parts (Fig. 6c). In the underground parts, the highest (0.374 mg CCE/g DW) condensed tannin content was produced in 2.5 µM NAA with MemTTHP treatment (Fig. 6d). In both plant parts evaluated, the highest level (c.a 6 mg CE/g DW) of flavonoids was observed in 2.5 µM NAA with mTTHP treatment (Fig. 6e, f). Apart from the 2.5 µM NAA with mT treatment with higher total phenolics in the underground parts, the aerial parts generally had higher or similar phenolic levels as compared to those quantified in the underground parts (Fig. 6g, h). As established in the current study, CK and auxin treatments have been reported to individually and interactively have a significant carry-over effect on phytochemical production in Aloe arborescens (Amoo et al. 2013) and Merwilla plumbea (Aremu et al. 2013).
Effect of PGRs on antioxidant potential of in vitro regenerants and acclimatized E. autumnalis subspecies autumnalis plants
The potential of in vitro plant culture systems for the production of an enormous variety of antioxidant compounds has been recognized (Matkowski 2008). Most in vitro antioxidant tests are easy, affordable and allows for high throughput screening, providing a motivation for the evaluation of antioxidant activity E. autumnalis subspecies autumnalis. Two test systems with different antioxidant mechanisms were used to accommodate for complexities involved in antioxidant processes (Huang et al. 2005). Using extracts from the in vitro regenerants, treatment with 5 µM NAA (DPPH assay) and BA (beta-carotene assays) treatments elicited the highest antioxidant activity (Table 1). Generally, the extracts demonstrated better antioxidant activity in the beta-carotene test system compared to the DPPH free-radical assay. For instance, plant extracts from 15 µM NAA as well as 2.5 or 15 µM NAA with MemTTHP had approximately fourfold higher antioxidant activity in the beta-carotene test system compared to the DPPH assay. Conversely, three of the treatments (2.5, 5 and 5 µM NAA with BA) had better antioxidant activity in DPPH compared to the beta-carotene assay. Based on the mechanisms of antioxidant test systems (Amarowicz et al. 2004; Huang et al. 2005), the current findings suggest that the antioxidant principles in in vitro regenerated E. autumnalis subspecies autumnalis are more favorable towards hydrogen atom transfer reactions (beta-carotene assay which involves inhibition of lipid peroxidation) than single electron transfer reactions (DPPH assay).
Cytokinin-derived (2 µM) regenerants without NAA had significantly higher antioxidant activity (beta-carotene assay) compared to the PGR-free treatment with exception to MemT (Table 1). Although NAA treatments (especially 2.5–10 µM) had similar antioxidant activity as the PGR-free, combination of NAA (10 and 15 µM) and topolins (mTTHP and MemT) significantly improved the antioxidant activity (beta-carotene assay) when compared with the use of the CK or NAA alone. Relative to other topolins (mT and MemTTHP), regenerants from BA and BA with NAA treatments exhibited higher antioxidant activity. As demonstrated in other studies (Hazarika and Chaturvedi 2013; Amoo et al. 2014), the current findings further emphasized the vital role of exogenously applied PGRs (types and concentration) on the resultant antioxidant potential of in vitro regenerants.
Despite the increase in number of recent studies (García-Pérez et al. 2012; Amoo et al. 2013; Aremu et al. 2013), the importance of better understanding the general physiology and series of events involved during and after micropropagation of valuable medicinal plants cannot be over-emphasized. Such information, especially the pharmacological activity of acclimatized plant, is vital from a conservation perspective. Table 2 shows the antioxidant activity of extracts from aerial and underground parts of the acclimatized E. autumnalis subspecies autumnalis. It is noteworthy that the antioxidant activity (mainly DPPH assay) elicited in the 4-month-old acclimatized material was higher (in the aerial part) when compared with similar treatments from in vitro regenerants. In contrast, García-Pérez et al. (2012) reported a 28 % increase in antioxidant activity of in vitro Poliomintha glabrescens when compared with the wild-type and acclimatized plants. It was shown that 5-month-old greenhouse-grown Artemisia judaica had a significantly higher antioxidant activity when compared with the 3-month-old in vitro regenerants (Liu et al. 2004). The type of CK and plant parts investigated significantly influenced the level of antioxidant activity in in vitro and acclimatized Merwilla plumbea (Aremu et al. 2013). In the current study, extracts from the aerial parts had better DPPH free-radical scavenging activity than the underground parts in all the treatments. Although PGR carry-over effects had no significant influence (when comparing any of the treatments with the control) on DPPH free-radical scavenging activity in aerial parts, 15 µM NAA with MemTTHP treatment had about 2.4-fold higher antioxidant activity than the control (PGR-free) in underground parts. In the beta-carotene test system, the highest antioxidant activity was observed with 2.5 µM NAA and 15 µM NAA with MemT treatments for the aerial and underground parts, respectively (Table 2).
Even though in vitro plants possess the possibility of producing standardized phytochemicals (with pharmacological properties), independent from environmental factors, the dynamics and accumulation of these compounds may be tilted under ex vitro conditions. According to Amoo et al. (2013), the type of PGR had a significant effect on antioxidant activity in tissue culture-derived Aloe arborescens after 2 months ex vitro growth. As a quality control measure, when compared with naturally grown Pelargonium sidoides, 1-year-old greenhouse (in vitro-derived regenerants) plants exhibited similar antioxidant activity (Moyo et al. 2013). Based on this evidence, it therefore follows that the acclimatized E. autumnalis subspecies autumnalis has the potential to exhibit similar pharmacological activities as the wild population.
Concluding remarks
The current findings provide an improved micropropagation protocol for E. autumnalis subspecies autumnalis with emphasis on the exogenously applied PGRs. Depending on the overall objectives, topolins can serve as suitable alternatives for conventional/commonly used BA for the species. However, evidence of the vital influence of NAA (either alone or in combination with CKs) on morphological growth and development during micropropagation and subsequent ex vitro acclimatization was established. The influence of the applied PGRs on secondary metabolites and antioxidant activity of E. autumnalis subspecies autumnalis was highlighted. In addition, when the in vitro regenerants were acclimatized, there was a gradual (several-fold higher) accumulation of quantified phytochemicals and antioxidant activity in the 4-month-old plants. Nevertheless, a detailed phytochemical profiling will be necessary to provide further insights on the identity of specific bioactive compounds in E. autumnalis subspecies autumnalis. Overall, the current findings highlight the need for an appropriate choice of PGR as it remains critical to enhance the micropropagation and conservation of E. autumnalis subspecies autumnalis.
Author contribution
N.A. Masondo conceived and was responsible for the experimental design and conducted the experiments. N.A. Masondo with the help of A.O. Aremu, J.F. Finnie and J. Van Staden drafted the manuscript and all the authors read and approved the final manuscript.
References
Ahmad N, Khan MI, Ahmed S, Javed SB, Faisal M, Anis M, Rehman S, Umair SM (2013) Change in total phenolic content and antibacterial activity in regenerants of Vitex negundo L. Acta Physiol Plant 35:791–800
Amâncio S, Rebordão J, Chaves M (1999) Improvement of acclimatization of micropropagated grapevine: photosynthetic competence and carbon allocation. Plant Cell Tissue Organ Cult 58:31–37
Amarowicz R, Pegg RB, Rahimi-Moghaddam P, Barl B, Weil JA (2004) Free-radical scavenging capacity and antioxidant activity of selected plant species from the Canadian prairies. Food Chem 84:551–562
Amoo SO, Van Staden J (2013) Influence of plant growth regulators on shoot proliferation and secondary metabolite production in micropropagated Huernia hystrix. Plant Cell Tissue Organ Cult 112:249–256
Amoo SO, Aremu AO, Van Staden J (2013) Shoot proliferation and rooting treatments influence secondary metabolite production and antioxidant activity in tissue culture-derived Aloe arborescens grown ex vitro. Plant Growth Regul 70:115–122
Amoo SO, Aremu AO, Moyo M, Szüčová L, Doležal K, Van Staden J (2014) Physiological effects of a novel aromatic cytokinin analogue in micropropagated Aloe arborescens and Harpagophytum procumbens. Plant Cell Tissue Organ Cult 116:17–26
Aremu AO, Bairu MW, Doležal K, Finnie JF, Van Staden J (2012a) Topolins: a panacea to plant tissue culture challenges? Plant Cell Tissue Organ Cult 108:1–16
Aremu AO, Bairu MW, Finnie JF, Van Staden J (2012b) Stimulatory role of smoke–water and karrikinolide on the photosynthetic pigment and phenolic contents of micropropagated ‘Williams’ bananas. Plant Growth Regul 67:271–279
Aremu AO, Bairu MW, Szüčová L, Doležal K, Finnie JF, Van Staden J (2012c) Assessment of the role of meta-topolins on in vitro produced phenolics and acclimatization competence of micropropagated ‘Williams’ banana. Acta Physiol Plant 34:2265–2273
Aremu AO, Gruz J, Šubrtová M, Szüčová L, Doležal K, Bairu MW, Finnie JF, Van Staden J (2013) Antioxidant and phenolic acid profiles of tissue cultured and acclimatized Merwilla plumbea plantlets in relation to the applied cytokinins. J Plant Physiol 170:1303–1308
Ault JR (1995) In vitro propagation of Eucomis autumnalis, E. comosa, and E. zambesiaca by twin-scaling. HortScience 30:1441–1442
Bairu MW, Amoo SO, Van Staden J (2011) Comparative phytochemical analysis of wild and in vitro-derived greenhouse-grown tubers, in vitro shoots and callus-like basal tissues of Harpagophytum procumbens. S Afr J Bot 77:479–484
Cheesman L, Finnie JF, Van Staden J (2010) Eucomis zambesiaca Baker: factors affecting in vitro bulblet induction. S Afr J Bot 76:543–549
Coenen C, Lomax TL (1997) Auxin-cytokinin interactions in higher plants: old problems and new tools. Trends Plant Sci 2:351–356
Coste A, Vlase L, Halmagyi A, Deliu C, Coldea G (2011) Effects of plant growth regulators and elicitors on production of secondary metabolites in shoot cultures of Hypericum hirsutum and Hypericum maculatum. Plant Cell Tissue Organ Cult 106:279–288
Doležal K, Popa I, Kryštof V, Spíchal L, Fojtíková M, Holub J, Lenobel R, Schmülling T, Strnad M (2006) Preparation and biological activity of 6-benzylaminopurine derivatives in plants and human cancer cells. Bioorg Med Chem 14:875–884
Doležal K, Popa I, Hauserová E, Spíchal L, Chakrabarty K, Novák O, Kryštof V, Voller J, Holub J, Strnad M (2007) Preparation, biological activity and endogenous occurrence of N6-benzyladenosines. Bioorg Med Chem 15:3737–3747
Dörnenburg H, Knorr D (1995) Strategies for the improvement of secondary metabolite production in plant cell cultures. Enzyme Microbial Technol 17:674–684
García-Pérez E, Gutiérrez-Uribe J, García-Lara S (2012) Luteolin content and antioxidant activity in micropropagated plants of Poliomintha glabrescens (Gray). Plant Cell Tissue Organ Cult 108:521–527
Gaspar T, Kevers C, Penel C, Greppin H, Reid D, Thorpe T (1996) Plant hormones and plant growth regulators in plant tissue culture. In Vitro Cell Dev Biol Plant 32:272–289
George EF, Hall MA, De Klerk G-J (2008) Plant growth regulators II: cytokinins, their analogues and antagonists. In: George EF, Hall MA, De Klerk G-J (eds) Plant propagation by tissue culture. Springer, Netherlands, pp 205–226
Hazarika BN (2006) Morpho-physiological disorders in in vitro culture of plants. Sci Hortic 108:105–120
Hazarika RR, Chaturvedi R (2013) Establishment of dedifferentiated callus of haploid origin from unfertilized ovaries of tea (Camellia sinensis (L.) O. Kuntze) as a potential source of total phenolics and antioxidant activity. In Vitro Cell Dev Biol Plant 49:60–69
Huang D, Ou B, Prior RL (2005) The chemistry behind antioxidant capacity assays. J Agr Food Chem 53:1841–1856
Kozai T, Kubota C, Ryoung Jeong B (1997) Environmental control for the large-scale production of plants through in vitro techniques. Plant Cell Tissue Organ Cult 51:49-56
Liu CZ, Murch SJ, El-Demerdash M, Saxena PK (2004) Artemisia judaica L.: micropropagation and antioxidant activity. J Biotechnol 110:63–71
Liu X-N, Zhang X-Q, Sun J-S (2007) Effects of cytokinins and elicitors on the production of hypericins and hyperforin metabolites in Hypericum sampsonii and Hypericum perforatum. Plant Growth Regul 53:207–214
Malá J, Máchová P, Cvrčková H, Karady M, Novák O, Mikulík J, Hauserová E, Greplová J, Strnad M, Doležal K (2009) Micropropagation of wild service tree (Sorbus torminalis [L.] Crantz): the regulative role of different aromatic cytokinins during organogenesis. J Plant Growth Regul 28:341–348
Masondo NA, Finnie JF, Van Staden J (2014) Pharmacological potential and conservation prospect of the genus Eucomis (Hyacinthaceae) endemic to southern Africa. J Ethnopharmacol 151:44–53
Matkowski A (2008) Plant in vitro culture for the production of antioxidants—a review. Biotechnol Adv 26:548–560
McCartan SA, Van Staden J (1995) In vitro propagation of the medicinal plant, Eucomis poleevansii N. E. Brown. J S Afr Soc Hortic Sci 5:73–75
Moyo M, Ndhlala AR, Finnie JF, Van Staden J (2010) Phenolic composition, antioxidant, and acetylcholinesterase inhibitory activities of Sclerocarya birrea and Harpephyllum caffrum (Anacardiaceae) extracts. Food Chem 123:69–76
Moyo M, Aremu AO, Gruz J, Šubrtová M, Szüčová L, Doležal K, Van Staden J (2013) Conservation strategy for Pelargonium sidoides DC: phenolic profile and pharmacological activity of acclimatized plants derived from tissue culture. J Ethnopharmacol 149:557–561
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Nordström A, Tarkowski P, Tarkowska D, Norbaek R, Ästot C, Dolezal K, Sandberd G (2004) Auxin regulation of cytokinin biosynthesis in Arabidopsis thaliana: a factor of potential importance for auxin-cytokinin-regulated development. Proc Natl Acad Sci USA 21:8039–8044
Nunes JDM, Pinhatti AV, Rosa LMG, von Poser GL, Rech SB (2009) Roles of in vitro plantlet age and growing period in the phenolic constituent yields of acclimatized Hypericum polyanthemum. Env Exp Bot 67:204–208
Podlešáková K, Zalabák D, Čudejková M, Plíhal O, Szϋčová L, Doležal K, Spíchal L, Strnad M, Galuszka P (2012) Novel cytokinin derivatives do not show negative effects on root growth and proliferation in submicromolar range. PLoS ONE 7:e39293. doi:10.1371/journal.pone.0039293
Pospíšilová J, Synková H, Haisel D, Semorádová S (2007) Acclimation of plantlets to ex vitro conditions: effects of air humidity, irradiance, CO2 concentration and abscisic acid (a review). Acta Hortic 748:29–38
Quiala E, Cañal M-J, Meijón M, Rodríguez R, Chávez M, Valledor L, de Feria M, Barbón R (2012) Morphological and physiological responses of proliferating shoots of teak to temporary immersion and BA treatments. Plant Cell Tissue Organ Cult 109:223–234
Ramachandra Rao S, Ravishankar GA (2002) Plant cell cultures: chemical factories of secondary metabolites. Biotechnol Adv 20:101–153
Strnad M, Hanus J, Vanek T, Kamínek M, Ballantine JA, Fussell B, Hanke DE (1997) Meta-topolin, a highly active aromatic cytokinin from poplar leaves (Populus × canadensis Moench., cv. Robusta). Phytochemistry 45:213–218
Szücová L, Spíchal L, Doležal K, Zatloukal M, Greplová J, Galuszka P, Krystof V, Voller J, Popa I, Massino FJ, Jørgensen J-E, Strnad M (2009) Synthesis, characterization and biological activity of ring-substituted 6-benzylamino-9-tetrahydropyran-2-yl and 9-tetrahydrofuran-2-ylpurine derivatives. Bioorg Med Chem 17:1938–1947
Tarkowská D, Doležal K, Tarkowski P, Åstot C, Holub J, Fuksová K, Schmülling T, Sandberg G, Strnad M (2003) Identification of new aromatic cytokinins in Arabidopsis thaliana and Populus × canadensis leaves by LC-(+)ESI-MS and capillary liquid chromatography/frit-fast atom bombardment mass spectrometry. Physiol Plant 117:579–590
Tarkowski P, Floková K, Václavíková K, Jaworek P, Raus M, Nordström A, Novák O, Doležal K, Šebela M, Frébortová J (2010) An improved in vivo deuterium labeling method for measuring the biosynthetic rate of cytokinins. Molecules 15:9214–9229
Taylor JLS, Van Staden J (2001) In vitro propagation of Eucomis L’Herit species—plants with medicinal and horticultural potential. Plant Growth Regul 34:317–329
Valero-Aracama C, Kane M, Wilson S, Philman N (2010) Substitution of benzyladenine with meta-topolin during shoot multiplication increases acclimatization of difficult- and easy-to-acclimatize sea oats (Uniola paniculata L.) genotypes. Plant Growth Regul 60:43–49
Van Huylenbroeck JM, Piqueras A, Debergh PC (2000) The evolution of photosynthetic capacity and the antioxidant enzymatic system during acclimatization of micropropagated Calathea plants. Plant Sci 155:59–66
Werbrouck SPO, Van der Jeugt B, Dewitte W, Prinsen E, Van Onckelen HA, Debergh PC (1995) The metabolism of benzyladenine in Spathiphyllum floribundum ‘Schott Petite’ in relation to acclimatisation problems. Plant Cell Rep 14:662–665
Acknowledgements
Financial support from the National Research Foundation (NAM), Claude Leon Foundation (AOA) and University of KwaZulu-Natal, South Africa is appreciated. Dr. K. Doležal and the team at Laboratory of Growth Regulators, Palacký University and Institute of Experimental Botany AS CR, Czech Republic for providing the topolins. Mrs. Alison Young (UKZN Botanical Garden) and Dr. C. Potgieter (Bews herbarium) assisted with the greenhouse experiments and plant identification, respectively. We are grateful to Drs. M. Moyo and S.O. Amoo for their valuable suggestions on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. J. Reigosa.
Rights and permissions
About this article
Cite this article
Masondo, N.A., Aremu, A.O., Finnie, J.F. et al. Plant growth regulator induced phytochemical and antioxidant variations in micropropagated and acclimatized Eucomis autumnalis subspecies autumnalis (Asparagaceae). Acta Physiol Plant 36, 2467–2479 (2014). https://doi.org/10.1007/s11738-014-1619-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-014-1619-4