Abstract
The effect of exogenous applied nitric oxide on photosynthesis under heat stress was investigated in rice seedlings. High temperature resulted in significant reductions of the net photosynthetic rate (P N) due to non-stomatal components. Application of nitric oxide donors, sodium nitroprusside (SNP) or S-nitrosoglutathione (GSNO), dramatically alleviated the decrease of P N induced by high temperature. Chlorophyll fluorescence measurement revealed that high temperature caused significant increase of the initial fluorescence (F o) and non-photochemical quenching (NPQ) whereas remarkable decrease of the maximal fluorescence (F m), the maximal efficiency of PSII photochemistry (F v/F m), the actual PSII efficiency (ΦPSII), and photochemical quenching (q p). In the presence of SNP or GSNO pretreatment, the increase of F o and decrease of F m, F v/F m, ΦPSII and q p were markedly mitigated, but NPQ was further elevated. Moreover, with SNP or GSNO pretreatment, H2O2 accumulation and electrolyte leakage induced by heat treatment were significantly reduced, whereas zeaxanthin content and carotenoid content relative to chlorophyll were elevated. The potassium salt of 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (cPTIO), a specific NO scavenger, arrested NO donors mediated effects. These results suggest that NO can effectively protect photosynthesis from damage induced by heat stress. The activation effect of NO on photosynthesis may be mediated by acting as ROS scavenging, or/and alleviating oxidative stress via maintaining higher carotenoid content relative to chlorophyll or/and enhancing thermal dissipation of excess energy through keeping higher level of zeaxanthin content under heat stress.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With increasing global warming, high temperature has been a major limitation to crop productivity (Mathur et al. 2011). High temperature induced accumulation of reactive oxygen species (ROS) in plants, including 1O2, H2O2, O −12 and ·OH, which led to lipid peroxidation, membrane injury, enzyme inactivation and consequent inhibition of the photosynthesis, respiration and plant growth (Wahid 2007). Photosynthesis is among the most thermolabile processes (Berry and Björkman 1980). High temperature was considered to impair photosynthesis by disturbing light energy capture, photosystem II- and photosystem I-mediated electron transfer, and Calvin cycle activity (Stasik and Jones 2007).
Photosystem II (PSII), which organizes the chlorophylls for light harvesting and harbors the electron transport cofactors needed for the oxidation of water, has long been considered the most heat-sensitive component of the photosynthetic apparatuses (Berry and Björkman 1980). Heat stress resulted in detachment of the light-harvesting complex, inactivity of reaction centers (RCs), loss of oxygen-evolving complex (OEC) function, and decreased probability of electron transport in PSII (Xue et al. 2011). Parameters of chlorophyll fluorescence have been frequently used as a rapid, non-destructive diagnostic method for detecting and quantifying damage to the leaf photosynthetic apparatus, particularly PSII activity, in response to environmental stress such as high temperature (Mathur et al. 2011), salt (Mehta et al. 2010) and osmotic stress (Singh-Tomar et al. 2012), which can provide information about changes taking place in the structure, conformation, and function of the photosynthetic apparatus, especially in PSII.
Nitric oxide (NO) is a highly reactive, membrane-permeant free radical that is a widespread intracellular and intercellular messenger with a broad spectrum of regulatory functions in many physiological processes in plants, including seed germination, maturation and senescence, stomatal movement (Neill et al. 2003). NO can act either as a cytotoxin or a cytoprotectant, which depends on its concentration and location (Siddiqui et al. 2011). NO injured membranes, proteins, and nucleic acids in plant cells and resulted in decrease of photosynthesis and respiration when applied at a relatively high dose (Siddiqui et al. 2011). NO also disturbed photosynthesis by slowing down electron transfer between QA and QB, and inhibiting charge recombination reactions of QA- with the S2 state of the water-oxidizing complex in PSII, steady-state photochemical, non-photochemical quenching (NPQ) processes (Wodala et al. 2008) and photophosphorylation (Takahashi and Yamasaki 2002). However, NO promotes normal growth and development of plants, and helps plants resist abiotic and biotic stresses such as drought, salt, heat, UV-B-radiation and disease infection at lower concentrations (Siddiqui et al. 2011).
The aim of this study was to evaluate the effect of NO on photosynthesis in rice seedlings under heat stress. Chlorophyll fluorescence measurements were used to investigate the changes of photosynthetic apparatuses in responses to NO application under heat stress. The pigment contents, H2O2 production, and ion leakage degree were determined to illustrate the mechanisms involved in the NO action on rice seedlings under high temperature.
Materials and methods
Plant material and heat and chemical treatment
Sterilized rice seeds of Zhong you No.9801 (Oryza sativa L.) were germinated on moist paper towels and planted in plastic pots containing a sterile mixture of soil: vermiculite (3:1, v/v). Plants were grown under a photoperiod of 16 h at 25 °C, and a dark period of 8 h at 20 °C. Irradiance was 350 μmol m−2 s−1 and relative humidity was 60 %. Sodium nitroprusside (SNP) and S-nitrosoglutathione (GSNO) were used as NO donors. 2-(4-Carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (cPTIO) was used as NO scavenger. Plants at the four leaves stage were sprayed with 10 μM SNP, 10 μM GSNO, 100 μM potassium ferricyanide (Fe(III)CN), and 200 μM cPTIO (sprayed together with SNP or GSNO) in the morning and evening over 3 days and then subjected to 40, 45 and 50 °C, separately, for 4 h in the dark. The distilled water sprayed plants without heat stress were referred as control (CK).
Photosynthetic analysis
Net photosynthetic rate (P n), transpiration rate (T r), stomatal conductance (g s), and intercellular CO2 concentration (C i) of the second fully expanded leaves (from up to down) were determined with a LI-COR 6400 portable photosynthesis system (LI-COR, Lincoln, NE, USA).
Activation state of Rubisco determination
Activation state of Rubisco was determined according to Haldimann and Feller (2004). Frozen leaf disks were rapidly (within 30 s) extracted using homogenizer in a buffer of 100 mM Tricine, pH 8.0, 5 mM MgCl2, 0.1 mM ethylenediaminetetraacetic acid, 5 mM dithiothreitol, 1 % (w/v) polyvinylpyrrolidone, 1 % (w/v) casein, 0.05 % (v/v) Triton X-100, 1 mM phenyl methyl sulphonyl fluoride, and 20 mM leupeptin. An aliquot (30 μL) of the resuspended extract was assayed at 30 °C either immediately, to determine initial Rubisco activity, or following a 10-min incubation period at 30 °C in an assay medium containing 10 mM NaHCO3 and Mg2+, but lacking ribulose-1,5-bisphosphate to determine the activity of fully carbamylated Rubisco (total Rubisco activity). Initial and total Rubisco assays were carried out following the methods of Salvucci and Anderson (1987), with the exception that Triton X-100 and casein were not included in the assay medium. Assays were terminated after 30 s and incorporation of 14CO2 into acid-stable products was determined essentially as described by Salvucci and Anderson (1987). The activation state of Rubisco (Perchorowicz et al. 1981) was calculated as the relative ratio of initial to total Rubisco activities.
Chlorophyll fluorescence
Chlorophyll fluorescence measurement was taken on the same leaves used for the photosynthetic analysis by a pulse-amplitude modulated chlorophyll fluorometer (PAM-2500, Walz, Effeltrich, Germany) at 30 °C. Leaves were dark-adapted for 30 min. The minimal fluorescence level in the dark-adapted state (F 0) was measured using the measuring light which is sufficiently low (0.8 μmol m−2 s−1) so as not to induce notable variable fluorescence. Far-red light (5 μmol m−2 s−1) was adopted to oxidize the PSII fully before measurement of the minimal fluorescence during illumination (F′0). Both the maximal fluorescence levels in the dark (F m) and under illumination (F′ m ) were obtained by a saturating pulse (8,000 μmol m−2 s−1). The steady-state fluorescence (F s) was recorded after actinic light illumination for approximately 3 min. The actual PSII efficiency (ΦPSII) was calculated from ΦPSII = (F′ m−F s)/F′ m (Genty et al. 1989). The maximum photochemical efficiency of PSII was determined from the ratio of variable (F v) to maximum (F m) fluorescence [F v /F m = (F m−F o)/F m] (Kitajima and Butler 1975). The photochemical fluorescence quenching efficiency (q p) was calculated from q p = (F′ m −F s)/(F′ m−F′ 0) (van Kooten and Snel 1990). NPQ was calculated from NPQ = F m/F′ m−1 (Bilger and Björkman 1990). All the above measurements were performed in a dark room with stable ambient conditions.
Determination of pigment content
The procedure was carried out at 4 °C and dark. A leaf sample (0.25 g) was mashed in a mortar and pestle with 80 % acetone (v/v), the extract was filtered through two layers of nylon and centrifuged in sealed tubes at 15,000 g for 5 min. The supernatant was collected and read at 663 and 647 nm for chlorophyll a and chlorophyll b, respectively, and at 470 nm for carotenoid content. The concentrations for chlorophyll a, chlorophyll b, and the sum of leaf carotenoids (xanthophylls and carotenes) were calculated according to the equations of Lichtenthaler and Buschmann (2001):
Zeaxanthin was analysed by the HPLC method as described by Rivas et al. (1989). Leaf disks frozen in liquid nitrogen were grounded in a mortar with acetone in the presence of sodium ascorbate. The extract was kept in the darkness at −80 °C until analysis. Chromatography was carried out on a 100 × 8 mm Waters Novapak C18 radial compression column (4-μm particle size). Samples were injected with a Rheodyne 7010 injector with a 20-μL loop, and mobile phases were pumped by a Waters M45 high-pressure pump at a flow of 2 mL/min. Peaks were detected at 450 nm by a Shimadzu UV–VIS detector and integrated with a Shimadzu CR3 A integrator. The column was equilibrated prior to injecting each sample by flushing with acetonitrile:methanol (7:1, v/v, mobile phase A) for 7 min. The sample was injected into the column and mobile phase A was pumped for another 2 min. A mixture of acetonitrile:methanol:water:ethyl acetate (7:0.96:0.04:2, by vol; mobile phase B) was then pumped for 1 min to achieve the resolution of lutein and zeaxanthin. Finally, acetonitrile:methanol:water:ethyl acetate (7:0.96:0.04:8, by vol; mobile phase C) was pumped until β-carotene was eluted (about 7 min). Typical working pressures with solvent flows of 2 mL/min were around 300 psi. Zeaxanthin was quantified using external calibration method.
H2O2 production
H2O2 contents were determined by the peroxidase-coupled assay according to Veljovic-Jovanovic et al. (2002). About 0.5 g rice seedling leaves were ground in liquid nitrogen, and the powder was extracted in 2 mL 1 M HClO4 in the presence of insoluble PVP (5 %). The homogenate was centrifuged at 12,000×g for 10 min and the supernatant was neutralized with 5 MK2CO3 to pH 5.6 in the presence of 50 μL 0.3 M phosphate buffer (pH 5.6). The solution was centrifuged at 12,000×g for 1 min and the sample was incubated for 10 min with 1 unit ascorbate oxidase (Sigma, St. Louis, USA) to oxidize ascorbate prior to assay. The reaction mixture consisted of 0.1 M phosphate buffer (pH 6.5), 3.3 mM dimethylamine borane (DMAB, Sigma, St. Louis, USA), 0.07 mM 3-Methyl-2-benzothiazolinonehydrazone hydrochloride hydrate (MBTH, Sigma, St. Louis, USA) and 0.3 U POX (Sigma, St. Louis, USA). The reaction was initiated by addition of sample. The absorbance change at 590 nm was monitored at 25 °C.
Relative ion leakage measurement
Relative ion leakage was determined according to Song et al. (2006). The rice leaves (0.2 g) were placed in Petri dishes with 10 mL of deionized water at 25 °C for 2 h. After the incubation, the conductivity in the bathing solution was determined (C1). Then, the samples were boiled for 15 min, and conductivity was read again in the bathing solution (C2). Relative ion leakage was expressed as a percentage of the total conductivity after boiling [Relative ion leakage (%) = C1/C2 × 100].
Statistical analysis
Each experiment was repeated at least three times. Statistical analysis was performed using ANOVA test.
Results
Effect of NO on net photosynthesis rate under heat stress
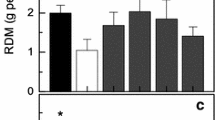
The net photosynthesis rate (P N) declined to 88.5, 76.5, and 58.3 % of the control after exposure to 40, 45, and 50 °C for 4 h, respectively. Pretreatment with SNP significantly alleviated the decrease of P N induced by high temperature. In addition, potassium ferricyanide (Fe(III)CN), which is a residual product of SNP (Oh and Mccaslin 1995), had little effect on P N under heat stress. Another NO donor, S-nitrosoglutathione (GSNO), was also able to evidently mitigate the decrease of P N under heat stress (Fig. 1A). In order to clarify the effect of NO, cPTIO (a specific NO scavenger) was used, which has no effect on rice seedlings under control condition or high temperature (data not shown). cPTIO completely blocked the effect of the NO donors on P N, which recovered to the level of heat treatment alone (Fig. 1A).
Effect of NO on net CO2 assimilation rate (P N) (A) and activation state of Rubisco (B) in rice leaves under heat stress. Plants at the four leaves stage were sprayed with 10 μM SNP, 10 μM GSNO, 100 μM potassium ferricyanide (Fe(III)CN), and 200 μM cPTIO (sprayed together with SNP or GSNO) in the morning and evening over 3 days and then subjected to 40, 45, and 50 °C, separately, for 4 h in the dark. These different treatments were CK (control), H (heat stress), H+S (heat stress+SNP), H+S+P (heat stress+SNP+cPTIO), H+G (heat stress+GSNO), H+G+P (heat stress+GSNO+cPTIO), H+F [heat stress+Fe(III)CN]. Mean values and SE were calculated from three independent experiments
Effect of NO on activation state of Rubisco under heat stress
High temperature resulted in a significant reduction of the Rubisco activation state, which decreased from about 86 % at 25 °C to 71 % at 40 °C, 52 % at 45 °C, and 39 % at 50 °C. In the presence of SNP or GSNO pretreatment, the Rubisco activation state recovered evidently. With cPTIO in combination with SNP or GSNO treatments, the Rubisco activation state kept at the level of heat treatment alone, indicating that the effect of SNP or GSNO was eliminated by cPTIO. Potassium ferricyanide (Fe(III)CN) had no influence on activation state of Rubisco under heat stress (Fig. 1B).
Effect of NO on intercellular CO2 concentration (C i) under heat stress
Heat treatment caused significant increase (13.2, 23.2, and 40.2 % higher than the control under 40, 45, and 50 °C, respectively) in intercellular CO2 concentration. Treatment with SNP or GSNO significantly prevented the increase of intercellular CO2 concentration induced by high temperature. cPTIO blocked the effect of SNP or GSNO treatments on intercellular CO2 concentration. Potassium ferricyanide (Fe(III)CN) had little effect on intercellular CO2 concentration under heat stress (Fig. 2A).
Effect of NO on intercellular CO2 concentration (C i) (A) stomatal conductance (g s) (B) and transpiration rate (T r) (C) in rice leaves under heat stress. Plants at the four leaves stage were sprayed with 10 μM SNP, 10 μM GSNO, 100 μM potassium ferricyanide (Fe(III)CN), and 200 μM cPTIO (sprayed together with SNP or GSNO) in the morning and evening over 3 days and then subjected to 40, 45 and 50 °C, separately, for 4 h in the dark. These different treatments were CK (control), H (heat stress), H+S (heat stress+SNP), H+S+P (heat stress+SNP+cPTIO), H+G (heat stress+GSNO), H+G+P (heat stress+GSNO+cPTIO), H+F [heat stress+Fe(III)CN]. Mean values and SE were calculated from three independent experiments
Effect of NO on stomatal conductance (g s) under heat stress
As shown in Fig. 2B, the stomatal conductance increased by 8.6, 17.6, and 24.5 %, after exposure to 40, 45, and 50 °C for 4 h, respectively. Pretreatment with SNP or GSNO obviously inhibited the increase of stomatal conductance. Potassium ferricyanide (Fe(III)CN) exercised no influence on P N under heat stress. In the presence of cPTIO in combination with SNP or GSNO, the stomatal conductances were close to the level of heat treatment alone.
Effect of NO on transpiration rate (T r) under heat stress
The transpiration rate increased to 191, 264, and 344 % of the control after exposure to 40, 45, and 50 °C for 4 h, respectively. Pretreatment with SNP or GSNO evidently prevented the increase of transpiration rate in rice leaves under heat stress, whereas potassium ferricyanide (Fe(III)CN) had no impact. cPTIO blocked the effect of SNP and GSNO on the transpiration rate under heat stress (Fig. 2C).
Effect of NO on PSII photochemical activities under heat stress
As shown in Fig. 3A, the initial fluorescence (F o) increased by 11.5, 26.9, and 38.4 % under 40, 45, and 50 °C, respectively, which were alleviated significantly by SNP or GSNO treatment. The maximal fluorescence (F m) was shown to be decreased by 14.7, 22.1, and 32.7 % under 40, 45, and 50 °C, respectively. SNP or GSNO pretreatment remarkably alleviated the decrease of F m (Fig. 3B). In addition, the maximal efficiency of PSII photochemistry (F v/F m) decreased by 9.4, 21.1, and 27 % under 40, 45, and 50 °C, respectively, while employment of SNP or GSNO evidently lightened the decline of F v/F m (Fig. 3C). Photochemical quenching (q p) decreased by 16, 30, and 45 %, whereas NPQ increased by 3.8, 9.1 and 10.3 % under 40, 45 and 50 °C, respectively. SNP or GSNO pretreatment significantly alleviated the decline of photochemical quenching (q p) (Fig. 4A) and increase of NPQ induced by high temperature (Fig. 4B). The actual PSII efficiency (ΦPSII) decreased greatly to 87, 73.8, and 60 % of the control under 40, 45, and 50 °C, respectively, which were obviously reversed by SNP or GSNO pretreatment (Fig. 4C). However, in the presence of cPTIO in combination with SNP or GSNO, F o, F m, F v/F m, ΦPSII, q p and NPQ resumed to the same level as that under heat stress alone, implying that cPTIO pretreatment blocked the action of SNP on F o, F m, F v/F m, ΦPSII, q p and NPQ. Potassium ferricyanide (Fe(III)CN) made no difference on F o, F m, F v/F m, ΦPSII, q p and NPQ under heat stress (Fig. 4).
Effect of NO on minimal fluorescence level (F 0) (A), maximal fluorescence (F m) (B) and maximum photochemical efficiency of PSII (F v/F m) (C) in rice leaves under heat stress. Plants at the four leaves stage were sprayed with 10 μM SNP, 10 μM GSNO, 100 μM potassium ferricyanide (Fe(III)CN), and 200 μM cPTIO (sprayed together with SNP or GSNO) in the morning and evening over 3 days and then subjected to 40, 45, and 50 °C, separately, for 4 h in the dark. These different treatments were CK (control), H (heat stress), H+S (heat stress+SNP), H+S+P (heat stress+SNP+cPTIO), H+G (heat stress+GSNO), H+G+P (heat stress+GSNO+cPTIO), H+F [heat stress+Fe(III)CN]. Mean values and SE were calculated from three independent experiments
Effect of NO on photochemical fluorescence quenching efficiency (q p) (A), non-photochemical quenching (NPQ) (B) and actual PSII efficiency (ΦPSII) (C) in rice leaves under heat stress. Plants at the four leaves stage were sprayed with 10 μM SNP, 10 μM GSNO, 100 μM potassium ferricyanide (Fe(III)CN) and 200 μM cPTIO (sprayed together with SNP or GSNO) in the morning and evening over 3 days and then subjected to 40, 45 and 50 °C, separately, for 4 h in the dark. These different treatments were CK (control), H (heat stress), H+S (heat stress+SNP), H+S+P (heat stress+SNP+cPTIO), H+G (heat stress+GSNO), H+G+P (heat stress+GSNO+cPTIO), H+F [heat stress+Fe(III)CN]. Mean values and SE were calculated from three independent experiments
Effects of NO on pigment contents under heat stress
Zeaxanthin content decreased evidently under heat stress, whereas recovered significantly in the presence of SNP or GSNO. With cPTIO in combination with SNP or GSNO treatment, the zeaxanthin content kept at the same level as that under heat treatment alone, demonstrating that cPTIO counteracted the effect of SNP or GSNO (Fig. 5A). Heat stress resulted in remarkable increase of the ratio of chlorophyll to carotenoid, which was alleviated evidently by SNP or GSNO pretreatment. There was no pronounced difference in the ratio of chlorophyll to carotenoid between heat treatment alone and SNP or GSNO plus cPTIO treatment under heat stress, suggesting that cPTIO arrested the effect of SNP or GSNO on the ratio of chlorophyll to carotenoid (Fig. 5B). Potassium ferricyanide (Fe(III)CN) had no effect on chlorophyll/carotenoid ratio and zeaxanthin content under heat stress (Fig. 5).
Effect of NO on zeaxanthin content (A) and chlorophyll/carotenoid ratio (B) in rice leaves under heat stress. Plants at the four leaves stage were sprayed with 10 μM SNP, 10 μM GSNO, 100 μM potassium ferricyanide (Fe(III)CN), and 200 μM cPTIO (sprayed together with SNP or GSNO) in the morning and evening over 3 days and then subjected to 40, 45, and 50 °C, separately, for 4 h in the dark. These different treatments were CK (control), H (heat stress), H+S (heat stress+SNP), H+S+P (heat stress+SNP+cPTIO), H+G (heat stress+GSNO), H+G+P (heat stress+GSNO+cPTIO), H+F [heat stress+Fe(III)CN]. Mean values and SE were calculated from three independent experiments
Effects of NO on H2O2 content under heat stress
H2O2 content increased by 26.7, 49.2, and 63.7 % under 40, 45, and 50 °C, respectively. In the presence of SNP or GSNO, H2O2 accumulation was obviously reduced compared with that under heat stress alone. With cPTIO in combination with SNP or GSNO pretreatment, the H2O2 accumulation was similar to that under heat stress alone. Potassium ferricyanide (Fe(III)CN) had no effect on H2O2 content under heat stress (Fig. 6A).
Effect of NO on H2O2 content (A) and relative ion leakage (B) in rice leaves under heat stress. Plants at the four leaves stage were sprayed with 10 μM SNP, 10 μM GSNO, 100 μM potassium ferricyanide (Fe(III)CN) and 200 μM cPTIO (sprayed together with SNP or GSNO) in the morning and evening over 3 days and then subjected to 40, 45, and 50 °C, separately, for 4 h in the dark. These different treatments were CK (control), H (heat stress), H+S (heat stress+SNP), H+S+P (heat stress+SNP+cPTIO), H+G (heat stress+GSNO), H+G+P (heat stress+GSNO+cPTIO), H+F [heat stress+Fe(III)CN]. Mean values and SE were calculated from three independent experiments
Effects of NO on relative electrolyte leakage under heat stress
High temperature resulted in cellular membrane injury and electrolyte leakage. Under heat treatment, the electrolyte leakage of rice leaves increased by 63, 112, and 151 % under 40, 45, and 50 °C, respectively, which were markedly alleviated by SNP or GSNO pretreatment. In the presence of cPTIO in combination with SNP or GSNO, the electrolyte leakage maintained at the same level as that under heat treatment alone. Potassium ferricyanide (Fe(III)CN) had no influence on electrolyte leakage under heat stress (Fig. 6B).
Discussion
Photosynthesis is one of the most heat-sensitive processes in plants (Camejo et al. 2005). In our work, high temperature resulted in significant decrease of P N. It is well established that, changes in the net photosynthesis rate reflect alterations in stomatal conductance, carboxylation efficiency and/or PSII activity (Efeoglu and Terzioglu 2009). In our case, stomatal conductance was observed to increase in stressed rice leaves. Meanwhile, leaf transpiration and internal CO2 concentration also rose evidently (Fig. 2), suggesting that the decrease of P N observed in the heat-treated rice leaves was not attributable to stomatal limitation, but to alterations in activity of Rubisco and/or PSII (Camejo et al. 2005).
Our results showed that the activation state of Rubisco decreases evidently under heat treatment (Fig. 1B), indicating that the inhibition of photosynthesis was related to the decline of Rubisco activity. The heat labile character of Rubisco activation has been reported to be due to the thermal sensitivity of activase (Crafts-Brandner and Salvucci 2002). Activase, which is responsible for maintaining Rubisco in its fully activated state, was reported to be one of the most heat-sensitive components of the photosynthetic apparatuses and was shown to aggregate, or redistribute from the soluble to the insoluble fraction of extracts (Salvucci et al. 2001) or alterate in quaternary structure from the more active associated state to the less active under heat stress (Crafts-Brandner et al. 1997). It is possible that the imbalance between accelerated deactivation of Rubisco at high temperature and reactivation by activase under heat stress resulted in heat sensitivity of Rubisco and decrease of photosynthetic rates (Salvucci and Crafts-Brandner 2004).
The effects of heat stress on the photosynthetic apparatus of rice, especially PSΠ, were evidenced through analysis of chlorophyll fluorescence. F o parameter reflects the state of the antenna chlorophyll and is a measure for the initial distribution of energy to PSII and the effectiveness of excitation capture in PSII. The results described here showed that F o increase significantly under heat stress, indicating irreversible damage in PSII associated with dissociation of light-harvesting complex, blocking of electron transport on the reductant side of PSII (Costa et al. 2002), and/or reduced energy transport effectiveness from antenna chlorophyll a to the reaction center of PSII (Bartošková et al. 1999), and/or the inhibition of Dl-protein of the PSII reaction center (Rintamaki et al. 1994). F v/F m indicates the maximal efficiency of PSII photochemistry and ΦPS2 means the actual efficiency of PSII photochemistry. This work showed that heat treatment resulted in significant decrease of F v/F m and ΦPS2 in rice, which might be related to the damage of D1 under high temperature (Asada et al. 1998).
In order to clarify the effect of NO on photosynthesis in rice seedlings, NO donors, SNP or GSNO, were applied exogenously. Results showed that SNP or GSNO application effectively prevented the decrease of activation state of Rubisco and P N induced by high temperature. Moreover, in the presence of SNP or GSNO, the increase of F o and the decrease of F v/F m and ΦPS2 induced by high temperature were significantly alleviated. To confirm the role of NO on photosynthesis, we used NO scavenger cPTIO in the experiment. The results showed that the protective effect of SNP and GSNO on photosynthesis was annihilated by the NO scavenger cPTIO, suggesting that exogenous NO indeed could protect photosynthesis from heat stress. Chloroplast proteins, including key enzymes of the Calvin-Benson cycle, such as glutamine synthase (Gln synthase), NADPH-dependent glyceraldehyde-3-phosphate dehydrogenase (GAPDH), Rubisco, and Rubisco activase, PSII reaction center proteins D1 and D2, as well as proteins of the energy transduction system in chloroplast thylakoids have been reported to be targets for NO (Lindermayr et al. 2005). NO might act as activator or inhibitor of enzymes, ion channels, or transcription factors by reacting with sulfhydryl groups and transition metals (Stamler 1994; Lindermayr et al. 2005). The effect of NO on photosynthesis under heat stress might be mediated by regulating activities of Rubisco, Rubisco activase and PSII through S-nitrosylation of cysteine (cys) residues.
As shown in Fig. 4A, photochemical quenching (q p) decreased much more under high temperature, which indicated a significant increase in the proportion of closed PSII reaction centers or the proportion of the reduced state of QA (Genty et al.1989). An increase in the fraction of QA in the reduced state suggested an increase in the excitation pressure on PSII under the steady state of photosynthesis, which would result in damage to PSII if not dissipated safely (Öquist and Huner 1993). NPQ is closely associated with the triggering of excess energy dissipation by non-radiative processes, which gives some protection to the photosynthetic apparatus (Yang et al. 2011). Our work showed that NPQ increased slightly under heat stress but in the presence of SNP or GSNO, NPQ further rose, indicating promotion effect of NO on excess energy dissipation. The results conflicted with that from heat stressed chrysanthemum, in which NPQ decreased in the presence of NO. Likewise, Hossain et al. (2011) reported that NO was involved in the decline of NPQ which is pronounced under heat stress conditions. Different treatment concentration of NO might explain the contradiction. Study on pea leaves indicated that NO, in a nanomolar concentration range, can assist to avoid the potential stress by inducing heat dissipation in the PS II antenna. In contrast, at higher concentrations, NO serves as a photosynthetic inhibitor (Wodala et al. 2005).
Xanthophylls have been proved to be involved in the NPQ of excess light energy in the antenna of PSII (Jahns and Holzwarth 2012). Especially in land plants, NPQ was strongly dependent on the xanthophyll zeaxanthin (Jahns and Holzwarth 2012). Previous work demonstrated that the zeaxanthin content exhibited a correlation with the activity of energy dissipation process while DTT, a known inhibitor for NPQ, treatment completely inhibited zeaxanthin formation as well as a large portion of non-photochemical chlorophyll fluorescence quenching (Demmig-Adams 1990). Zeaxanthin might act as a direct acceptor of energy from excited chlorophyll a or interact and deactivate ROS or change conformation of light-harvesting complexes, and thus results in an enhanced thermal dissipation of excess energy (Štepigová et al. 2007). In this experiment, high temperature resulted in remarkably decrease of zeaxanthin content, which was alleviated significantly by SNP or GSNO application. Moreover, the effect of SNP or GSNO on zeaxanthin content and NPQ was reversed by cPTIO, which verified the effect of NO on energy dissipation of photosynthesis apparatus under high temperature. Thus, it is highly possible that the activation effect of NO on photosynthesis may be mediated by enhanced NPQ resulting from increased level of zeaxanthin content under heat stress.
High temperature is known to generate oxidative stress through the ROS formation. Overproduction of ROS, such as superoxide radicals, hydroxyl radicals, and hydrogen peroxide, inevitably causes lipid peroxidation and consequently membrane injury, enzyme inactivation. (Wahid 2007). Components of the thylakoid membranes are particularly sensitive to heat stress (Haldimann and Feller 2004. Increase in the permeability of the thylakoid membranes induced by high temperature has been reported to be related to photosynthetic activity reduction in plant cells (Bukhov et al. 1999). In our work, H2O2 level and ion leakage increased significantly under high temperature, whereas P N decreased evidently. In the presence of SNP or GSNO, H2O2 content and ion leakage were significantly lower, while P N was remarkably higher than those under heat stress only. cPTIO application removed the effect of SNP or GSNO on H2O2 content and ion leakage, suggesting alleviated membrane damage and photosynthesis inactivation by NO under high temperature. Carotenoids are known to play a crucial role in deactivating triplet chlorophyll (3Chl*) and singlet oxygen (1O2*) (Jahns and Holzwarth 2012). In this work, the ratio of chlorophyll to carotenoid was observed to increase in rice leaves under heat stress. SNP or GSNO pretreatment significantly counteracted the influence of high temperature on the ratio of chlorophyll to carotenoid (Fig. 5B), but the effect was abolished by cPTIO. It is possible that the protection role of NO in photosynthesis was mediated by increased carotenoid content relative to chlorophyll and the enhanced ROS scavenging ability.
In conclusion, Rubisco inactivation, photochemical activity decrease, and thylakoid membranes damage are associated with photosynthesis inhibition under high temperature. SNP or GSNO pretreatments significantly alleviated the inactivation of Rubisco, disorder of PSII photochemistry reaction and accumulation of ROS and membranes damage induced by high temperature. NO might play an important protective role in photosynthesis by regulating activities of Rubisco, Rubisco activase, and PSII, or/and enhancing thermal dissipation of excess energy through keeping higher level of zeaxanthin content or/and alleviating ROS accumulation via maintaining higher relative content of carotenoid under heat stress.
Author contribution
LLS, HQZ and MFH designed the research; LLS, LLY, HQZ and MFH conducted the research; LLS, LLY and HQZ analysed the data; LLS and MFH wrote the paper; LLS had primary responsibility for the final content. All authors have read and approved the final manuscript.
Abbreviations
- PSII:
-
Photosystem II
- RCs:
-
Reaction centers
- OEC:
-
Oxygen-evolving complex
- P N :
-
Net photosynthetic rate
- T r :
-
Transpiration rate
- g s :
-
Stomatal conductance
- C i :
-
Intercellular CO2 concentration
- F o :
-
Initial fluorescence
- NPQ:
-
Non-photochemical quenching
- F m :
-
The maximal fluorescence
- F v/F m :
-
The maximal efficiency of PSII photochemistry
- ΦPSII :
-
Actual PSII efficiency
- q p :
-
Photochemical quenching
- SNP:
-
Sodium nitroprusside
- cPTIO:
-
2-(4-Carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide
- GSNO:
-
S-nitrosoglutathione
- ROS:
-
Reactive oxygen species
- Gln synthase:
-
Glutamine synthase
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
References
Asada K, Endo T, Mano J, Miyake C (1998) Molecular mechanism for relaxation of and protection from light stress. In: Saton K, Murata N (eds) Stress responses of photosynthetic organisms. Elsevier, Amsterdam, pp 37–52
Bartošková H, Komenda J, Nauš J (1999) Functional changes of photosystem II in the moss Rhizomnium punctatum (Hedw.) induced by different rates of dark desiccation. J Plant Physiol 154:597–604
Berry J, Björkman O (1980) Photosynthetic response and adaptation to temperature in higher plants. Annu Rev Plant Physiol 31:491–543
Bilger W, Björkman O (1990) Role of the xanthophyll cycle in photoprotection elucidated by measurements of light-induced absorbance changes, fluorescence and photosynthesis in Hedera canariensis. Photosynth Res 25:173–185
Bukhov NG, Wiese C, Neimanis S, Heber U (1999) Heat sensitivity of chloroplasts and leaves: leakage of protons from thylakoids and reversible activation of cyclic electron transport. Photosynth Res 59:81–93
Camejo D, Rodríguez P, Morales MA, Dell’Amico JM, Torrecillas A, Alarcón JJ (2005) High temperature effects on photosynthetic activity of two tomato cultivars with different heat susceptibility. J Plant Physiol 162:281–289
Costa ES, Bressan-Smith R, Oliveira JG, Campostrini E, Pimentel C (2002) Photochemical efficiency in bean plants during recovery from high temperature stress. Braz J Plant Physiol 14:105–110
Crafts-Brandner SJ, Salvucci ME (2002) Sensitivity of photosynthesis in a C4 plant, maize, to heat stress. Plant Physiol 129:1773–1780
Crafts-Brandner SJ, van de Loo FJ, Salvucci ME (1997) The two forms of ribulose-1,5-bisphosphate carboxylase/oxygenase activase differ in sensitivity to elevated temperature. Plant Physiol 114:439–444
Demmig-Adams B (1990) Carotenoids and photoprotection in plants: a role for the xanthophyll zeaxanthin. Biochim Biophys Acta 1020:1–24
Efeoglu B, Terzioglu S (2009) Photosynthetic responses of two wheat varieties to high temperature. Eurasia J BioSci 3:97–106
Genty B, Briantais JM, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990:87–92
Haldimann P, Feller U (2004) Inhibition of photosynthesis by high temperature in oak (Quercus pubescens L.) leaves grown under natural conditions closely correlates with a reversible heat-dependent reduction of the activation state of ribulose-1,5-bisphosphate carboxylase/oxygenase. Plant Cell Environ 27:1169–1183
Hossain KK, Nakamura T, Yamasaki H (2011) Effect of nitric oxide on leaf non-photochemical quenching of fluorescence under heat stress conditions. Russ J Plant Physiol 58(4):629–633
Jahns P, Holzwarth AR (2012) The role of the xanthophyll cycle and of lutein in photoprotection of photosystem II. Biochim Biophys Acta 1817:182–193
Kitajima M, Butler WL (1975) Excitation spectra for photosystem I and photosystem II in chloroplasts and the spectral characteristics of the distributions of quanta between the two photosystems. Biochim Biophys Acta 408:297–305
Lichtenthaler HK, Buschmann C (2001) Chlorophylls and carotenoids: measurement and characterization by UV–VIS spectroscopy. In: Wrolstad RE, Acree TE, An H, Decker EA, Penner MH, Reid DS, Schwartz SJ, Shoemaker CF, Sporns P (eds) Current protocols in food analyticial chemistry (CPFA). Wiley, New York
Lindermayr C, Saalbach G, Durner J (2005) Proteomic identification of S-nitrosylated proteins in Arabidopsis. Plant Physiol 137:921–930
Mathur S, Jajoo A, Mehta P, Bharti S (2011) Analysis of elevated temperature-induced inhibition of photosystem II using chlorophyll a fluorescence induction kinetics in wheat leaves (Triticum aestivum). Plant Biol 13:1–6
Mehta P, Jajoo A, Mathur S, Bharti S (2010) Chlorophyll a fluorescence study revealing effects of high salt stress on Photosystem II in wheat leaves. Plant Physiol Biochem 48:16–20
Neill SJ, Desikan R, Hancock JT (2003) Nitric oxide signalling in plants. New Phytol 159(1):11–35
Oh S, Mccaslin PP (1995) The iron component of sodium-nitroprusside blocks NMDA-induced glutamate accumulation and intracellular Ca2+ elevation. Neurochem Res 20:779–784
Öquist G, Huner NPA (1993) Cold-hardening-induced resistance to photoinhibition of photosynthesis in winter rye is dependent upon an increased capacity for photosynthesis. Planta 189:150–156
Perchorowicz JT, Raynes DA, Jensen RG (1981) Light limitation of photosynthesis and activation of ribulose bisphosphate carboxylase in wheat seedlings. Proc Natl Acad Sci USA 78:2985–2989
Rintamaki E, Salo R, Eva-mari ARo (1994) Rapid turnover of the D1 reaction-centre protein of photosystem II as a protection mechanism against photoinhibition in a moss, Ceratodon purpureus (Hedw.) Brid. Planta 193:520–529
Rivas J, Abadia A, Abadfa J (1989) A new reversed phase HPLC method resolving all major higher plant photosynthetic pigments. Plant Physiol 91:190–192
Salvucci ME, Anderson JC (1987) Factors affecting the activation state and the level of total activity of ribulose bisphosphate carboxylase in tobacco protoplasts. Plant Physiol 85:66–71
Salvucci ME, Crafts-Brandner SJ (2004) Inhibition of photosynthesis by heat stress: the activation state of Rubisco as a limiting factor in photosynthesis. Physiol Plant 120:179–186
Salvucci ME, Osteryoung KW, Crafts-Brandner SJ, Vierling E (2001) Exceptional sensitivity of Rubisco activase to thermal denaturation in vitro and in vivo. Plant Physiol 127:1053–1064
Siddiqui MH, Al-Whaibi MH, Basalah MO (2011) Role of nitric oxide in tolerance of plants to abiotic stress. Protoplasma 248:447–455
Singh-Tomar R, Mathur S, Allakhverdiev SI, Jajoo A (2012) Changes in PSII heterogeneity in response to osmotic and ionic stress in wheat leaves (Triticum aestivum). J Bioenerg Biomembr 44:411–419
Song LL, Ding W, Zhao MG, Sun BT, Zhang LX (2006) Nitric oxide protects against oxidative stress under heat stress in the calluses from two ecotypes of reed. Plant Sci 171(4):449–458
Stamler JS (1994) Redox signaling: nitrosylation and related target interactions of nitric oxide. Cell 78:931–936
Stasik O, Jones HG (2007) Response of photosynthetic apparatus to moderate high temperature in contrasting wheat cultivars at different oxygen concentrations. J Exp Bot 58(8):2133–2143
Štepigová J, Vráblíková H, Lang J, Večeřová K, Barták M (2007) Glutathione and zeaxanthin formation during high light stress in foliose lichens. Plant Soil Environ 53(8):340–344
Takahashi S, Yamasaki H (2002) Reversible inhibition of photophosphorylation in chloroplasts by nitric oxide. FEBS Lett 512:145–148
van Kooten O, Snel JFH (1990) The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynth Res 25:147–150
Veljovic-Jovanovic S, Noctor G, Foyer CH (2002) Are leaf hydrogen peroxide concentrations commonly overestimated? The potential influence of artefactual interference by tissue phenolics and ascorbate. Plant Physiol Biochem 40:501–507
Wahid A (2007) Physiological implications of metabolite biosynthesis for net assimilation and heat-stress tolerance of sugarcane (Saccharum officinarum) sprouts. J Plant Res 120:219–228
Wodala B, Deák Z, Vass I, Erdei L, Horváth F (2005) Nitric oxide modifies photosynthetic electron transport in pea leaves. Acta Biol Szeged 49(1–2):7–8
Wodala B, Deák Z, Vass I, Erdei L, Altorjay I, Horváth F (2008) In vivo target sites of nitric oxide in photosynthetic electron transport as studied by chlorophyll fluorescence in pea leaves. Plant Physiol 146:1920–1927
Xue W, Li XY, Lin LS, Wang YJ, Li L (2011) Effects of elevated temperature on photosynthesis in desert plant Alhagi sparsifolia S. Photosynthetica 49(3):435–447
Yang W, Sun Y, Chen S, Jiang J, Chen F, Fang W, Liu Z (2011) The effect of exogenously applied nitric oxide on photosynthesis and antioxidant activity in heat stressed Chrysanthemum. Biol Plantarum 55(4):737–740
Acknowledgments
This work was supported by the Key Course Construction Program of Shanghai Municipal Education Commission: Plant Physiology; and Alliance Plan of Shanghai Promotion Association of Science and Technology Achievements: The design and application of root-control box.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Garstka.
Rights and permissions
About this article
Cite this article
Song, L., Yue, L., Zhao, H. et al. Protection effect of nitric oxide on photosynthesis in rice under heat stress. Acta Physiol Plant 35, 3323–3333 (2013). https://doi.org/10.1007/s11738-013-1365-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-013-1365-z