Abstract
The present study was aimed to assess the effect of nitric oxide (NO) and the interaction of NO with hydrogen peroxide (H2O2) on plant tolerance to low temperatures in cucumber seedlings. Exogenous NO significantly increased the endogenous NO content, initial and total activities of ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO), RuBisCO carboxylation rate (Vc,max), RuBP regeneration rate (Jmax) and the transcript levels of related genes in cucumber seedlings under low temperatures (11 °C/7 °C); however, the effect of NO was blocked by PTIO (NO scavenger). In addition, the SNP treatment significantly improved the contents of glucose, fructose, sucrose, starch, and the activities of sucrose phosphate synthase (SPS), acid invertase (AI), sucrose synthase (SS), as well as the expression levels of SUCROSE PHOSPHATE SYNTHASE 1&2 (SPS1, SPS2), SUCROSE TRANSPORTER 1&2 (SUT2, SUT4), β-starch hydrolase (BAM), and invertase gene (INVERTASE) in cucumber leaves under low temperatures, and the positive effect of NO was impaired by PTIO. Furthermore, we found that the H2O2, induced by NO, participated in NO-induced elevation of ascorbic acid (AsA), glutathione (GSH), and increased activities of related enzymes in the AsA-GSH cycle at low temperatures. However, the positive effect of NO was blocked by l-NAME (NOS inhibitor), PTIO, DPI (inhibitor of NADPH oxidase), and DMTU (reactive oxygen species scavenger). Taken together, our findings indicate that NO increased the low temperature tolerance of cucumber seedlings via H2O2 by improving the efficiency of the Calvin cycle, which in turn increased the carbohydrates content and accelerated the AsA-GSH cycle to enhance ROS scavenging.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Most tropical and subtropical plant species lack the ability to cope with low temperatures and are typically injured by temperatures below 15 °C. Cucumber (Cucumis sativus L.) is a typical subtropical plant, which is susceptible to low temperature stress, particularly during off-season cultivation (September to December). Low temperature is one of the most critical abiotic factors limiting the growth and production of plants (Puyaubert and Baudouin 2014). Increasing evidence indicates that low temperatures can cause an imbalance between reactive oxygen species (ROS) production and scavenging. If not detoxified in time, the excess ROS will increase the membrane permeability and membrane lipid peroxidation, DNA damage and protein denaturation (Ruelland et al. 2009). In addition, excessive generation of ROS caused by low temperatures could further severely impair the photosynthetic activity, including inhibition or abnormality of ribulose-1,5-bisphosphate (RuBP) carboxylase/oxygenase (RuBisCO) activity, carbon fixation, and chloroplast morphologies (Ruelland et al. 2009; Zhang et al. 2014). Plants have evolved a series of strategies to cope with low-temperature stress, such as strengthening the induction of antioxidant systems and synthesis of protective molecules (e.g., sugar, reduced glutathione and polyamines) (Theocharis et al. 2012).
H2O2 is the most stable ROS molecule, which was thought to be a toxic byproduct of aerobic metabolism previously (Ahammed et al. 2020a; Dat et al. 2000; Wang et al. 2010). In contrast with earlier views, H2O2 has also been implicated in a multitude of cellular signaling networks in plants (Mittler 2017). H2O2 acts as signaling molecule for the activation of defense response when present at non-toxic levels (Ahammed et al. 2020b; Arfan et al. 2019; Zhou et al. 2014). In addition, studies have reported that RBOH1-dependent H2O2 production is critical for the epigallocatechin-3-gallate-induced tolerance to abiotic stress (Li et al. 2019; Zhang et al. 2020a). Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase is the main enzymatic sources for the generation of H2O2 in the cell membrane of plants (Ahammed et al. 2020b). Rapid H2O2 production in the root tips of tolerant wheat genotype in response to aluminum (Al) stress is likely to be resulted, at least in part, from NADPH oxidase (Sun et al. 2018). Therefore, the diphenyleneodonium (DPI, an inhibitor of NADPH oxidase) is widely used to study the effects of NADPH-dependent H2O2 in plants.
A large body of evidence demonstrates that nitric oxide (NO) is a gaseous signaling molecule, which acts as a potential mediator of numerous biochemical and physiological processes in plants under biotic and abiotic stresses (Kotapati et al. 2017; Sharma et al. 2020a, b). Studies have reported that NO and its related RNS have the capacity to govern every single step of plant developmental process by balancing antioxidants and ROS under stress conditions (Begara-Morales et al. 2018). NO is synthesized endogenously in plants (Castello et al. 2019; Fancy et al. 2017; Zhang et al. 2020a, b). The main sites of NO production in plants are cytoplasm, chloroplasts, mitochondria, and peroxisomes, and both biotic and abiotic stresses can alter NO production (Terron-Camero et al. 2020). Nitric oxide synthase (NOS)-and nitrite reductase (NR)-based pathways are the major NO production routes, although the existence of NOS genes in plants still remains controversial (Astier et al. 2016). Notably, NO itself is a reactive nitrogen species and its roles in cells have been proven to be either as a potent oxidant or as an effective antioxidant; nonetheless, the positive effects of NO in plants largely depend on its local concentrations and spatial accumulation patterns (Fatma and Khan 2014; Sami et al. 2018). More interestingly, numerous studies have reported cross-talk between NO and other signaling molecules such as H2O2 during exposure of plants to environmental stress (Arfan et al. 2019; Si et al. 2017; Sun et al. 2018).
In recent years, NO along with ROS are supposed to accomplish developmental and stress responses (Nidhi et al. 2020). For instance, NO regulates the H2O2 generation under salt stress, and H2O2 potentially acts downstream of NO to regulate the activity of plasma membrane (PM) H+-ATPase under salt stress (Mazid et al. 2011). However, in some other conditions, H2O2 mediates NO production, and NO as a downstream signal of H2O2, increases the activities of antioxidant enzymes (Sun et al. 2018). As observed by Tanou et al. (2010), the protein network interfered by H2O2 and NO mainly belongs to photosynthesis and especially the Calvin–Benson cycle.
In our previous study, we showed that NO could enhance the chilling tolerance of cucumber seedlings (Zhang et al. 2020b). To our knowledge, as yet, only a small number of studies have reported the effects of NO on photosynthetic carbon assimilation in response to chilling stress in cucumber seedlings. Therefore, in the present study, we investigated the effect of NO on carbohydrate metabolism and the Calvin-Benson cycle. Additionally, we analyzed the influence of NO and the interaction of NO produced by the NOS-dependent pathway with H2O2 on the AsA-GSH cycle in cucumber seedlings under low temperature stress.
Materials and Methods
Plant Materials
The experiments were carried out in the solar greenhouse and laboratory of the Experiment Station of the Agricultural College, (longitude 86° and latitude 44.18° N), Shihezi University, China. Cucumber (C. sativus L.) cultivar ‘Jinyan No. 4’ was used for the study. Healthy and uniform seeds of cucumber were selected and disinfected by soaking in 55 °C hot water, then the seeds were further soaked for 6 h at room temperature, followed by photophobic incubation at 28 °C. Cucumber seeds were germinated in a growth medium filled with a mixture of peat and vermiculite (2:1, v:v). The seedlings were transplanted into plastic pots (diameter × height, 120 × 110 mm) containing a mixture of peat and vermiculite (2:1 by volume, with one seedling per container), when the two cotyledons were fully expanded. Thereafter, the seedlings were irrigated daily and fertilized every three days with Hoagland’s nutrient solution. When the second true leaves of seedlings were fully expended, the seedlings were transferred to RXZ intelligent artificial incubator (Ningbo, China) and used for the experiment. The environmental conditions were as follows: light cycle 12 h, the temperature of 25 °C/20 °C (day/night), photosynthetic photon flux density (PPFD) of 120 μmol m−2 s−1, and relative humidity was controlled at around 70%.
Experimental Procedures
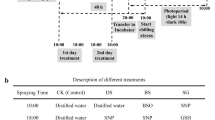
To investigate the effects of NO on photosynthetic carbon assimilation and carbohydrate metabolism in cucumber seedlings under low temperature stress, the seedlings were treated with distilled water, sodium nitroprusside (SNP, 200 μmol L−1), and PTIO (200 μmol L−1) combined with SNP (Cui et al. 2011; Zhang et al. 2020b). The treatments were classified as presented in Table 1. After 24 h of treatment, low temperature treatment was carried out (11 °C with a 12 h-light, 7 °C with a 12 h-dark cycle, light intensity 120 μmol m−2 s−1). The second leaves of cucumber seedlings from the bottom were harvested at various durations of low temperature stress (2 h, 5 h, 7 h, 24 h, and 48 h) and immediately frozen in liquid nitrogen for subsequent analyses.
To investigate the interrelation of NO and H2O2 in the low temperature-induced lipid peroxidation and redox state, the cucumber seedlings were pre-treated with 200 μmol L−1 l-NAME, 200 μmol L−1 PTIO, 100 μmol L−1 DPI (diphenyleneodonium, a NADPH oxidase inhibitor) or 5 mmol L−1 DMTU (dimethylthiourea, a H2O2 and OH· scavenger), and 8 h after the pre-treatment, the plants were sprayed with 200 μmol L−1 SNP (Cui et al. 2011). The treatments were classified as presented in Table 1. Sixteen hours after the SNP treatment, the seedlings were exposed to low temperature (11 °C with a 12 h-light, 7 °C with a 12 h-dark cycle, light intensity 120 μmol m−2 s−1). Cucumber seedlings were harvested at various durations of low temperature stress (0 h, 2 h, 5 h, 7 h, 24 h, and 48 h) and leaf samples were immediately frozen in liquid nitrogen.
Nitric Oxide Fluorescence Detection
According to the method of Corpas et al. (2004), the DAF-FM DA (4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate), a probe highly specific to NO, was used to detect the NO fluorescence. Cucumber leaf segments (approximately 20–25 mm2) were incubated in 10 μmol L−1 DAF-FM DA (prepared in 10 μmol L−1 Tris–HCl, pH 7.4) at 25 °C for 1 h in the dark and then washed at least twice with the same buffer for 15 min each. The confocal laser scanning microscope system (ZEISS LSM 510 META, Germany) was used for detecting NO fluorescence, and the standard filters and collection modalities for DAF-FM DA green fluorescence (excitation 495 nm; emission 515 nm) were also used.
Determination of Activity and Carboxylation of RuBisCO and Regeneration Rate of RuBP
RuBisCO activity was measured according to the improved method of Lilley and Walker (1974). The assay of RuBisCO initial activity was assayed by determining the rate of change in the absorbance at 340 nm over 90 s. Added 10 μL of the enzyme solution to 0.1 mL reaction solution. The reaction mixture consisted of 5 mmol L−1 Heps-NaOH (pH 8.0) buffer, 1 mmol L−1 NaHCO3, 2 mmol L−1 MgCl2, 0.25 mmol L−1 DTT, 0.1 mmol L−1 EDTA, 1 U inositol kinase, 1 U 3-phosphoglycerate kinase, 1 U GAPDH, 0.5 mmol L−1 ATP, 0.015 mmol L−1 NADH2, 0.5 mmol L−1 inositol phosphate and 0.06 mmol L−1 RuBP. The enzyme activation reaction (30 °C, 15 min) was carried out before the RuBisCO total activity measurement. The activated reaction solution contained 33 mmol L−1 Tris–HCl (pH 7.5), 0.67 mmol L−1 EDTA, 33 mmol L−1 MgCl2, and 10 mmol L−1 NaHCO3. After the activation, the reaction solution was added. When the temperature reached the measurement temperature, RuBP was quickly added, and the absorbance of 340 nm was recorded according to the initial viability method.
The gas exchange parameters including net photosynthetic rate (Pn), stomatal conductance (Gs), intercellular CO2 concentration (Ci) and transpiration rate (Tr) were measured using a LI-6400 Portable Photosynthesis system (LI-COR Inc., Lincoln, NE, USA). The carboxylation rate (Vc,max) of RuBisCO and the regeneration rate (Jmax) of RuBP were calculated by the method of Ethier and Livingston (2004) based on the gas exchange parameters.
Determination of Carbohydrates and Related Enzyme Activity
The contents of carbohydrates were measured according to the method of Buysse and Merckx (1993) as described in detail previously (Zhang et al. 2020b). The carbohydrates were extracted from 0.1 g of fresh material, and incubated with 4 mL of 80% ethanol for 30 min at 85 °C, then centrifuged at room temperature for 30 min. The precipitate was repeatedly extracted three times (80% ethanol 2 mL for each time) and the supernatant was combined. After the pigment of extract was adsorbed by activated carbon (0.1 g), the extract was brought to a volume of 50 mL. The resulting solution was used to determine the content of glucose, fructose and sucrose, and the content of starch was measured using the residue after plant sugar extraction.
Sucrose phosphate synthase (SPS) and sucrose synthase (SS) were extracted at 0–4 °C, according to the method described by Lowell et al. (1989). SPS activity was measured at a wavelength of 620 nm in 37 °C. Acid invertase (AI) was extracted as described by Schaffer et al. (1989). The reaction mixture consisted of 4% sucrose, 50 mmol L−1 sodium acetate buffer (pH 4.5), and an aliquot of enzyme solution in a total volume of 1 mL. The activity of the AI was measured by incubating the reaction solution at 30 °C for 15 min.
Histochemical Staining of H2O2
In situ H2O2 accumulation was assayed by histochemical staining of cucumber leaves with 3,3-diaminobenzidine (DAB) according to the method of Thordal et al. (1997) as described previously (Hasan et al. 2019).
Determination of Lipid Peroxidation
Lipid peroxidation was determined by quantifying the content of leaf malondialdehyde (MDA). The content of MDA was determined based on the method of Health and Packer (1968), based on the thiobarbituric acid (TBA) reaction. The MDA contents were calculated based on absorption at 532 and 600 nm, with an extinction coefficient of 155 mM−1 cm−1.
Determination of the Related Substances and Enzyme Activities in AsA-GSH Cycle
Frozen leaf tissues (0.3 g) were homogenized in 3 mL ice-cold 6% metaphosphoric acid containing 0.2 mmol L−1 EDTA. The homogenate was centrifuged at 4 °C for 15 min at 12,000×g and the supernatant was used for antioxidant analysis. The content of reduced ascorbate (AsA) and oxidized ascorbate (dehydroascorbate, DHA) were detected based on the method of Law et al. (1983). Absorbance was recorded at 525 nm. The contents of reduced glutathione (GSH) and oxidized glutathione (GSSG) were determined according to the method by Griffith (1980) based on the changes in absorption at 412 nm.
Cucumber leaves (0.3 g frozen leaf tissues) were homogenized in 3 mL ice-cold phosphate (pH 7.8) buffer containing 0.2 mmol L−1 EDTA, 2% PVP (W/V). The homogenate was centrifuged at 4 °C for 20 min at 12,000×g and the extract was used for determining the activities of antioxidant enzyme. Ascorbate peroxidase (APX) activity was measured according to Nakano and Asada (1981). The reaction mixture (2 mL) contained the enzyme extract (0.1 mL), 25 mmol L−1 sodium phosphate buffer (pH 7.0), 0.5 mmol L−1 ascorbate, 0.1 mmol L−1 H2O2, and 0.1 mmol L−1 EDTA. The reaction was started by adding H2O2.
The activity of glutathione reductase (GR) was measured according to Foyer and Halliwell (1976). The activity of GR was determined by the reduction rate of NADPH at 340 nm. The reaction mixture consisted of 25 mmol L−1 HEPES buffer (pH 7.0), 0.5 mmol L−1 oxidized glutathione (GSSG), 0.12 mmol L−1 NADPH, 0.2 mmol L−1 EDTA, and 0.1 mL enzyme extract.
The activity of dehydroascorbate reductase (DHAR) was detected by measuring the increase of absorbance at 265 nm due to dehydroascorbate (DHA) formation. The reaction mixture of 2 mL contained 25 mmol L−1 sodium phosphate buffer (pH 7.0), 2.5 mmol L−1 reduced glutathione (GSH), 0.4 mmol L−1 DHA, and 0.1 mL enzyme extract.
The activity of monodehydroascorbate reductase (MDAR) was analyzed by determining the rate of decrease in the absorbance at 340 nm due to NADH oxidation using a UV-3900 (Japan) spectrophotometer.
Total RNA Extraction and Gene Expression Analysis
The extraction of total RNA from cucumber leaves was carried out using Trizol reagent according to the supplier’s recommendation. The nucleic acid content was detected by Nanodrop 2000, and 2% agarose gel electrophoresis was performed to verify RNA integrity. Total RNA (1 μg) was reverse-transcribed using ReverTra Ace qRT-PCR first Strand cDNA Synthesis kit (TOYOBO, Japan). The gene-specific primers were designed based on the sequence of CDS, and the primers were presented in Table 2.
Quantitative real-time PCR was performed in the PCR detection system of iCycler iQ Multicolor Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA). The SYBR Green Realtime PCR Master Mix (TOYOBO, Japan) was used for PCRs. The PCR conditions consisted of denaturation at 95 °C for 30 s, followed by 40 cycles of denaturation at 95 °C for 10 s, annealing at 56 °C for 30 s and extension at 72 °C for 1 min. The analysis of the dissolution profile was carried out at 65 °C to 95 °C. To minimize sample variations, mRNA expression of a target gene was normalized relative to the expression of housekeeping gene Actin. All experiments were repeated three times using cDNA prepared from three biological replicates of cucumber leaves. The fold changes in expression level relative to the control were expressed as \(2^{{ - \Delta \Delta C_{{\text{T}}} }}\) (Livak and Schmittgen 2001).
Statistical Analysis
All treatments were conducted at least three replicates. The data were analyzed using SPSS version 19.0 (SPSS, Inc., Chicago, IL). Results were expressed as mean ± standard deviation (SD). The Tukey test (HSD) was used for significance analysis. Differences between treatments were considered to be significant, when the P value was less than 0.05 (P < 0.05).
Results
SNP Induces NO Accumulation Under Low Temperature
To investigate whether the exogenous SNP can increase endogenous NO accumulation in cucumber seedlings under low temperature stress, we pre-treated cucumber leaves with SNP and PTIO. The NO signals in cucumber leaves after 2 h, 5 h and 7 h of low temperature were shown in Fig. 1. Compared with CK, SNP treatment alone greatly increased the NO signal, but PTIO diminished the positive effect of SNP to some extent.
Nitric oxide (NO) accumulation in cucumber leaves as influenced by SNP (NO donor) and PTIO (NO scavenger). The seedlings were treated with distilled water (CK), 200 μmol L−1 SNP and 200 μmol L−1 PTIO + 200 μmol L−1 SNP. After 24 h of the first spray, low temperature treatment (10 °C/12 h during the day and 6 °C/12 h at night) was imposed. Segments of the second leaves were loaded with diaminofluorescein-FM diacetate (DAF-FM DA) after 2, 5 and 7 h of low temperature treatment, and NO accumulation was detected by confocal laser scanning microscope system (scale bars = 100 μm)
Effect of NO on the Carboxylation Rate of RuBisCO and Regeneration Rate of RUBP
To study the effect of NO on photosynthetic carbon assimilation after 24 h of low temperature stress, we detected the carboxylation rate (Vc,max) of RuBisCO and regeneration rate (Jmax) of RuBP after 24 h of low temperature treatment. As presented in Table 3, compared with CK (11 °C/7 °C for 24 h), exogenous SNP significantly improved the Vc,max and Jmax by 25.3% and 19.9%, respectively. However, the PTIO in combination with SNP treatment, lead to a significant decrease in Vc,max of RuBisCO and Jmax of RuBP by 24.3% and 24.3%, respectively, when compared with the SNP treatment alone.
Effect of NO on the Activity of RuBisCO
To further determine whether NO could induce the process of carbon dioxide (CO2) fixation after 24 h of low temperature stress, we examined the initial and total activity of RuBisCO in cucumber seedlings that were pre-treated with SNP and PTIO. As shown in Fig. 2, pre-treatment of cucumber seedlings with SNP significantly enhanced the initial (Fig. 2A) and total activities (Fig. 2B) of RuBisCO by 43.86% and 38.16% compared with CK (11 °C/7 °C for 24 h), while PTIO significantly suppressed the effect of NO on initial and total activities of RuBisCO by 65.79% and 42.54%, respectively.
Effect of NO on the Transcript Levels of Calvin–Benson Cycle-Related Genes
Next, we analyzed the changes in the transcript levels of some genes encoding for enzymes related to photosynthetic carbon assimilation in cucumber seedlings after 24 h of low temperature stress (Fig. 3). Compared with CK (11 °C/7 °C for 24 h), SNP pretreatment significantly increased the expression levels of RCA, rbcS, FBPaldolase, FBPase, and SBPase by 3, 3, 2.5, 2, and 1.3 fold, respectively, while the expression level of rbcL decreased in cucumber seedlings by 0.7 fold under low temperature stress. In addition, the PTIO in combination with SNP treatments significantly attenuated the expression levels of RCA, rbcS, FBPase, SBPase, and FBPaldolase, and increased the rbcL expression level.
Effect of NO on the Accumulation of Carbohydrates Under Low Temperature
As presented in Fig. 4, the content of glucose, fructose, sucrose, and starch in cucumber seedlings increased with the prolongation of low temperature stress. In addition, compared with the CK (11 °C/7 °C for 24 h), exogenous NO significantly increased the content of glucose, fructose, sucrose and starch in cucumber leaves by 30.1%, 22.2%, 4.8% and 27.5%, respectively at 24 h of low temperature treatment. In addition, NO induced the content of glucose, fructose, sucrose and starch by 46.5%, 50.9%, 33.6%, and 26.6% at 48 h of low temperature stress when compared to CK (11 °C/7 °C for 48 h), respectively. However, the effect of exogenous NO on the accumulation of carbohydrates was either attenuated or reversed by PTIO.
Effect of NO on the Metabolism of Sucrose
In order to clarify the role of NO in the regulation of sucrose metabolism, we determined the activity of sucrose metabolism related enzymes. Table 4 showed that NO induced the activity of sucrose phosphate synthase (SPS) by 12.7% and 8.2% at 24 h and 48 h of low temperature stress, respectively. Importantly, these results were consistent with the content of sucrose in Fig. 4c. In addition, NO induced the activities of acid invertase (AI) and sucrose synthase (SS) by 15.6% and 12.4% at 24 h of low temperature stress, and by 13.6% 13.4% at 48 h of low temperature stress, respectively. However, the positive effect of NO on sucrose metabolism was attenuated by PTIO treatment.
Effect of NO on the Expression Levels of Carbohydrate Metabolism-Related Genes
To investigate the effect of NO on sucrose synthesis and transport in low temperature-stressed cucumber seedlings, we assessed the transcript levels of β-starch hydrolase gene (BAM) (it often plays an important role in decomposition of starch to sucrose in plants), invertase gene (Invertase), SUCROSE PHOSPHATE SYNTHASE 1&2 (SPS1, SPS2), SUCROSE TRANSPORTER 1&2 (SUT2, SUT4). As evident from Fig. 5, compared with CK, the expression levels of SPS1, SPS2, SUT2, and SUT4 were significantly increased by NO in cucumber seedlings regardless of the duration of low temperature stress. In particular, the transcript levels of SPS1, SPS2, SUT2, SUT4, and Invertase increased 0.18, 1.53, 0.57, 0.54, and 0.52 fold, respectively, in SNP-pretreated seedlings at 24 h of low temperature stress. NO also induced the transcript levels of SPS1, SPS2, SUT2 and SUT4 by 0.54, 0.85, 0.74, and 0.89-fold at 48 h of low temperature stress, respectively. There was no significant difference in the transcript level of Invertase at 48 h of low temperature stress. Apart from these, NO significantly increased the expression level of BAM by 0.37 fold after 24 h of low temperature stress; however, the transcript levels of BAM significantly decreased by 0.4 fold after 48 h of low temperature stress in cucumber seedlings when compared with CK. Notably, compared with SNP treatment alone, PTIO in combination with SNP treatment significantly diminished the effect of SNP on the transcript levels of SPS1, SPS2, SUT2 and SUT4.
Transcript levels of SUCROSE PHOSPHATE SYNTHASE 1&2 (SPS1) (A), SPS2 (B), SUCROSE TRANSPORTER 1&2 (SUT2) (C), SUT4 (D), β-starch hydrolase gene (BAM) (E), and invertase gene (Invertase) (F) in leaves of low temperature-stressed cucumber seedlings as affected by exogenous SNP and PTIO. Error bars represent SD (n = 3). Bars with different letters within a sampling time are significantly different (P < 0.05)
H2O2 is Involved in NO-Induced Alleviation of Oxidative Stress in Cucumber Seedlings
To further determine whether H2O2 is involved in NO-induced low temperature tolerance of cucumber seedlings, we investigated the in situ H2O2 accumulation in cucumber leaves by histochemical staining with DAB. The l-NAME, PTIO, DPI, and DMTU were used to understand the relationship between NO and H2O2. As shown in Fig. 6, pretreatment of cucumber seedlings with SNP enhanced the H2O2 accumulation during the first 7 h when compared with CK, while simultaneous application of l-NAME and SNP, PTIO and SNP reduced the SNP-induced H2O2 production up to 7 h of low temperature treatments. However, compared to CK, the content of H2O2 was greatly decreased by SNP after 24 h and 48 h of low temperature treatments. But compared to SNP treatment alone, l-NAME and PTIO in combination with SNP treatments partly blocked the H2O2 content under low temperature for 24 h and 48 h. Notably, pretreatment of cucumber seedlings with DPI and DMTU along with SNP also attenuated the SNP-induced H2O2 levels under low temperature for 2 h, 5 h and 7 h when compared with SNP treatment alone.
To further investigate the effect of H2O2 in NO-induced tolerance to low temperature in cucumber seedlings, we analyzed the content of MDA, an important marker of lipid peroxidation and relevant oxidative stress. As shown in Fig. 7, compared with CK, pre-treatment of cucumber seedlings with SNP alone significantly reduced the MDA content by 40% and 42.1% when cucumber seedlings were exposed to low temperature for 24 h and 48 h, respectively. In addition, l-NAME in combination with SNP, and PTIO in combination with SNP, significantly increased the content of MDA, whether in normal condition or under low temperature stress, when compared with SNP alone. Intriguingly, compared with SNP, the DPI plus SNP and DMTU plus SNP also significantly increased the content of MDA after exposure of cucumber seedlings to low temperature for 24 h and 48 h.
H2O2 is Involved in NO-Induced Changes in Ascorbate–Glutathione (AsA-GSH) Cycle
To determine whether the H2O2 plays a role in NO-induced stress tolerance via the AsA-GSH cycle, we analyzed the effects of NO and ROS scavenger on NO-induced changes in key components of AsA-GSH cycle in cucumber seedlings. Compared with CK, SNP alleviated the low temperature-induced declines of reduced glutathione (GSH), reduced ascorbic acid (AsA), and the ratios of cellular redox status (GSH/GSSG and AsA/DHA) in cucumber leaves throughout the experiment (P < 0.05) (Fig. 8A, C, D, F). For instance, SNP increased the ratios of GSH/GSSG and AsA/DHA by 36.8% and 45.5% at 24 h of low temperature stress, respectively, compared with the CK. However, when the cucumber leaves were pre-treated with l-NAME and PTIO, and then treated with SNP, the effects of SNP on GSH, AsA, GSH/GSSG, and AsA/DHA were completely abolished. In addition, the content of AsA and GSH, and the ratios of GSH/GSSG and AsA/DHA in combined treatment of DPI with SNP and DMTU with SNP remarkably attenuated the SNP effect on AsA and GSH contents during the entire experiment (P < 0.05). In contrast, the contents of DHA and GSSG were significantly declined by SNP throughout the experiment when compared with CK. Compared with SNP treatment alone, cucumber seedlings that were pre-treated with l-NAME, PTIO, DPI and DMTU followed by SNP treatment, showed significantly increased contents of DHA and GSSG throughout the experiment.
Ascorbate (AsA) content (A), dehydroascorbate (DHA) content (B), Ascorbate/dehydroascorbate (AsA/DHA) rate (C), glutathione (GSH) content (D), oxidized glutathione (GSSG) content (E), and glutathione/oxidized glutathione GSH/GSSG (F) in low temperature-stressed cucumber seedlings as affected by various treatments. Error bars represent SD (n = 3). Bars with a different letter within a sampling date are significantly different (P < 0.05)
H2O2 is Involved in NO-Induced Increases in Activities of Antioxidant Enzymes
Since we found that H2O2 is involved in NO-induced changes in GSH and AsA contents, we analyzed the temporal changes in the activities of enzymes related to the AsA-GSH cycle in cucumber seedlings under low temperature. The application of SNP alone exerted positive effects on the activity of these enzymes in cucumber seedlings during low temperature (Fig. 9). When compared with SNP, the application of l-NAME plus SNP and PTIO plus SNP significantly decreased the activity of APX, GR, MDAR, and DHAR. Moreover, treatment with DPI plus SNP and DMTU plus SNP markedly decreased the activity of APX, GR, MDAR, and DHAR in cucumber seedlings, when compared to treatment with SNP alone.
Activities of Ascorbate peroxidase (APX) (A), glutathione reductase (GR) (B), monodehydroascorbate reductase (MDAR) (C), and dehydroascorbate reductase (DHAR) (D) in chilling-stressed cucumber seedlings as affected by various treatments. Error bars represent SD (n = 3). Bars with a different letter within a sampling date are significantly different (P < 0.05)
Discussion
NO-Enhanced Tolerance to Low Temperature is Attributed to Stimulation of the Carbohydrate Metabolism and Calvin–Benson Cycle
Many studies have reported that NO is an important signal for transducing information, and can alleviate the injuries of plants under low temperatures (Liu et al. 2016; Zhao et al. 2009). In the present study, we used NO-sensitive fluorescent molecular probes, DAF-FM DA, combined with laser confocal microscopy to observe the accumulation of NO in cucumber leaves after the exposure of plants to low temperature for 2 h, 5 h, and 7 h. Consistent with Cui et al. (2011) and Dong et al. (2018), our results showed that exogenous SNP could increase NO accumulation, whereas the positive effect of SNP on cucumber leaves under low-temperature stress was reversed by the addition of PTIO (Fig. 1). These results suggest that exogenous SNP could further induce the increase of endogenous NO in plants under low temperature. The rapid increases in NO levels during early hours of low temperature stress may act as a signal, which could further regulate the physiological and biochemical processes of plants, and thus enhancing the low temperature tolerance in cucumber plants.
In plants, many physiological and metabolic processes are impaired by low temperatures (Karimi and Ershadi 2015). Previous studies have shown that the accumulation of water-soluble carbohydrates and starch is an essential strategy for plant adaptations to low temperature stress (Hajihashemil et al. 2018; Shin et al. 2015; Theocharis et al. 2012; Yamdeu et al. 2016). In addition, NO could strongly stimulate the physiological processes including carbohydrates metabolism of plants under various environmental stresses (Sehar et al. 2019; Wang et al. 2017). All of these reports indicate that the increase of carbohydrate content is induced by low temperature, while NO also participates in the low temperature-induced accumulation of water-soluble carbohydrates and starch in many plants (Amooaghaie and Nikzad 2013). Consistent with previous studies, here, we showed that the content of water-soluble carbohydrates and starch increased to varying degrees at low temperature. Apart from this, exogenous NO promoted the accumulation of starch, sucrose, glucose, and fructose in cucumber leaves, but PTIO blocked the effect of NO at low temperature stress.
The initial products of photosynthesis in plants include sucrose and temporary starch that are synthesized in cytoplasmic matrix and chloroplasts, respectively. Sucrose is the main (or even only) form of carbohydrates for long-distance transport in plants. Krapp et al. (1993) reported that when the synthesis of sucrose in leaves is greater than the ability to export sucrose in plants, excessive accumulation of sucrose could inhibit the expression level of photosynthetic genes, thereby attenuating photosynthesis. Therefore, sucrose metabolism and transport are very important in regulating many physiological processes of plants. In the present study, sucrose accumulation in the cucumber leaves continued to increase under low temperature stress along with the prolongation of stress conditions. In addition, low temperature-stressed cucumber seedlings treated with exogenous NO exhibited increased content of sucrose, activities of SPS, AI and SS, and transcription levels of SPS1, SPS2, SUT2, and SUT4. These results are in agreement with the reports by Wang et al. (2013) and Yu et al. (2015), in peach fruits during cold storage. However, the positive effect of NO was offset by PTIO. So it is highly plausible that NO-induced enhancements of glucose and fructose contents under low temperature were probably caused by NO-induced increased metabolism of sucrose and starch. This also suggested that the products of photosynthesis were influenced by NO under environmental stress, and a relatively higher sucrose content was induced by NO alongside its higher transportation and decomposition activity. Besides, the inverse relationship between starch content and the expression level of BAM under low temperature stress may also suggest that the hydrolysis of starch is responsible for the increased levels of glucose and fructose. All these results indicated that NO participated in low temperature-induced increased carbohydrates content, sucrose metabolism, and polysaccharide hydrolysis. Moreover, an increased accumulation of water-soluble carbohydrates further contributed to enhanced tolerance to low temperature in cucumber seedlings. Our results are in conformity with an earlier study in cucumber, suggesting that NO generated by the NOS-dependent pathway may alleviate the damage caused by chilling stress through elevating the soluble sugar content (Liu et al. 2016).
The photosynthetic carbon assimilation can also affect the accumulation of carbohydrates in plants, and RuBisCO is the essential carboxylase in carbon assimilation. The improvement of carbohydrate content induced by NO can be partly explained by the changes in RuBisCO activities and the carboxylation rate in this study. The initial and total RuBisCO activity was decreased to a lesser extent than CO2 assimilation by various environmental stresses (Chen and Cheng 2003; Li et al. 2010). However, it has been revealed that NO could play a protective role in the regulation of plant responses to abiotic stress by enhancing the RuBisCO activity (Khairy et al. 2016). The present study discovered that exogenous NO significantly improved the activity and carboxylation rate of RuBisCO, the regeneration rate of RuBP, and the expression levels of RCA and rbcS, and decreased the expression level of rbcL in cucumber leaves under low temperature. The differential expression of rbcL and rbcS as induced by NO may be due to the different coding positions of the two genes in plants, rbcL is encoded by the chloroplast gene, and rbcS is encoded by the nuclear gene (Silverthorne et al. 1990). However, PTIO blocked the effect of NO throughout the experiment (Table 3, Figs. 2 and 3). These results suggested that NO may play a critical role in photosynthetic carbon assimilation of cucumber seedlings by improving the RuBisCO activity and carboxylation rate, thereby enhancing the tolerance of plants to low temperature stress. In addition, Puyaubert et al. (2014) reported that the S-nitrosylation level of the large and small subunits of RuBisCO as well as its regulator, RuBisCO activity, is strongly increased by NO in Arabidopsis. From this point of view, it is possible that NO regulates the activity of RuBisCO and carboxylation rate through post-transcriptional modifications, thereby enhancing cold tolerance of cucumber seedlings. The activities of several enzymes such as FBPaldolase, FBPase, and SBPase play an essential role in the RuBP regeneration phase. The present study showed that exogenous NO also increased the RuBisCO regeneration rate and the expression levels of FBPaldolase, FBPase, and SBPase in cucumber seedlings under low temperature. However, PTIO diminished the positive effect of SNP. These results further demonstrate that NO can effectively alleviate the inhibitory effect of low temperature stress on the Calvin cycle by improving the initial and total activities of RuBisCO, RuBisCO carboxylation and the regeneration rate of RuBP in cucumber seedlings.
H2O2 is Involved in NO-Induced Low Temperature Tolerance of Cucumber
Previously, ROS were mainly regarded as toxic byproducts, but in contrast with earlier views, H2O2 has been shown to be a necessary and vital signaling molecule in normal metabolism as well as stress metabolism in plants (Ahammed et al. 2020a; Guo et al. 2019; Mittler 2017; Shi et al. 2016). Previous studies show that H2O2 participates in NO-induced chilling tolerance in tomato seedlings (Diao et al. 2017) and stomatal closure in Arabidopsis (Shi et al. 2015). In addition, Qiao et al. (2014) reported that the production of H2O2 and NO under abiotic stress conditions occurs in short succession to one another or parallelly. A plethora of reports evidenced that the NO is required for the H2O2 production. For instance, cPTIO (NO scavenger) or l-NAME could inhibit the endogenous H2O2 generation during the development of adventitious roots (Liao et al. 2011). Consistent with this, in the present study, the elevation of H2O2 in cucumber leaves was induced by NO under low temperature for 2 h, 5 h, and 7 h. However, the application of exogenous NO showed an opposite effect on H2O2 content after 24 h and 48 h at low temperatures as compared to the untreated ones. On the other hand, l-NAME and PTIO blocked the effect of SNP throughout the treatment period (Fig. 6). This also indicates that both exogenous NO and NO produced by the NOS-dependent pathway might mediate the increased production of H2O2 under short-term low-temperature stress.
The environmental stress-induced ROS accumulation could oxidize proteins and membrane lipids, resulting in abnormalities in cells (Petrov et al. 2015; Wu et al. 2017). MDA content is a well-known index that indirectly reflects the membrane fluidity, integrity and membrane peroxidation (Rui et al. 2010). In our study, the MDA content in the control seedlings increased quickly with the extension of low temperature treatment, while exogenous NO significantly inhibited the increase of MDA content (Fig. 7). Similar to our results, a study in walnut indicated that NO could decline the MDA concentration under low temperature (Dong et al. 2018). However, the application of l-NAME, PTIO, DPI and DMTU in combination with SNP aggravates the oxidative stress induced by low temperature as compared to the SNP treatment alone, implicating an enhanced oxidative degradation of lipids. These results suggest that H2O2 is partially involved in NO-induced potential delay of membrane lipid peroxidation, thus enhancing the low temperature tolerance of cucumber seedlings.
Plants have evolved enzymatic and non-enzymatic antioxidant defense systems to protect themselves against oxidative stress. It is well known that the AsA-GSH cycle is a key metabolic pathway to scavenge ROS, and it maintains redox equilibrium in plants. A higher efficiency of the AsA-GSH cycle under abiotic and biotic stress is indispensable for alleviating damage to plants (Asada 2006). In particular, H2O2 produced in plants is mainly eliminated by the ASA-GSH cycle located in the cytosol, mitochondria, chloroplast, and peroxisomes (Foyer and Noctor 2011). A recent report revealed that NO could promote the metabolism of ROS in peroxisomes (Corpas et al. 2019) and maintain the stability of the cell membrane by enhancing the reducing ability of the ASA-GSH cycle. Notably, GSH and AsA are the vital cellular antioxidants, and high concentrations of GSH and AsA in plants are conducive to maintaining an appropriate redox environment and reducing the damage caused by abiotic and biotic stresses. Consistent with this, our study also showed that exogenous NO enhanced the tolerance to low temperature in cucumber seedlings by decreasing the content of DHA and GSSG, and increasing the content of AsA, GSH and the ratio of AsA/DHA and GSH/GSSG. Notably, DPI and DMTU diminished the effect of SNP, implying that H2O2 participated in NO-induced promotion of GSH and AsA content. The intracellular redox state is essential for plants to resist the damage of biotic and abiotic stress (Dietz 2008). Therefore, maintaining a high reducing power of GSH and AsA is essential for plants to remove excess ROS. To attain a high reducing power, relatively high activities of APX, GR, MDAR, and DHAR are required (Mittler 2002). In AsA-GSH cycle, APX is the most important enzyme for removing excessive H2O2. GR, MDAR, and DHAR are responsible for reducing the DHA and GSSG to AsA and GSH, respectively, and thus providing substrates for APX. Recently, Ma et al. (2019) reported that NO could enhance the reducing ability of the AsA-GSH cycle and maintain a high antioxidant capacity of peach during cold storage. In tomato seedlings, the activities of the enzymes related to the AsA-GSH cycle could be enhanced by the application of exogenous NO under cadmium stress (Ahmad et al. 2018). The present study also showed that the enhanced activities of APX, GR, MDAR, and DHAR were induced by NO at low temperature, while l-NAME and PTIO blocked the effect of SNP. Apart from this, as mentioned by Kolupaev et al. (2015), NO and H2O2 signaling pathways are closely linked in plant cells under control conditions as well as during the overall responses of plants to environmental stimuli. In the present study, we found that the application of DPI and DMTU can also diminish the activities of APX, GR, MDAR, and DHAR induced by NO at low temperature, suggesting that H2O2 is involved in NO-induced improvement of the reducing ability of the AsA-GSH cycle.
In conclusion, we have revealed that exogenous NO is involved in the enhancement of plant tolerance to low temperature stress in cucumber seedlings by increasing the initial and total activities of RuBisCO and RuBP regeneration rate, thereby improving the content of carbohydrates. Furthermore, NO could induce the production of H2O2, which plays a crucial role in stimulating the AsA-GSH cycle. H2O2 is involved in the process of NO-induced enhancement of GR, APX, DHAR, and MDAR activities. The NO-induced improvement in enzyme activities further contributed to the increased content of ASA, GSH, and the ratio of AsA/DHA, GSH/GSSG, thereby alleviating the oxidative damage of cucumber seedlings under low temperature. Our results suggest that H2O2 participates in NO-mediated signaling pathways in response to low temperature stress, which may provide an understanding of plant response mechanisms to other environmental stresses as well.
References
Ahammed GJ, Wu MJ, Wang YQ, Yan YR, Mao Q, Ren JJ, Ma RH, Liu AR, Chen SC (2020a) Melatonin alleviates iron stress by improving iron homeostasis, antioxidant defense and secondary metabolism in cucumber. Sci Hortic 265:109205. https://doi.org/10.1016/j.scienta.2020.109205
Ahammed GJ, Li X, Yang YX, Liu CC, Zhou GZ, Wan HJ, Cheng Y (2020b) Tomato WRKY81 acts as a negative regulator for drought tolerance by modulating guard cell H2O2-mediated stomatal closure. Environ Exp Bot. https://doi.org/10.1016/j.envexpbot.2019.103960
Ahmad P, Ahanger MA, Alyemeni MN, Wijaya L, Alam P (2018) Exogenous application of nitric oxide modulates osmolyte metabolism, antioxidants, enzyme of ascorbate–glutathione cycle and promotes growth under cadmium stress in tomato. Protoplasma 255:79–93. https://doi.org/10.1007/s00709-017-1132-x
Amooaghaie R, Nikzad K (2013) The role of nitric oxide in priming-induced low-temperature tolerance in two genotypes of tomato. Seed Sci Res 23:123–131. https://doi.org/10.1017/S0960258513000068
Arfan M, Zhang DW, Zou LJ, Luo SS, Tan WR, Zhu T, Lin HH (2019) Hydrogen peroxide and nitric oxide crosstalk mediates brassinosteroids induced cold stress tolerance in Medicago truncatula. Int J Mol Sci 20:144. https://doi.org/10.3390/ijms20010144
Asada K (2006) Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol 141:391–396. https://doi.org/10.1104/pp.106.082040
Astier J, Gross I, Durner J (2016) Nitric oxide production in plants: an update. J Exp Bot 69:3041–3041. https://doi.org/10.1093/jxb/erx420
Begara-Morales JC, Chaki M, Valderrama R, Sánchez-Calvo B, Mata-Pérez C, Padilla MN, Corpas FJ, Barroso JB (2018) Nitric oxide buffering and conditional nitric oxide release in stress response. J Exp Bot 69:3425–3438. https://doi.org/10.1007/s10725-019-00543-w
Buysse J, Merckx R (1993) An improved colorimetric method to quantify sugar content of plant tissue. J Exp Bot 44:1627–1629. https://doi.org/10.1093/jxb/44.10.1627
Castello FD, Nejamkin A, Cassia R, Correa-Aragunde N, Fernández B, Foresi N, Lombardo C, Ramirez L, Lamattina L (2019) The era of nitric oxide in plant biology: twenty years tying up loose ends. J Exp Bot 85:17–27. https://doi.org/10.1016/j.niox.2019.01.013
Chen LS, Cheng LL (2003) Carbon assimilation and carbohydrate metabolism of “Concord” grape (Vitis labrusca L.) leaves in response to nitrogen supply. J Am Soc Hortic Sci 128:754–760. https://doi.org/10.21273/JASHS.128.5.0754
Corpas FJ, Barroso JB, Alfonso C, Miguel Q, León AM, Romero-Puertas MC, Esteban FJ, Raquel V, Palma JM, Sandalio LM (2004) Cellular and subcellular localization of endogenous nitric oxide in young and senescent pea plants. Plant Physiol 136:2722–2733. https://doi.org/10.1104/pp.104.042812
Corpas FJ, Río LAD, Palma JM (2019) Impact of nitric oxide (NO) on the ROS metabolism of peroxisomes. Plants (Basel) 8:37. https://doi.org/10.3390/plants8020037
Cui JX, Zhou YH, Ding JG, Xia XJ, Shi K, Chen SC, Asami T, Chen ZX, Yu JQ (2011) Role of nitric oxide in hydrogen peroxide-dependent induction of abiotic stress tolerance by brassinosteroids in cucumber. Plant Cell Environ 34:347–358. https://doi.org/10.1111/j.1365-3040.2010.02248.x
Dat J, Vandenabeele S, Vranova E, Montagu VM, Inze D, Breusegem VF (2000) Dual action of the active oxygen species during plant stress responses. Cell MolLife Sci 57:779–795. https://doi.org/10.1007/s000180050041
Diao QN, Song YJ, Shi DM, Qi HY (2017) Interaction of polyamines, abscisic acid, nitric oxide, and hydrogen peroxide under chilling stress in tomato (Lycopersicon esculentum Mill.) seedlings. Front Plant Sci. https://doi.org/10.3389/fpls.2017.00203
Dietz KJ (2008) Redox signal integration: from stimulus to networks and genes. Physiol Plant 133:459–468. https://doi.org/10.1111/j.1399-3054.2008.01120.x
Dong N, Li Y, Qi J, Chen Y, Hao Y (2018) Nitric oxide synthase-dependent nitric oxide production enhances chilling tolerance of walnut shoots in vitro via involvement chlorophyll fluorescence and other physiological parameter levels. Sci Hortic 230:68–77. https://doi.org/10.1016/j.scienta.2017.11.016
Ethier GJ, Livingston NJ (2004) On the need to incorporate sensitivity to CO2 transfer conduetance into the Farquhar-von Caemmerer-Berry leaf photosynthesis model. Plant Cell Environ 27:137–153. https://doi.org/10.1111/j.1365-3040.2004.01140.x
Fancy NN, Bahlmann AK, Loake GJ (2017) Nitric oxide function in plant abiotic stress. Plant Cell Environ 40:462–472. https://doi.org/10.1111/pce.12707
Fatma M, Khan NA (2014) Nitric oxide protects photosynthetic capacity inhibition by salinity in indian mustard. J Funct Environ Bot 4:106–116. https://doi.org/10.5958/2231-1750.2014.00009.2
Foyer CH, Halliwell B (1976) The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta 133:21–25. https://doi.org/10.1007/BF00386001
Foyer CH, Noctor G (2011) Ascorbate and glutathione: the heart of the redox hub. Plant Physiol 155:2–18. https://doi.org/10.1104/pp.110.167569
Griffith OW (1980) Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem 106:207–212. https://doi.org/10.1016/0003-2697(80)90139-6
Guo DL, Wang ZG, Li Q, Gu SC, Zhang GH, Yu YH (2019) Hydrogen peroxide treatment promotes early ripening of Kyoho grape. Aust J Grape Wine Res 25:357–362. https://doi.org/10.1111/ajgw.12399
Hajihashemil S, Noedoostl F, Geuns JMC, Djalovic L, Siddique KHM (2018) Effect of cold stress on photosynthetic traits, carbohydrates, morphology, and anatomy in nine cultivars of Stevia rebaudiana. Front Plant Sci. https://doi.org/10.3389/fpls.2018.01430
Hasan MK, Ahammed GJ, Sun S, Li M, Yin H, Zhou J (2019) Melatonin inhibits cadmium translocation and enhances plant tolerance by regulating sulfur uptake and assimilation in Solanum lycopersicum L. J Agric Food Chem 67:10563–10576. https://doi.org/10.1021/acs.jafc.9b02404
Health RL, Packer L (1968) Photoperoxidation in isolated chloroplast: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198. https://doi.org/10.1016/0003-9861(68)90654-1
Karimi R, Ershadi A (2015) Role of exogenous abscisic acid in adapting of ‘Sultana’ grapevine to low-temperature stress. Acta Physiol Plant 37:151. https://doi.org/10.1007/s11738-015-1902-z
Khairy AIH, Oh MJ, Lee SM, Kim DS, Roh KS (2016) Nitric oxide overcomes Cd and Cu toxicity in invitro-grown tobacco plants through increasing contents and activities of RuBisCO and RuBisCO activase. Biochimie Open 2:41–51. https://doi.org/10.1016/j.biopen.2016.02.002
Kolupaev Y, Karpets Y, Dmitriev A (2015) Signal mediators in plants in response to abiotic stress: calcium, reactive oxygen and nitrogen species. Cytol Genet 49:338–348. https://doi.org/10.3103/S0095452715050047
Kotapati KV, Palaka BK, Ampasala DR (2017) Alleviation of nickel toxicity in finger millet (Eleusine coracana L.) germinating seedlings by exogenous application of salicylic acid and nitric oxide. Crop Journal 5:240–250. https://doi.org/10.1016/j.cj.2016.09.002
Krapp A, Hofmann B, Schäfer C, Stitt M (1993) Regulation of the expression of rbcS and other photosynthetic genes by carbohydrates: a mechanism for the “sink regulation” of photosynthesis? Plant J 3:817–828. https://doi.org/10.1111/j.1365-313X.1993.00817.x
Law MY, Charles SA, Halliwell B (1983) Glutathione and ascorbic acid in spinach (Spinacea oleracea) chloroplasts. The effect of hydrogen peroxide and of paraquat. Biochem J 210:899–903. https://doi.org/10.1042/bj2100899
Li Q, Chen LS, Jiang HX, Tang N, Yang LT, Lin ZH, Li Y, Yang GH (2010) Effects of manganese-excess on CO2 assimilation, ribulose-1,5-bisphosphate carboxylase/oxygenase, carbohydrates and photosynthetic electron transport of leaves, and antioxidant systems of leaves and roots in Citrus grandis seedlings. BMC Plant Biol 10:42. https://doi.org/10.1186/1471-2229-10-42
Li X, Li Y, Ahammedd GJ, Zhang XN, Ying L, Zhang L, Yan P, Zhang LP, Li QY, Han WY (2019) RBOH1-dependent apoplastic H2O2 mediates epigallocatechin-3-gallateinduced abiotic stress tolerance in Solanum lycopersicum L. Environ Exp Bot 161:357–366. https://doi.org/10.1016/j.envexpbot.2018.11.013
Liao WB, Huang GB, Yu JH, Zhang ML, Shi XL (2011) Nitric oxide and hydrogen peroxide are involved in indole-3-butyric acid-induced adventitious root development in marigold. J Horticult Sci Biotechnol 86:159–165. https://doi.org/10.1080/14620316.2011.11512742
Lilley RM, Walker DA (1974) An improved spectrophotometric assay for ribulosebisphosphat carboxylase. Biochem Biophys Acta 358:226–229. https://doi.org/10.1016/0005-2744(74)90274-5
Liu XW, Liu B, Xue SD, Cai YL, Qi WZ, Jian C, Xu S, Wang T, Ren HZ (2016) Cucumber (Cucumis sativus L) nitric oxide synthase associated gene 1 (CsNOA1) plays a role in chilling stress. Front Plant Sci 7:1652. https://doi.org/10.3389/fpls.2016.01652
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Lowell CA, Tomlinson PT, Koch KE (1989) Sucrose-metabolizing enzymes in transport tissues and adjacent sink structures in developing citrus fruit. Plant Physiol 90:1394–1402. https://doi.org/10.1104/pp.90.4.1394
Ma YY, Huang DD, Chen CB, Zhu SH, Gao JG (2019) Regulation of ascorbate–glutathione cycle in peaches via nitric oxide treatment during cold storage. Sci Hortic 247:400–406. https://doi.org/10.1016/j.scienta.2018.12.039
Mazid M, Khan TA, Mohammad F (2011) Role of Nitric oxide in regulation of H2O2 mediating tolerance of plants to abiotic stress: a synergistic signaling approach. J Stress Physiol Biochem 7:34–74
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410. https://doi.org/10.1016/S1360-1385(02)02312-9
Mittler R (2017) ROS are good. Trends Plant Sci 22:11–19. https://doi.org/10.1016/j.tplants.2016.08.002
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880. https://doi.org/10.1093/oxfordjournals.pcp.a076232
Nidhi V, Santwana T, Vijay PS, Sheo MP (2020) Nitric oxide in plants: an ancient molecule with new tasks. Plant Growth Regul 90:1–13. https://doi.org/10.1007/s10725-019-00543-w
Petrov V, Hille J, Mueller-Roeber B, Gechev TS (2015) ROS-mediated abiotic stress-induced programmed cell death in plants. Front Plant Sci. https://doi.org/10.3389/fpls.2015.00069
Puyaubert J, Baudouin E (2014) New clues for a cold case: nitric oxide response to low temperature. Plant Cell Environ 37:2623–2630. https://doi.org/10.1111/pce.12329
Puyaubert J, Fares A, Rézé N, Peltier JB, Baudouin E (2014) Identification of endogenously S-nitrosylated proteins in Arabidopsis plantlets: effect of cold stress on cysteine nitrosylation level. Plant Sci 215–216:150–156. https://doi.org/10.1016/j.plantsci.2013.10.014
Qiao WH, Li CN, Fan LM (2014) Cross-talk between nitric oxide and hydrogen peroxide in plant responses to abiotic stresses. Environ Exp Bot 100:84–93. https://doi.org/10.1016/j.envexpbot.2013.12.014
Ruelland E, Vaultier MN, Zachowski A, Hurry V (2009) Cold signaling and cold acclimation in plants. Adv Bot Res 49:35–150. https://doi.org/10.1016/S0065-2296(08)00602-2
Rui HJ, Cao SF, Shang HT, Jin P, Wang KT, Zheng YH (2010) Effects of heat treatment on internal browning and membrane fatty acid in loquat fruit in response to chilling stress. J Sci Food Agric 90:1557–1561. https://doi.org/10.1002/jsfa.3993
Sami F, Faizan M, Faraz A, Siddiqui H, Yusuf M, Hayat S (2018) Nitric oxide-mediated integrative alterations in plant metabolism to confer abiotic stress tolerance, NO crosstalk with phytohormones and NO-mediated post translational modifications in modulating diverse plant stress. Nitric Oxide 73:22–38. https://doi.org/10.1016/j.niox.2017.12.005
Schaffer AA, Rylski I, Fogelman M (1989) Carbohydrate content and sucrose metabolism in developing Solanum muricatum fruits. Phytochemistry 28:737–739. https://doi.org/10.1016/0031-9422(89)80105-0
Sehar Z, Masood A, Khan NA (2019) Nitric oxide reverses glucose-mediated photosynthetic repression in wheat (Triticum aestivum L.) under salt stress. Environ Exp Bot 161:277–289. https://doi.org/10.1016/j.envexpbot.2019.01.010
Sharma A, Kapoor D, Wang J, Landi M, Zheng B, Yan D, Yuan H (2020a) Nitric oxide mediated mechanisms adopted by plants to cope with salinity. Biol Plant 64:512–518. https://doi.org/10.32615/bp.2020.070
Sharma A, Soares C, Sousa B, Martins M, Kumar V, Shahzad B, Sidhu GPS, Bali AS, Asgher M, Bhardwaj R, Thukral AK, Fidalgo F, Zheng B (2020b) Nitric oxide-mediated regulation of oxidative stress in plants under metal stress: a review on molecular and biochemical aspects. Physiol Plant 168:318–344. https://doi.org/10.1111/ppl.13004
Shi CY, Qi C, Ren HY, Huang AX, Hei SM, She XP (2015) Ethylene mediates brassinosteroid-induced stomatal closure via Gα protein-activated hydrogen peroxide and nitric oxide production in Arabidopsis. Plant J 82:280–301. https://doi.org/10.1111/tpj.12815
Shi J, Shi G, Tian Z (2016) Effect of exogenous hydrogen peroxide or ascorbic acid on senescence in cut flowers of tree peony (Paeonia suffruticosa Andr.). J Hortic Sci Biotechnol 90:689–694. https://doi.org/10.1080/14620316.2015.11668732
Shin H, Oh Y, Kim D (2015) Differences in cold hardiness, carbohydrates, dehydrins and related gene expressions under an experimental deacclimation and reacclimation in Prunus persica. Physiol Plant 154:485–499. https://doi.org/10.1111/ppl.12293
Si T, Wang X, Wu L, Zhao CZ, Zhang LN, Huang M, Cai J, Zhou Q, Dai TB, Zhu JK, Jiang D (2017) Nitric oxide and hydrogen peroxide mediate wounding-induced freezing tolerance through modifications in photosystem and antioxidant system in wheat. Front Plant Sci 8:17. https://doi.org/10.3389/fpls.2017.01284
Silverthorne J, Wimpee CF, Yamada T, Rolfe SA, Tobin EM (1990) Differential expression of individual genes encoding the small subunit of ribulose-1,5-bisphosphate carboxylase in Lemna gibba. Plant Mol Biol 15:49–58. https://doi.org/10.1007/BF00017723
Sun CL, Liu LJ, Lu LI, Jin CW, Lin XY (2018) Nitric oxide acts downstream of hydrogen peroxide in regulating aluminum-induced antioxidant defense that enhances aluminum resistance in wheat seedlings. Environ Exp Bot 145:95–103. https://doi.org/10.1016/j.envexpbot.2017.10.020
Tanou G, Job C, Belghazi M, Molassiotis A, Diamantidis G, Job D (2010) Proteomic signatures uncover hydrogen peroxide and nitric oxide cross-talk signaling network in citrus plants. J Proteome Res 9:5994–6006. https://doi.org/10.1021/pr100782h
Terron-Camero LC, Rodriguez-Serrano M, Sandalio LM, Romero-Puertas MC (2020) Nitric oxide is essential for cadmium-induced peroxule formation and peroxisome proliferation. Plant Cell Environ. https://doi.org/10.1111/pce.13855
Theocharis A, Clement C, Barka EA (2012) Physiological and molecular changes in plants grown at low temperatures. Planta 235:1091–1105. https://doi.org/10.1007/s00425-012-1641-y
Thordal CH, Zhang ZG, Wei YD, Collinge DB (1997) Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J 11:1187–1194. https://doi.org/10.1046/j.1365-313X.1997.11061187.x
Wang HH, Hou JH, Li Y, Zhang YH, Huang JH, Liang WH (2017) Nitric oxide-mediated cytosolic glucose-6-phosphate dehydrogenase is involved in aluminum toxicity of soybean under high aluminum concentration. Plant Soil 416:39–52. https://doi.org/10.1007/s11104-017-3197-x
Wang HZ, Zhang LH, Ma J, Li XY, Li Y, Zhang RP, Wang RQ (2010) Effects of water stress on reactive oxygen species generation and protection system in rice during grain-filling stage. Agric Sci China 9:633–641. https://doi.org/10.1016/s1671-2927(09)60138-3
Wang K, Shao XF, Gong YF, Zhu Y, Wang HF, Zhang XL, Yu DL, Yu F, Qiu ZY, Lu H (2013) The metabolism of soluble carbohydrates related to chilling injury in peach fruit exposed to cold stress. Postharvest Biol Technol 86:53–61. https://doi.org/10.1016/j.postharvbio.2013.06.020
Wu LB, Ueda Y, Lai SK, Frei M (2017) Shoot tolerance mechanisms to iron toxicity in rice (Oryza sativa L.). Plant Cell Environ 40:570–584. https://doi.org/10.1111/pce.12733
Yamdeu JHG, Gupta PH, Patel NJ, Shah AK, Talati JG (2016) Effect of storage temperature on carbohydrate metabolism and development of cold-induced sweetening in Indian potato (Solanum tuberosum L.) varieties. J Food Biochem 40:71–83. https://doi.org/10.1111/jfbc.12190
Yu F, Ni ZM, Shao XF, Yu LN, Liu HX, Xu F, Wang HF (2015) Differences in sucrose metabolism in peach fruit stored at chilling stress versus nonchilling stress temperatures. HortScience 50:1542–1548. https://doi.org/10.21273/HORTSCI.50.10.1542
Zhang GX, Liu YF, Ni Y, Meng ZJ, Lu T, Li TL (2014) Exogenous calcium alleviates low night temperature stress on the photosynthetic apparatus of tomato leaves. PLoS ONE. https://doi.org/10.1371/journal.pone.0097322
Zhang XN, Liao YWK, Wang XR, Zhang L, Ahammede GJ, Li QY, Li X (2020a) Epigallocatechin-3-gallate enhances tomato resistance to tobacco mosaic virus by modulating RBOH1-dependent H2O2 signaling. Plant Physiol Biochem 150:263–269. https://doi.org/10.1016/j.plaphy.2020.03.008
Zhang ZW, Wu P, Zhang WB, Yang ZF, Liu HY, Ahammed GJ, Cui JX (2020b) Calcium is involved in exogenous NO-induced enhancement of photosynthesis in cucumber (Cucumis sativus L.) seedlings under low temperature. Sci Hortic 261:108953. https://doi.org/10.1016/j.scienta.2019.108953
Zhao MG, Chen L, Zhang LL, Zhang WH (2009) Nitric reductase-dependent nitric oxide production is involved in cold acclimation and freezing tolerance in Arabidopsis. Plant Physiol 151:755–767. https://doi.org/10.1104/pp.109.140996
Zhou J, Wang J, Li X, Xia XJ, Zhou YH, Shi K, Chen ZX, Yu JQ (2014) H2O2 mediates the crosstalk of brassinosteroid and abscisic acid in tomato responses to heat and oxidative stresses. J Exp Bot 65:4371–4383. https://doi.org/10.1093/jxb/eru217
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant Numbers 31560571, 31160404 to Jinxia Cui); Natural Science Project of Shihezi University, Xinjiang, China (Grant Number ZZZc201616 to Jinxia Cui); and the High-level Talent Startup Project of Shihezi University, Xinjiang, China (Grant Number RCZX201213 to Jinxia Cui).
Author information
Authors and Affiliations
Contributions
This work was carried out in collaboration between all the authors. J.C., P.W., and C.X. defined the research theme and designed the experimental setup. P.W., C.X., B.H., W.Z. and Z.Y. performed the experiments. P.W. and C.X. analyzed the data, and interpreted the results. P.W. and C.X. wrote the initial draft of the manuscript. J.C., P.W., G.J.A., H.L. and H.C. revised the manuscript and edited it to present form. All authors have contributed to, read, and approved it for submission.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Pei Wu and Chunyan Xiao contributed equally to this study.
Rights and permissions
About this article
Cite this article
Wu, P., Xiao, C., Cui, J. et al. Nitric Oxide and Its Interaction with Hydrogen Peroxide Enhance Plant Tolerance to Low Temperatures by Improving the Efficiency of the Calvin Cycle and the Ascorbate–Glutathione Cycle in Cucumber Seedlings. J Plant Growth Regul 40, 2390–2408 (2021). https://doi.org/10.1007/s00344-020-10242-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-020-10242-w