Abstract
The increased frequency of heavy rains as a result of global climate change can lead to flooding and changes in light availability caused by the presence of thick clouds. To test the hypothesis that reduction in light availability can alleviate the harmful effects of soil flooding on photosynthesis, the authors studied the effects of soil flooding and acclimation from high to low light on the photosynthetic performance of Eugenia uniflora. Seedlings acclimated to full sunlight (about 35 mol m−2 d−1) for 5 months were transferred to partial sunlight (about 10 mol m−2 d−1) and were either subjected to soil flooding or not flooded. Chlorophyll fluorescence was measured throughout the flooding period and leaf gas exchange was measured 16 days after flooding was initiated. Minimal fluorescence yield (Fo) was significantly higher and the quantum efficiency of open PSII centres (Fv/Fm) was significantly lower in flooded than in non-flooded plants in full sunlight. Sixteen days after flooding was initiated, stomatal conductance (gssat) and net photosyntheses expressed on a leaf area (Asat-area), weight (Asat-wt) and chlorophyll (Asat-Chl) basis decreased in response to soil flooding. Flooding decreased stomatal conductance by similar amounts in full and partial sunlight, but Asat-area in partial and full sunlight was 3.4 and 16.8 times lower, respectively, in flooded than in non-flooded plants. These results indicate that changes from full to partial sunlight during soil flooding can alleviate the effects of flooding stress on photosynthesis in E. uniflora seedlings acclimated to full sunlight. The responses of photosynthesis in trees to flooding stress may be dependent on changes in light environment during heavy rains.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plants have different strategies to survive and grow in high or low light environments (Smith 1982; Valladares and Niinemets 2008). In general, leaves have great phenotypic plasticity in relation to the light environment and high capacity to acclimate to changes in light availability (Walters 2005; Valladares and Niinemets 2008). Leaves acclimated to high light are very different in terms of their physiology, anatomy and morphology than shade-acclimated leaves. In general, sun leaves have greater leaf weight per area and chlorophyll a/b ratio, lower chlorophyll content, less nitrogen allocated to the light-harvesting complexes and more nitrogen allocated to the enzymes of the Calvin cycle than shade leaves (Evans and Poorter 2001; Valladares and Niinemets 2008). Photosynthetic acclimation of shaded leaves to full sun, or vice versa, may involve physiological adjustments within hours, days or weeks, or structural changes within weeks or months (Bongers and Popma 1990; Walters 2005; Mielke and Schaffer 2010a).
Flooding is a major environmental stress to terrestrial plants in ecosystems prone to high rainfall or poor soil drainage, as well as in ecosystems susceptible to high water table fluctuations (Schaffer 1998; Pezeshki 2001; Kozlowski 2002; Kreuzwieser et al. 2004). Soil flooding induces oxygen deficiency and low soil redox potential which affect different aspects of plant physiology and development, such as premature leaf senescence and abscission, changes in leaf formation and expansion, suppression of root metabolism and growth and absorption of macronutrients (Pezeshki 2001; Kozlowski 2002; Kreuzwieser et al. 2004). Changes in CO2 assimilation is one of the first measurable responses of plants to soil flooding, which can be attributed to stomatal and non-stomatal limitations to photosynthesis (Mielke et al. 2003; Herrera et al. 2008). Studies have shown that the photosynthetic performance and growth of different plant species to soil flooding may be influenced by the interaction of flooding with light intensity (Wagner and Dreyer 1997; Gardiner and Krauss 2001; Lavinsky et al. 2007; Mielke and Schaffer 2010a, b).
In many tropical areas, high rainfall is accompanied by the presence of thick clouds, causing a large reduction in light available for CO2 assimilation and growth (Zotz and Winter 1994; Graham et al. 2003). In addition, in tropical and subtropical regions the increased frequency of heavy rains as a result of global climate change (Vera et al. 2006; Marengo et al. 2009) can lead to flooding (Michener et al. 1997; Milly et al. 2002; Hirabayashi et al. 2008) and changes in light availability caused by the presence of thick clouds. Thus, in ecosystems prone to soil flooding, it is probable that reductions in light caused by the presence of clouds can reduce the harmful effects of flooding on the photosynthetic performance of plants. Although there are many references on photosynthetic acclimation of plants transferred from shade to full sun (i.e., Bongers and Popma 1990; Evans and Poorter 2001; Krause et al. 2001; Houter and Pons 2005; Guo et al. 2006; Naramoto et al. 2006), and interactive effects of light environment and soil flooding (Wagner and Dreyer 1997; Gardiner and Krauss 2001; Lavinsky et al. 2007; Mielke and Schaffer 2010a, b), the authors are aware of no published studies on the effects of changes from high to low light on the physiological responses of plants subjected to soil flooding.
Eugenia uniflora L. (Myrtaceae) is a typical shrub or small tree species native to the Restingas (Margis et al. 2002), a marginal ecosystem of the Brazilian Atlantic Rainforest (Scarano 2002). Restingas occur along the coastline, including marshes, dry and swamp forests, and open clumped vegetation, where the occurrence of drought and soil flooding are often limiting factors for plant growth (Henriques et al. 1986). Studies have shown that E. uniflora is a high light demanding species and moderately sensitive to soil flooding (Mielke and Schaffer 2010a, b). Mielke and Schaffer (2010a) observed that the light availability during flooding and the pre-acclimation to different light intensities affected the physiological performance and growth of seedlings subjected to soil flooding. In an experiment in which E. uniflora plants cultivated in a shade house were transferred to full or partial sunlight and subjected to soil flooding, the photosynthetic apparatus of leaves developed in shade had a relatively limited capacity to acclimate to changes in light intensity (Mielke and Schaffer 2010b). In that experiment, both stomatal and non-stomatal limitations to photosynthesis were related to the low capacity of photosynthetic acclimation of flooded seedlings after transference from shade to full sun.
To test the hypothesis that changes in light availability, that can be caused by the presence of thick clouds, can alleviate the harmful effects of soil flooding on photosynthesis of trees during periods of heavy rains, we conducted an experiment aimed at analyzing the effects of soil flooding and acclimation from high to low light on chlorophyll fluorescence, leaf chlorophyll content and leaf gas exchange of E. uniflora seedlings.
Materials and methods
The experiment was conducted at the Tropical Research and Education Center, University of Florida (TREC/UF), Homestead, Florida, USA (25.5oN 80.5oW). In March 2008, E. uniflora L. seedlings were obtained from a commercial nursery located in Homestead, Florida, USA. Plants were cultivated in 10-l plastic containers (ten to twelve plants per container) with a standard nursery substrate of 65 pine bark, 25 Florida peat and 10% coarse sand by volume. Plants were carefully selected to obtain a uniform sample. The seedlings were about 1 year old at the time of transference to TREC/UF. At this stage, the average height of the plants per container was 0.5–0.8 m and the average stem diameter 0.10 m above the soil surface was 3–10 mm. In an open field at TREC/UF, 3 × 2 m (an area sufficient for ten containers) blocks were selected perpendicular to the daily track of the sun, permitting plants in full sunlight to receive almost all solar radiation during the day. Shade cages (3 × 2 × 1.7 m) were constructed with PVC tubes covered with one layer of a neutral shade netting (25–30% of full sunlight) and placed on four blocks. The remaining four blocks were left open (no cages). Cages were spaced far enough apart so that they did not shade the open blocks.
The experiment was arranged in a completely randomized design with a 2 × 2 factorial arrangement consisting of two light treatments (partial and full sunlight), two flood treatments (flooded and non-flooded) and four replications (blocks) per treatment. The containers were kept in full sunlight from March 2008 to October 2008. On 16 October 2008, half of the containers were transferred to the shade cages and half remained in full sunlight. Half of the plants in each light treatment were flooded and the remaining plants were maintained with soil water content near field capacity using an automatic irrigation system. Plants in the flooded treatment were flooded by placing each container in a 19-l plastic bucket filled with tap water to 50–100 mm above the soil surface to ensure complete inundation of the root system. To ensure that the irrigation system was sufficient to maintain the soil water content of non-flooded plants at field capacity, tensiometers (Soil Moisture Equipment Corp., Santa Barbara, California, USA) were installed in three containers in the full sun treatment.
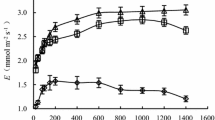
During the period of acclimation to full sunlight and throughout the experiment, the photosynthetic photon flux density (PPFD) was measured in each light treatment with model LI-190SA quantum sensors connected to LI-1000 data loggers (Li-Cor, Inc., Lincoln, Nebraska, USA). In full and partial sunlight treatments, air temperature (Ta) and relative humidity (RH) were monitored and recorded with Hobo H8 Pro Series dataloggers (Onset Computer, Bourne, Massachusetts, USA), and the vapour pressure deficit (VPD) calculated as described by Landsberg (1986). Total daily rainfall during the experiment was obtained from a weather station of the Florida Automated Weather Network (http://fawn.ifas.ufl.edu/) located 50 m from the experiment. The same averages of Ta and VPD for the partial and full sunlight treatments were reached during the flooding period, i.e., 22.1°C and 0.5 kPa, respectively. Total daily PPFD during plant acclimation was between 4.7 mol m−2 d−1 (18 August 2008) and 56.5 mol m−2 d−1 (13 May 2008). The average value of rainfall during the period of soil flooding was 0.1 mm. The maximum, minimum and average values of total daily PPFD were 11.4, 3.4 and 7.9 mol m−2 d−1 in partial sunlight and 41.0, 12.3 and 28.4 mol m−2 d−1 in full sunlight (Fig. 1). In this experiment the authors did not measure the soil redox potential (Eh), but in a parallel experiment (Mielke and Schaffer 2010b) the values of soil Eh decreased to −80 mV and remained close to this value in both partial and full sunlight treatments 1 week after the soil was initially flooded.
One, five and eleven days after seedlings were flooded, chlorophyll fluorescence was measured for two recently matured, fully-expanded leaves per container (one container per replication) with a portable fluorescence system (model OS-30, Opti-Sciences Inc., Hudson, New Hampshire, USA) between 12 and 13:00 h. Total daily and maximum instantaneous values of PPFD during days in which chlorophyll fluorescence was measured are presented in Table 1. Leaves were acclimated in the dark for 30 min prior to each chlorophyll fluorescence measurement. Sixteen days after seedlings were flooded chlorophyll fluorescence, leaf chlorophyll content, leaf gas exchange and leaf weight per area (LWA) were measured on one recently matured, fully-expanded leaf per container (one container per replication). Chlorophyll fluorescence was measured as previously described between 8 and 9:00 am. The minimal (Fo), maximum (Fm) and variable (Fv) fluorescence yields and the quantum efficiency of open PSII centres (Fv/Fm) were measured and calculated as described by Maxwell and Johnson (2000). After chlorophyll fluorescence measurements, leaf gas exchange variables were measured with a portable photosynthesis system (CIRAS-2, PP Systems, Amesbury, Massachusetts, USA). Prior to each measurement, leaves were acclimated to the environmental conditions inside the leaf chamber for about 20 min. The air flow rate, Ta, VPD, PPFD inside the leaf chamber and the reference CO2 concentration (Ca) during measurements were 200 ml min−1, 25°C, 1 kPa, 1000 μmol photons m−2 s−1 and 375 μmol mol−1, respectively. The value of PPFD used in this experiment was above the light saturation point for E. uniflora (Mielke and Schaffer 2010b). The light saturated net photosynthetic rate on a leaf area basis (Asat-area) and stomatal conductance to water vapour (gssat) were calculated using the values of CO2 and humidity variation inside the cuvette. Intrinsic water use efficiency (A/gs) was calculated by dividing Asat-area by gssat.
After leaf gas exchange measurements, total leaf chlorophyll content (Chl) was estimated with a SPAD meter (Model 502, Minolta Inc., Osaka, Japan). SPAD values were converted to total chlorophyll (Chl a + b) content using the equation derived by Mielke et al. (2010) for E. uniflora. Immediately after SPAD measurements, the leaves were collected and total leaf area and leaf dry weight were determined for each plant. Leaf area was determined with a leaf area meter (model LI-3000, Li-Cor Inc., Lincoln, Nebraska, USA) and leaf dry weight was obtained after oven-drying leaves at 75°C until a constant weight was reached. Leaf weight per area (LWA) was calculated by dividing the leaf dry weight by the leaf area. Light saturated net photosynthetic rates based on leaf weight (Asat-wt) and leaf chlorophyll (Asat-Chl) were calculated by dividing Asat-area by LWA and Chl a + b, respectively.
Interactions between light and flood treatments were analyzed by a two-way ANOVA. When interactive effects of light × flood treatments were observed, a non-paired T-test was used for comparisons of light effects within flood treatments and flood effects within light environments. When interactive effects of light × flood treatments were not observed, only comparisons between flood treatments within light environments were analyzed by a non-paired T-test.
Results
From the first to the fifth day after flooding was initiated there were non-significant differences in Fo and Fv/Fm among treatments (Table 2). Eleven days after flooding, the average values of Fo were significantly higher and the average Fv/Fm lower (P ≤ 0.05) in flooded plants in full sunlight compared with flooded plants in partial sunlight. Also, from the first to the eleventh day after flooding there was an increase of 102% in the average values of Fo and 39% decrease of Fv/Fm in the leaves of flooded plants in full sun. Sixteen days after transferring plants from full to partial sunlight and initiating flooding treatments (Table 1), there were significant differences between full and partial sunlight treatments for Fv/Fm (P ≤ 0.01) and significant effects of flooding on Fv/Fm (P ≤ 0.01) and Fo (P ≤ 0.05). The average Fv/Fm and was significantly higher in non-flooded than in flooded plants only in full sunlight. No significant difference (P > 0.05) was observed between Fo in flooded and non-flooded plants in partial sunlight, but a significant difference (P ≤ 0.05) for this variable was observed between flooded and non-flooded plants in full sunlight. In the full sunlight treatment, the average value of Fo was 35% higher in flooded than in non-flooded plants. The average values of Fv/Fm were, respectively, 1.1 and 1.3 times higher in non-flooded than in flooded plants in partial and in full sunlight.
Sixteen days after transferring plants from full to the partial sunlight and flooding (Table 3), there were significant differences between full and partial sunlight treatments for Chl a + b, gssat, Asat-area, Asat-wt (P ≤ 0.01) and A/gs (P ≤ 0.05) and significant effects of flooding on gssat, Asat-area, Asat-wt, Asat-Chl (P ≤ 0.01) and A/gs (P ≤ 0.05). There were significant interactions (P ≤ 0.01) between light and flooding treatments for A/gs. The average values of Chl a + b, gssat, Asat-area, Asat-wt and Asat-Chl were significantly higher (P ≤ 0.05) in non-flooded than in flooded plants in both full and partial sunlight; whereas A/gs was significantly higher in non-flooded than in flooded plants only in full sunlight. In the full sunlight treatment, the average A/gs was 72% higher in non-flooded than in flooded plants. In partial sunlight, the average Chl a + b, gssat, Asat-area, Asat-wt and Asat-Chl in non-flooded plants were, respectively, 1.1, 4.0, 3.4, 3.2 and 3.1 times higher than in flooded plants, whereas full sunlight the same differences were, respectively, 1.5, 4.3, 16.8, 21.0 and 14.0 times higher.
Discussion
In this experiment, transferring plants acclimated to full sun to partial sunlight was aimed at simulating changes in light availability due to the appearance of thick clouds during tropical storms or heavy rains. Thus, plants acclimated to full sunlight were subjected to continuous flooding and full and partial (30%) sunlight treatments for 16 days in an open field experiment. There are few references reporting changes in light availability caused by the presence of clouds on photosynthesis of tropical trees. In a seasonal rainforest in Panama, Graham et al. (2003) reported that monthly average values of PPFD during the wet season were about 70% of the values measured during the dry season and that changes in light availability was an important factor limiting CO2 assimilation and growth of trees during rainy season. However, considering the variations between clear and cloudy days, these differences can be much larger. On heavily clouded days, the total daily solar radiation can reach values at or lower than 30% of full sun. For example, during the acclimation of plants to full sunlight (data not shown), it was calculated a maximum total daily PPFD of 56.5 mol m−2 d−1 on 13 May 2008, when the total daily rainfall was 0 mm, and a minimum value of 4.7 mol m−2 d−1 on 18 August 2008, when the total daily rainfall was 68 mm; a percentage difference of about 92% (or 8% in relation to full sunlight).
Despite variations in the total daily values of PPFD in full sun during the experiment, on the first 3 days of chlorophyll fluorescence measurements PPFD values were within the range found in two previous studies conducted by Mielke and Schaffer (2010a, b). Moreover, the maximum daily values of PPFD in full and partial sunlight were, respectively, higher and lower than the values of the light saturation point for E. uniflora (Mielke and Schaffer 2010b).
Significant differences observed between flooded and non-flooded plants in full sunlight for Fo and Fv/Fm indicate that the leaves of flooded plants in full sunlight were more susceptible to the photoinhibition of photosynthesis (Baker 2008). Photoinhibition occurs when the rate of absorption of light energy by photosynthetic pigments exceeds the utilization rate in chloroplasts and several studies have shown the occurrence of synergistic effects between high light and other stress factors on photoinhibition of photosynthesis (Murata et al. 2007). Changes in Fv/Fm in plants subjected to soil flooding and high light have been reported by others (Lavinsky et al. 2007; Mielke and Schaffer 2010a). In this experiment, 11 and 16 days after transferring plants from full to the partial sunlight and the onset of flooding, significant differences in Fv/Fm and Fo between flooded and non-flooded plants in full sun were observed. On day 5, although differences were not significant between flooded and non-flooded treatments for Fv/Fm and Fo, those variables were, respectively, 31% lower and 35% higher in flooded than in non-flooded plants. Even though other complementary methods should be used to access the extension of photoinhibition in leaves (Logan et al. 2007), the changes in Fv/Fm and Fo indicated that flooding plants in full sunlight affected the flow of energy through the photosynthetic processes, probably making these plants susceptible to photoinhibition (Baker 2008).
Leaf weight per area is a very important morphological indicator of acclimation to changes in light availability and is often associated with plasticity in relation to light acclimation (Evans and Poorter 2001; Aranda et al. 2004; Valladares and Niinemets 2008). In general, sun leaves have higher LWA than shade leaves which is related to thicker leaves and/or reduced leaf area of sun leaves compared to shade leaves. The increased thickness of leaves is usually associated with anatomical changes, such as more photosynthetic cells per unit of leaf area (Evans and Poorter 2001) causing a higher photosynthetic capacity in sun than in shade leaves (Meir et al. 2008). In this experiment all photosynthetic measurements were done on leaves that had been grown in full sunlight and LWA did not vary substantially after transferring plants from full to partial sunlight. The average values of LWA varied between 109.6 and 123.4 g m−2 and were similar to values observed for leaves of E. uniflora acclimated to full sunlight and higher than values for leaves acclimated to partial sunlight in previous experiments conducted under similar conditions (Mielke and Schaffer 2010a, b). Thus, the differences among treatments cannot be explained by changes in leaf structure.
Leaf chlorophyll content is known to be higher in shade than in sun leaves (Valladares and Niinemets 2008). The estimated average Chl values in E. uniflora leaves acclimated to full sunlight were lower than those of leaves acclimated to partial sunlight (Mielke and Schaffer 2010b). The average leaf Chl a + b was approximately 32% higher in partial than in full sunlight. These results indicate that short-term acclimation to partial sunlight in E. uniflora leaves is related to an increase in chlorophyll content. Losses of chlorophyll and leaf chlorosis, in contrast, are stress symptoms observed in tropical or temperate tree species subjected to soil flooding (Gravatt and Kirby 1998; Gardiner and Krauss 2001; Oliveira and Joly 2010). Sixteen days after flooding, despite the 9% difference in leaf Chl a + b content between flooded and non-flooded plants in partial sunlight, a 47% difference was observed between flooded and non-flooded plants in full sunlight. In addition, the average Chl a + b value was 20% higher in flooded plants in partial sunlight than in non-flooded plants in full sunlight, indicating that changes from high to low light may alleviate the chlorophyll loss in plants subjected to soil flooding. Also, such differences may be related to the effects of soil flooding on the ability of leaf acclimation after transference from full to partial sunlight.
The values of Asat-area, Asat-wt and gssat in leaves of non-flooded plants in partial sunlight were similar to values obtained in two previous experiments in which E. uniflora plants were grown in the same shade cages used in this experiment (Mielke and Schaffer 2010a, b). In general, sun-acclimated leaves have higher Asat-area and gssat, and lower Asat-wt than shade-acclimated leaves (Valladares and Niinemets 2008). Interestingly, in this study there was an increase in Asat-area after the transference of plants from full to partial sunlight. It is possible that the increase in Asat-area may be related to increases in Chl and gssat, since Chl and gssat increased after plants were transferred from full to partial sunlight. The values of Asat-Chl are often higher in sun than in shade acclimated leaves (Pons and Anten 2004), which is related to increased activity of the photochemistry of photosynthesis at the expense of activity of the Calvin cycle (Pearcy 2000). The Asat-area, Asat-wt and Asat-Chl declined as a result of flooding treatments in both full and partial sunlight. Reductions in net CO2 assimilation and gssat are common responses of tree species to soil flooding (Gravatt and Kirby 1998; Pezeshki and DeLaune 1998; Nuñez-Elisea et al. 1999; Gardiner and Krauss 2001; Mielke et al. 2003; Lavinsky et al. 2007). In many flood-tolerant plant species, increases in A/gs due to stomatal limitation of photosynthesis are related to decreases in stomatal conductance associated with maintenance of high photosynthetic rates (Mielke et al. 2003; Lavinsky et al. 2007). The dramatic decrease in A/gs in flooded plants in full sunlight and the 17% increase in A/gs observed in flooded plants when compared with non flooded plants in partial sunlight 16 days after flooding indicate that stomatal limitation of photosynthesis occurred only in the plants transferred to the partial sunlight.
The non-significant difference between light treatments on day 16 for Fo may be related to the fact that chlorophyll fluorescence was measured in the early morning whereas on days 1, 5 and 11 the chlorophyll fluorescence was measured around midday. Also, the non-significant difference between light treatments on day 16 for Asat-Chl, at same time in which Asat-area and Asat-weight were significantly higher in partial than in full sunlight, could be related to the increase in chlorophyll content after the transference of the plants from full to partial sunlight. As discussed previously, shade leaves have higher leaf area, higher chlorophyll content and lower leaf weight per area than sun leaves (Valladares and Niinemets 2008). The rapid increase in chlorophyll content after the transference from full to partial sunlight can be interpreted as a mechanism of acclimation of the photosynthetic apparatus since the time after transference was not sufficient for the plants to produce new leaves morphologically acclimated to shade.
In addition to reporting photosynthetic variables, other longer-term studies of the effects of flooding in tropical trees have included growth responses (Davanso et al. 2002; Mielke et al. 2003; Lavinsky et al. 2007; Medina et al. 2009). In this study, the authors did not analyze the effects of flooding on plant growth because the duration of the study (about 2 weeks) was too short to expect sufficient plant growth to allow for comparisons between treatments. However, in a previous study in which E. uniflora trees were pre-acclimated to full and partial sunlight and flooded for 36 days, soil flooding caused significant decreases in plant growth (Mielke and Schaffer 2010). Thus, the long-term effects of flooding and decreases in light availability caused by occasional heavy rains warrants further investigation.
There are many references predicting increases in the frequency of heavy rains in tropical and subtropical regions as a result of global climate change (Vera et al. 2006; Marengo et al. 2009). Field studies have shown that cloud cover and changes in light intensity during rainy season limits net CO2 assimilation in sun leaves (Zotz and Winter 1994; Graham et al. 2003). Thus, the changes in light caused by the presence of thick clouds can be a disadvantage for CO2 assimilation and growth of trees. On the other hand, the increased frequency of heavy rains may be followed by an increase in the frequency of floods (Michener et al. 1997; Milly et al. 2002; Hirabayashi et al. 2008). In addition, the existence of interactive effects of light environment and soil flooding on photosynthesis and growth of temperate and tropical tree species has been reported by several authors (Wagner and Dreyer 1997; Gardiner and Krauss 2001; Lavinsky et al. 2007; Mielke and Schaffer 2010a, b). Despite some limitations in this experimental procedure, especially in relation to the continuous exposure of flooded plants to low and high light and the absence of a precise control of light intensity during the experiment, the results of chlorophyll fluorescence, leaf chlorophyll content and leaf gas exchange were sufficient to support the hypothesis that changes in light availability can alleviate the harmful effects of soil flooding on photosynthesis of E. uniflora leaves acclimated to full sun. To explore the extensiveness of the hypothesis tested in this study, additional studies should analyze the effects of changes in light availability and soil flooding at the whole plant and ecosystems levels. Furthermore, similar studies with other tree species should be conducted to analyze the impacts of changes in rainfall on the ecophysiological responses of cultivated and native species in areas prone to soil flooding.
In summary, these results indicate that changes in light availability during soil flooding can alleviate the effects of flooding stress on photosynthesis in E. uniflora seedlings acclimated to full sunlight, demonstrating that the responses of trees to flooding stress may be dependent on changes in light environment during heavy rains. Interactions between flooding stress and sun/shade acclimation on photosynthesis and growth of trees should be considered in studies aimed at predicting changes in the plant production and native vegetation distribution as a function of changes in rainfall associated with global climate change.
References
Aranda I, Pardo F, Gil L, Pardos JA (2004) Anatomical basis of the change in leaf mass per area and nitrogen investment with relative irradiance within the canopy of eight temperate tree species. Acta Oecol 25:187–195
Baker NR (2008) Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annu Rev Plant Biol 59:89–113
Bongers F, Popma J (1990) Leaf dynamics of seedlings of rain forest species in relation to canopy gaps. Oecologia 82:122–127
Davanso VM, Souza LA, Medri ME, Pimenta JA, Bianchini E (2002) Photosynthesis, growth and development of Tabebuia avellanedae Lor. ex Griseb. (Bignoniaceae) in flooded soil. Braz Arch Biol Technol 45:375–384
Evans JR, Poorter H (2001) Photosynthetic acclimation of plants to growth irradiance: the relative importance of specific leaf area and nitrogen partitioning in maximizing carbon gain. Plant Cell Environ 24:755–767
Gardiner ES, Krauss KW (2001) Photosynthetic light response of flooded cherrybark oak (Quercus pagoda) seedlings grown in two light regimes. Tree Physiol 21:1103–1111
Graham EA, Mulkey SS, Kitajima K, Phillips NG, Wright SJ (2003) Cloud cover limits net CO2 uptake and growth of a rainforest tree during tropical rainy seasons. Proc Nat Acad Sci 100:572–576
Gravatt DA, Kirby CJ (1998) Patterns of photosynthesis and starch allocation in seedlings of four bottomland hardwood tree species subjected to flooding. Tree Physiol 18:411–417
Guo XR, Cao KF, Xu ZF (2006) Acclimation to irradiance in seedlings of three tropical rain forest Garcinia species after simulated gap formation. Photosynthetica 44:193–201
Henriques RPB, Araujo DSD, Hay JD (1986) Descrição e classificação dos tipos de vegetação da restinga de Carapebus, Rio de Janeiro. Rev Bras Bot 9:173–189
Herrera A, Tezara W, Marín O, Rengifo E (2008) Stomatal and non-stomatal limitations of photosynthesis in trees of a tropical seasonally flooded forest. Physiol Plant 134:41–48
Hirabayashi Y, Kanae S, Emori S, Oki T, Kimoto M (2008) Global projections of changing risks of floods and droughts in a changing climate. Hydrolog Sci J 53:754–772
Houter NC, Pons TL (2005) Gap size effects on photoinhibition in understorey saplings in tropical rainforest. Plant Ecol 179:43–51
Kozlowski TT (2002) Physiological-ecological impacts of flooding on riparian forest ecosystems. Wetlands 22:550–561
Krause GH, Koroleva OY, Dalling JW, Winter K (2001) Acclimation of tropical tree seedlings to excessive light in simulated tree-fall gaps. Plant Cell Environ 24:1345–1352
Kreuzwieser J, Papadopoulou E, Rennenberg H (2004) Interaction of flooding with carbon metabolism of forest trees. Plant Biol 6:299–306
Landsberg JJ (1986) Physiological ecology of forest production. Academic, London
Lavinsky AO, Sant’Ana CS, Mielke MS, Almeida A-AF, Gomes FP, França S, Silva DC (2007) Effects of light availability and soil flooding on growth and photosynthetic characteristics of Genipa americana L. seedlings. New For 34:41–50
Logan BA, Adams WW III, Demmig-Adams B (2007) Avoiding common pitfalls of chlorophyll fluorescence analysis under field conditions. Funct Plant Biol 34:853–859
Marengo JA, Jones R, Alves LM, Valverde MC (2009) Future change of temperature and precipitation extremes in South America as derived from the PRECIS regional climate modeling system. Int J Climatol 29:2241–2255
Margis R, Felix D, Caldas JF, Salgueiro F, De Araujo DSD, Breyne P, Van Montagu M, De Oliveira D, Margis-Pinheiro M (2002) Genetic differentiation among three neighboring Brazilcherry (Eugenia uniflora L.) populations within the Brazilian Atlantic rain forest. Biodivers Conserv 11:149–163
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence: a practical guide. J Exp Bot 51:659–668
Medina CL, Sanches MC, Tucci MLS, Sousa CAF, Cuzzuol GRF, Joly CA (2009) Erythrina speciosa (Leguminosae-Papilionoideae) under soil water saturation: morphophysiological and growth responses. Ann Bot 104:671–680
Meir P, Kruijt B, Broadmeadow M, Barbosa E, Kull O, Carswell F, Nobre A, Jarvis PG (2008) Acclimation of photosynthetic capacity to irradiance in tree canopies in relation to leaf nitrogen concentration and leaf mass per unit area. Plant Cell Environ 25:343–357
Michener WK, Blood ER, Bildstein KL, Brinson MM, Gardner LR (1997) Climate change, hurricanes and tropical storms, and rising sea level in coastal wetlands. Ecol Appl 7:770–801
Mielke MS, Schaffer B (2010a) Leaf gas exchange, chlorophyll fluorescence and pigment indexes of Eugenia uniflora L. in response to changes in light intensity and soil flooding. Tree Physiol 30:45–55
Mielke MS, Schaffer B (2010b) Photosynthetic and growth responses of Eugenia uniflora L. seedlings to soil flooding and light intensity. Environ Exp Bot 68:113–121
Mielke MS, Almeida A-AF, Gomes FP, Aguilar AG, Mangabeira PAO (2003) Leaf gas exchange, chlorophyll fluorescence and growth responses of Genipa americana seedlings to soil flooding. Environ Exp Bot 50:221–231
Mielke, Schaffer B, Li C (2010) Use of a SPAD meter to estimate chlorophyll content in Eugenia uniflora L. leaves as affected by contrasting light environments and soil flooding. Photosynthetica 48(3):332–338
Milly PCD, Wetherald RT, Dunne KA, Delworth TL (2002) Increasing risk of great floods in a changing climate. Nature 415:514–517
Murata N, Takakashi S, Nishiyama Y, Alakhverdiev SI (2007) Photoinhibition of photosystem II under environmental stress. Bioch Biophys Acta 1767:414–421
Naramoto M, Katahata S-I, Mukai Y, Kakubari Y (2006) Photosynthetic acclimation and photoinhibition on exposure to high light in shade-developed leaves of Fagus crenata seedlings. Flora 201:120–126
Nuñez-Elisea R, Schaffer B, Fisher JB, Colls AM, Crane JH (1999) Influence of flooding on net CO2 assimilation, growth and stem anatomy of Annona species. Ann Bot 84:771–780
Oliveira VC, Joly CA (2010) Flooding tolerance of Calophyllum brasiliense Camb. (Clusiaceae): morphological, physiological and growth responses. Trees 24:185–193
Pearcy RW (2000) Acclimation to sun and shade. In: Raghavendra AS (ed) Photosynthesis. A Comprehensive Treatise. Cambridge University, Cambridge, pp 19–263
Pezeshki SR (2001) Wetland plant responses to soil flooding. Env Exp Bot 46:299–312
Pezeshki SR, DeLaune RD (1998) Responses of seedlings of selected woody species to soil oxidation-reduction conditions. Env Exp Bot 40:123–133
Pons TL, Anten NPR (2004) Is plasticity in partitioning of photosynthetic resources between and within leaves important for whole-plant carbon gain in canopies? Funct Ecol 18:802–811
Scarano FR (2002) Structure, function and floristic relationships of plant communities in stressful habitats marginal to the Brazilian Atlantic Rainforest. Ann Bot 90:517–524
Schaffer B (1998) Flooding responses and water-use efficiency of subtropical and tropical fruit trees in an environmentally-sensitive wetland. Ann Bot 81:475–481
Smith H (1982) Light quality, photoperception, and plant strategy. Ann Rev Plant Physiol 33:481–518
Valladares F, Niinemets U (2008) Partial sunlight tolerance, a key plant feature of complex nature and consequences. Annu Rev Ecol Evol Syst 39:237–257
Vera C, Silvestri G, Liebmann B, González P (2006) Climate change scenarios for seasonal precipitation in South America from IPCC-AR4 models. Geophys Res Lett. doi:10.1029/2006GL025759
Wagner PA, Dreyer R (1997) Interactive effects of waterlogging and irradiance on the photosynthetic performance of seedlings from three oak species displaying different sensitivities (Quercus robur, Q. petraea and Q. rubra). Ann Sci For 54:409–429
Walters RG (2005) Towards an understanding of photosynthesis acclimation. J Exp Bot 56:435–447
Zotz G, Winter K (1994) Photosynthesis of a tropical canopy tree, Ceiba pentandra, in a lowland tropical forest in Panama. Tree Physiol 14:1291–1301
Acknowledgments
The authors gratefully thank Chunfang Li, S. Michael Gutierrez, Manny Soto, Stella Grinberg Mielke and Henrique Grinberg Mielke for assisting with the experiment installation, maintenance and data collection. Marcelo S. Mielke also thanks Capes (Brazilian Higher Education Council) for a grant to support his postdoctoral work at the Tropical Research and Education Center, University of Florida, USA.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R. Aroca.
Rights and permissions
About this article
Cite this article
Mielke, M.S., Schaffer, B. Effects of soil flooding and changes in light intensity on photosynthesis of Eugenia uniflora L. seedlings. Acta Physiol Plant 33, 1661–1668 (2011). https://doi.org/10.1007/s11738-010-0702-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-010-0702-8