Abstract
A comparative evaluation of As accumulation and subsequent effects upon exposure to arsenite [As(III)] was performed in three species of Ocimum. Plants accumulated high amount of As (μg g−1 dry weight; dw) (662 in O. tenuiflorum, 764 in O. basilicum and 831 in O. gratissimum at 100 μM As(III) after 10 days) with the order of accumulation being roots > stem > leaves. A significant reduction in plant height and biomass was observed. However, essential oil yield and major oil constituents, such as eugenol, methyl chevicol, and linalool, increased at lower As(III) concentrations [mostly up to 25 μM As(III)] in all three species. Positively, no detectable amount of As was found in oil of any species. The study proposes that Ocimum may be used as a phytoremediator and at the same time as a source of essential oils under proper regulation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arsenic is a ubiquitous and an extremely toxic metalloid. It is of environmental and health concern due to its known chronic and epidemic toxicity (Hossain 2006; Jomova et al. 2011). The WHO guideline for permissible limit of As in drinking water is 10 μg L−1 (Meharg and Raab 2004) and maximum tolerable weekly intake limit of As is 15 μg kg−1 body weight day−1 (Saper et al. 2008). However, As exposure affects millions of people worldwide through drinking water and food having higher than safe level of As particularly in Bangladesh, Vietnam, India, and China (Mukherjee et al. 2006). Arsenic exposure has been linked with various types of cancer, cardiovascular diseases, diabetes, neurological disorders, dermal effects, genotoxicity, and chromosomal aberrations (Tsai et al. 2003; Jomova et al. 2011).
Remediating As-contaminated soil and groundwater using currently available engineering methods is costly and difficult. This led to the development of environmentally friendly and cost-effective plant-based remediation technology namely phytoremediation. Earlier studies have suggested that some essential aromatic and medicinal crops might be capable of accumulating heavy metals from contaminated soil (Scora and Chang 1997; Zheljazkov et al. 2008), suggesting that such plants could be used in the phytoremediation of contaminated soils. However, the effect of these contaminants on essential oil crops is not well known. Many of the medicinal herbs usually grow as weed in the wastelands receiving contaminated municipal and industrial wastewater, which may have high metal concentrations. The medicinal plants constitute a large group of plants (both lower and higher) providing raw material for the use in drug formulation and related industries. If such plants are either naturally grown or cultivated in metal-contaminated regions, there is a danger that the heavy metal accumulation by plants of medicinal value may cause serious health hazards to patients using metal adulterated herbal drugs (Rai et al. 2004). There are reports on heavy metal accumulation by some essential oil yielding and other medicinal plants (Arpadjan et al. 2008). The contamination of heavy metals in market samples of some plant-based drugs has also been reported (Rai et al. 2001). Hence, it becomes necessary that medicinal plants are first tested for metal contamination before exploiting them for medicinal uses. Very recently, it has been reported that traditional Chinese herbal products, deliberately fortified with As for therapeutic purposes, may represent a serious health hazard (Martena et al. 2010). In a recent study, native plants collected from West Bengal including some medicinal plants were found to contain As higher than the permissible limits of 1 mg kg−1 dw (Tripathi et al. 2012). This study clearly suggests that naturally growing medicinal plants are accumulating higher than safe limit of As in their tissues that is alarming to human health.
The genus Ocimum, a member of Lamiaceae family, is ranked high among some of the herbs with medicinal potentialities. Among the various Ocimum species, O. tenuiflorum, O. basilicum and O. gratissimum are widely distributed. These plants have great medicinal value, such as antiseptic, antispasmodic, antibacterial, and insect repellent properties (Gupta et al. 2002; Anand et al. 2011). These are also commercially cultivated for essential oil production in India and abroad, which constitutes some highly valuable compounds, such as methyl chevicol, linalool, eugenol, 1,8‐cineole, methyl eugenol, and camphor (Keita et al. 2000). Family Lamiaceae has been reported as hyperaccumulator of Co (Sharma 2011). Lately, metal accumulation properties of Ocimum have been explored (Rai et al. 2004; Zheljazkov et al. 2006; Chaiyarat et al. 2011) and possible use of plants for phytoremediation purposes has been suggested. However, at the same time, this warns us about the commercial exploitation of plants, growing in contaminated areas, for medicinal purposes (Saper et al. 2008). The present study was planned to analyze the As accumulation potential of Ocimum species and subsequent impacts on growth, biomass, essential oil yield, and essential oil constituents. This is imperative considering medicinal uses of Ocimum and associated threats to human health. In contrast, if oil does not contain As, these plants may be suitable for utilization, under proper guidance and control, in phytoremediation projects and may still provide some economical benefits (in terms of oil yield).
Materials and methods
Plant material and treatment conditions
Seeds of test plants (O. tenuiflorum, O. basilicum and O. gratissimum) were obtained from CSIR—Central Institute of Medicinal and Aromatic Plants, Lucknow, India. Seeds were grown in plastic pots (12 cm diameter) filled with acid washed sand (0.01 M HCl) placed in glasshouse receiving normal light and dark conditions, temperature, and humidity. These pots were irrigated regularly with nutrient solution based on the Long Ashton formula (Hewitt 1966). The composition of supplied nutrient solution was 4 mM KNO3, 4 mM Ca(NO3)2, 2 mM MgSO4, 1.5 mM NaH2PO4, 0.1 mM NaCl, 100 μM Fe-EDTA, 30 μM H3BO3, 10 μM MnSO4, 1 μM CuSO4, 1 μM ZnSO4, 0.2 μM Na2MoO4, 0.1 μM NiSO4 and 0.1 μM CoSO4. After 15 days, seedlings showing improper growth pattern were removed and ten plants of approximately same height were allowed to grow till 40 days. At 40 days, five plants of same height for a species (15–20 cm, as three species had different growth) were treated with different concentrations of As(III) (up to 100 μM) prepared using NaAsO2 (J. T. Baker, UK) for a period of 10 days. Experiments were set up in triplicate. After harvesting, plants were washed with double distilled water, blotted to remove water and then separated into leaves, stem, and roots for the analysis of various parameters.
Determination of arsenic
For analyzing the level of absorbed As, plants were initially washed with ice-cold Milli-Q water to remove the adsorbed As followed by drying to constant weight at 80 °C for 2 days in a hot air oven. Samples were prepared and analyzed by following the method of Bleekar et al. (2003). Dried and powdered plant material (100 mg) was digested in 2 mL of 37 % (v/v) HCl: 65 % (v/v) HNO3 (1:4) at 140 °C for 7 h and diluted with 10 mL of Milli-Q water. Arsenic concentrations were determined using an atomic absorption spectrophotometer via hydride generation (Perkin-Elmer, Analyst 200). The absorption wavelength for As was 193.7 nm and detection limit was 0.001 ppm.
Plant growth parameters
Growth parameters included measurement of plant height and dry biomass. Plant height was measured using a metric scale. Dry biomass of As-treated and control plants was recorded after drying the plants to achieve constant weight at 80 °C for 2 days in a hot air oven.
Analysis of essential oil
Extraction of essential oils
Essential oil content was determined by method of Langenau (1948). A sample of 100 g of fresh leaves and aerial plant parts was subjected to hydrodistillation in Clevenger type apparatus for 4 h. The oil was collected in glass vials, dried over anhydrous sodium sulphate and stored at 4 °C until analysis. The oil concentration in the leaves is expressed on percentage basis (mL of oil obtained from 100 g of fresh leaves and aerial plant parts). The oil constituents were quantified using Gas Chromatograph equipped with Mass Spectrometry (GC–MS) (Perkin Elmer, model 3920 B Series, Mass Selective Detector, equipped with a cross linked methyl silicone gum phase capillary column, 25 m × 0.32 mm). Helium was used as the carrier gas at a flow rate of 1 mL min−1. The temperature programming was set with initial oven temperature at 40 °C and held for 3 min and the final temperature of the oven was 280 °C with the rate of increase in temperature being 10 °C min−1. The injector and source temperatures were 210 °C. Total run time for a sample was 45 min. The injection volume was 0.06 μl neat and split ratio was 1:30. MS were taken at 70 eV with an EI source with mass range of m/z 40–500. The identification of compounds was done on the basis of retention time, retention indices (relative to n-alkane, C9-C24), MS Library search (NIST & WILEY) and by comparing mass spectra with the MS literature data (Adams 1995).
Quantification of arsenic in essential oil samples
Oil samples (5 mL), obtained from control and As(III)-treated samples, were reduced to ash at 400 °C for 4 h. After cooling, samples were digested in 2 mL of HCl/HNO3 as mentioned above till the white fumes appeared. After digestion, the volume was made up to 10 mL with 1 % HNO3 (Lobinski and Adams 1993) and As concentrations were determined as described earlier.
Statistical analyses
Experiments were performed in a complete randomized block design involving five treatments and two durations. A two-way analysis of variance was performed to confirm the validity of the data except for essential oil data. Comparison of means of control and different treatments was done by Duncan’s multiple range test.
Results
Arsenic accumulation
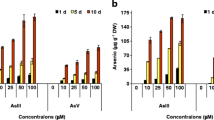
The accumulation of As was found to be concentration- and duration-dependent phenomenon and was in the order of roots > stem > leaves (Fig. 1a–c). The maximum accumulation of As was observed at 100 μM As(III) after 10 days of treatment in all three Ocimum species. In O. tenuiflorum, the maximum accumulation was 662 μg As g−1 dry weight; dw (whole plant) of which about 62 % was retained in roots while about 22 % and 16 % of the total As was translocated to stem and leaves, respectively. In O. basilicum, total accumulation was 764 μg g−1 dw of which 58 % was retained in roots and about 23 and 19 % of the total As was translocated to stem and leaves, respectively. In O. gratissimum, total accumulation was 831 μg g−1 dw of which 54 % was retained in roots and about 24 and 22 % of the total As was translocated to stem and leaves, respectively. The maximum As was accumulated by O. gratissimum followed by O. basilicum and O. tenuiflorum. Further, O. gratissimum also showed a greater tendency to translocate As to above-ground shoot and leaf parts in comparison to O. tenuiflorum and O. basilicum.
Arsenic accumulation in root (a), stem (b) and leaves (c) of O. tenuiflorum, O. basilicum and O. gratissimum after 5 and 10 days of exposure to different concentrations of arsenite. Values represent the mean of three technical and five biological replicates. Different letters indicate significant difference between means for a particular Ocimum spp. (DMRT, P < 0.05)
Effect of arsenic on plant growth
Arsenic uptake by Ocimum species significantly (ANOVA, P < 0.05) affected the growth of the plant, which was reflected by the decrease in height and biomass (Table 1) in dose- and duration-dependent manner. However, after exposure to 10 μM As(III), there was slight enhancement in height and biomass up to 5 days of treatment in all three Ocimum species compared to control. The maximum decrease in height and biomass was 56 and 58 %, respectively, after 10 days at 100 μM As(III) in O. tenuiflorum followed by O. basilicum (47 and 49 %) and O. gratissimum (42 and 43 %) compared to control.
Effect of arsenic on essential oil of Ocimum species
Essential oil yield
Essential oil content increased at lower concentrations [10 and 25 μM As(III)] in comparison to control in O. tenuiflorum and O. basilicum, thereafter it decreased significantly (Table 2). In O. gratissimum, oil yield increased in treatments of 50 μM As(III) or less compared to control. The maximum increase in oil content was at 10 μM As(III) in O. tenuiflorum (23 %) while at 25 μM As(III) in O. basilicum (29 %) and O. gratissimum (41 %) in comparison to control. The maximum decrease in oil yield at 100 μM As(III) was in order O. tenuiflorum (55 %) > O. basilicum (48 %) > O. gratissimum (40 %) compared to control.
Eugenol, methyl eugenol, β-caryophyllene, and β-ocimene were the major and common chemical constituents identified in three Ocimum species. Besides, carvacrol identified in O. tenuiflorum, linalool in O. basilicum and 1,8-cineole and germacrene–D in O. gratissimum were other important chemical constituents identified in essential oil. In three species, the maximum increase in most of the chemical constituents was observed at 25 μM As(III) compared to control. The maximum loss in essential oil constituents at 100 μM As(III) was observed in O. tenuiflorum and the least in O. gratissimum.
In essential oil of O. tenuiflorum, the main constituents were eugenol, methyl eugenol, and methyl chevicol (Table 3; Fig. 2a). Eugenol and carvacrol were found to increase in treatment of 50 μM As(III) or less compared to control with the maximum increases being at 25 μM As(III) (44 %) and 10 μM As(III) (22 %), respectively. Methyl eugenol and methyl chevicol also increased up to 25 μM As(III). β-Caryophyllene showed maximum level at 25 μM As(III) (24 % more than control), while β-ocimene showed increase only at 10 μM As(III) (13 %). The maximum decline in all constituents was observed at 100 μM As(III) (eugenol 37 %, methyl eugenol 21 %, methyl chevicol 22.8 %, β-caryophyllene 42 %, β-ocimene 51 %, and carvacrol 20 %).
Total ion chromatogram (TIC) of essential oils of O. tenuiflorum (a), O. basilicum (b) and O. gratissimum (c) analyzed by gas chromatography mass spectrometry (GC–MS) in plants exposed to different concentrations of arsenite (0, 25, 100 μM). For O. tenuiflorum, peaks denote 1 β-ocimene, 2 β-caryophyllene, 3 eugenol, 4 methyl eugenol, 5 methyl chevicol, 6 carvacrol. For O. basilicum, peaks denote 1 linalool, 2 β-ocimene, 3 β-caryophyllene, 4 eugenol, 5 methyl eugenol, 6 methyl chevicol. For O. gratissimum, peaks denote 1 1,8-cineole, 2 β-ocimene, 3 β-caryophyllene, 4 germacrene 5 eugenol, 6 methyl eugenol
In essential oil of O. basilicum, all major chemical constituents namely eugenol, methyl eugenol, β-caryophyllene, and β-ocimene showed their maximum level at 25 μM As(III) (eugenol 25 %, methyl eugenol 20 %, β-caryophyllene 28 %, and β-ocimene 29 % higher than control) (Table 4; Fig. 2b). The maximum decrease of 40 % in eugenol, 23 % in methyl eugenol, 42 % in β-caryophyllene and 31 % in β-ocimene compared to control was observed at 100 μM As(III). Linalool and methyl chevicol increased up to 50 μM As(III); however, the maximum increase occurred at 25 μM As(III) in both chemical constituents (31 and 39 %, respectively).
In essential oil of O. gratissimum, eugenol, β-ocimene, germacrene-D, and 1,8-cineole were increased up to 50 μM As(III) with the maximum increase occurring at 25 μM As(III) (eugenol 48 %, β-ocimene 38 %, germacrene-D 32 %, and 1,8-cineole 27 %) (Table 5; Fig. 2c). The maximum decrease occurred at 100 μM As(III) in all chemical constituents (eugenol 31 %, β-ocimene 22 %, germacrene-D 25 %, and 1,8-cineole 22 %) in comparison to control. Methyl eugenol and β-caryophyllene increased up to 25 μM As(III) and declined thereafter. Methyl eugenol increased by 29 % and β-caryophyllene by 16 % at 25 μM As(III), while the maximum decline of 19 % in methyl eugenol and 28 % in β-caryophyllene was noticed at 100 μM As(III).
Concentration of arsenic in essential oil
No detectable amount of As was found in oil samples, which indicates that metalloid was not removed from the tissues during the process of steam distillation.
Discussion
In India, Ocimum plants are collected from their natural habitats throughout the country. Among these areas, some are highly As contaminated, such as West Bengal, Sahebgunj district of Jharkhand, Bhojpur district of Bihar, Dhemaji and Karimganj districts of Assam, Rajnandgaon district of Chattisgarh, and Balia district of Uttar Pradesh (Mondal et al. 2006). Thus, it is imperative to study As accumulation potential of these plants as they appear to possess tolerance to As. In this study, significant accumulation of As was observed in three species of Ocimum in the order of O. gratissimum > O. basilicum > O. tenuiflorum (Fig. 1a–c). The accumulation of significant amount of As might be due to several reasons. Arsenite is taken up through specific channels known as aquaglyceroporinsas a neutral molecule (Ali et al. 2009) and suffers no competition with any other major nutrient and this might be the reason for the rapid uptake and considerable accumulation of As(III). Well developed and branched tap root system of Ocimum species and high solubility of As(III) in water might be other reasons contributing to significant accumulation of As in plants (Sharma 2011). In most of the plants, As translocation is generally low, as observed in this study, due to the immobilization of As through efficient chelation and subsequent compartmentalization in vacuoles (Mishra et al. 2011). Angelova et al. (2007) reported that maximum part of Pb, Cd, and Zn is retained by the roots of O. basilicum and only a small quantity moves to the surface portion. Other studies on Ocimum with heavy metals also showed more accumulation in roots than in leaves, such as Cr in O. tenuiflorum (Rai et al. 2004) and Cd/Zn in O. gratissimum (Chaiyarat et al. 2011). Ocimum is an easily harvestable plant and can be completely uprooted. Thus, the total accumulation of As observed in this study puts forward its potential for the phytoremediation efforts. Earlier studies also suggested the use of Ocimum species for remediation purposes. Seeds of O. basilicum show significant uptake of 137Cs and 90Sr (Chakraborty et al. 2007) and potential for biosorbtion of Cr (Melo and D’Souza 2004).
However, a good potential for metal accumulation also indicates towards possible risks associated with the indiscriminative use of Ocimum for medicinal purposes. Traditionally, leaves of Ocimum species are taken as herbal tea, fresh leaves, and dried powder. Its root, stem, and seeds are also used as household medicine or used for making crude extract. We calculated probable daily ingestion of As on the basis of As accumulation observed in this study and considering that plants from contaminated sites are used. This analysis speculates that if 2.5–3 g dry powder (as recommended dose of some commercial products based on Ocimum) or crude extract of leaves or plant parts (as practiced at household level) are consumed, the As load would exceed the 150 μg As day−1 threshold for a 70 kg adult set by Food and Agricultural Organization/World Health Organization Joint Expert Committee on Food Additives (Saper et al. 2008). However, the As exposure concentrations used in this study are very high, while in natural soil solutions the levels of As are generally low. The calculation, therefore, only highlights that care should be taken for using Ocimum plants for traditional or commercial purposes.
Arsenic uptake by Ocimum species significantly (ANOVA, P < 0.05) affected the growth of the plant, which may be attributed to impaired uptake of nutrients like P, Cu, Mn, Fe, etc. (Dwivedi et al. 2010). Arsenic is known to interfere with the functioning of enzymes of metabolic pathways viz., those of carbohydrate and nitrogen metabolism, which may result in impaired growth and reduced biomass (Jha and Dubey 2004; Singh et al. 2009). Essential oil content increased at lower concentrations in comparison to control up to 25 μM in O. tenuiflorum and O. basilicum and up to 50 μM As(III) in O. gratissimum. Such an increase in oil content might be attributed to a decline in the primary metabolites due to the effects of heavy metal stress, causing intermediary products to become available for secondary metabolite synthesis (Morales et al. 1993). Stancheva et al. (2009) also reported an increase in oil yield in Salvia officinalis as a result of heavy metal stress. The decrease in essential oil content at higher concentrations is attributable to increasing toxicity and more negative impact to metabolism of plants. Reduced oil content relative to the control in O. tenuiflorum and O. basilicum at higher concentrations but not in O. gratissimum (where it increased up to 50 μM) indicates that elevated concentration of metals in growth medium variably affected essential oil content of the three species. Zheljazkov et al. (2006) reported a reduction in essential oil content of O. basilicum and Anethum graveolens relative to the control as a result of Cd, Pb, and Cu toxicity and suggested that elevated concentration of metals in growth medium affected essential oil content in some aromatic species but not in others. GC analyses of the essential oils indicated some variation in chemical constituents of all three species of Ocimum, however, with no clear trend. The accumulation of As induced some constituents, such as eugenol, methyl chevicol, linalool, 1,8-cineole, and germacrene-D up to 50 μM in treated plant compared to control, which might be part of defense strategy adapted by plants against As toxicity to protect themselves by formation of secondary metabolites (Trease and Evans 1989). Rai et al. (2004) reported induced level of eugenol in O. tenuiflorum under Cr stress. An increase in the levels of linalool and α-terpineol in basil with the application of high Cu compost has been reported by Zheljazkov and Warman (2003). Variation observed with respect to alteration of chemical constituents of essential oil and accumulation of metalloid in three Ocimum species might be due to environmental and genetic factors that influence genetic expression (Bernath 1986).
Our analysis of As in oil samples revealed that As was not present in detectable quantities, which indicates that metalloid was not removed from the tissues during the process of steam distillation. Immobilization of As through efficient chelation and subsequent compartmentalization in vacuoles might also be a reason for this. Zheljazkov et al. (2006) also reported that no detectable amount of Cd, Cu, or Pb in essential oils of any of the three species A. graveolens, Menthax piperita, and O. basilicum was found. These results confirm the understanding that high metal concentrations in the growth medium may increase metal accumulation in plant tissue, but not in the essential oil, which is the final marketable product (Scora and Chang 1997).
Conclusion
It may be concluded from the present study that Ocimum species can be grown in As-affected sites with the perspective of remediation as they can accumulate high amount of As in their plant parts and further whole plants can be easily uprooted. Interestingly, As-stress induced the production of essential oil and the level of major essential oil constituents at lower concentrations. Our results demonstrate that As was not removed from the tissues during the process of steam distillation; hence, essential oil, the final commercial product, is free from As. Thus, the use of Ocimum for phytoremediation would give dual benefits in terms of clean up of site and economic benefits as oil yield. However, As accumulation potential is alarming as well from the point of their consumption for medicinal purposes. Hence, the use of Ocimum for phytoremediation should be practiced under strict regulation. Present study also concluded that O. gratissimum is the best candidate for phytoremediation followed by O. basilicum and O. tenuiflorum.
Author contribution
F. Siddiqui and S.K. Krishna conducted all experiments and analyzed data. P.K. Tandon conceptualized and planned the study. F. Siddiqui and S. Srivastava contributed in preparing the final manuscript.
References
Adams RP (1995) Identification of essential oil components by gas chromatography/mass spectrometry. Allured Publishing Corporation, Carol Stream
Ali W, Isayenkov SV, Zhao F-J, Maathuis FJM (2009) Arsenite transport in plants. Cell Mol Life Sci 66:2329–2339
Anand AK, Mohan M, Haider SZ, Sharma A (2011) Essential oil composition and antimicrobial activity of three Ocimum species from Uttarakhand (India). Int J Pharm Sci 3:223–225
Angelova V, Ivanova R, Ivanov K (2007) Heavy metals uptake by plants from family Lamiaceae growing in the polluted soils. Geophys Res Abstr 9:05206
Arpadjan S, Celik G, Taskesen S, Gucer S (2008) Arsenic, cadmium and lead in medicinal herbs and their fractionation. Food Chem Toxicol 46:2871–2875
Bernath J (1986) Production ecology of secondary plant products. In: Craker LE, Simon J (eds) Herbs, spices, and medicinal plants: recent advances in botany, horticulture, and pharmacology, vol 1. Oryx Press, AZ, pp 185–234
Bleeker PM, Schat H, Vooijs R, Verkleij JAC, Ernst WHO (2003) Mechanisms of arsenate tolerance in Cytisus striatus. New Phytol 157:33–38
Chaiyarat R, Rujira S, Narupot P, Maleeya K, Prayad P (2011) Effects of soil amendments on growth and metal uptake by Ocimum gratissimum grown in Cd/Zn-contaminated soil. Water Air Soil Pollut 214:383–392
Chakraborty D, Maji S, Bandyopadhyay A, Basu S (2007) Biosorption of cesium-137 and strontium-90 by mucilaginous seeds of Ocimum basilicum. Bioresour Technol 98:2949–2952
Dwivedi S, Tripathi RD, Srivastava S, Singh R, Kumar A, Tripathi P, Dave R, Rai UN, Chakrabarty D, Trivedi PK, Tuli R, Adhikari B, Bag MK (2010) Arsenic affects mineral nutrients in grains of various Indian rice (Oryza sativa L.) genotypes grown on arsenic-contaminated soils of West Bengal. Protoplasma 245:113–124
Gupta SK, Prakash J, Srivastava S (2002) Validation of traditional claim of Tulsi, Ocimum sanctum Linn. as a medicinal plant. Indian J Exp Biol 40:765–773
Hewitt EJ (1966) Sand and water culture method used in the study of plant nutrition. Common Wealth Agric, Bureau, 2nd edn. England
Hossain MF (2006) Arsenic contamination in Bangladesh—an overview. Agric Ecosyst Environ 113:1–16
Jha AB, Dubey RS (2004) Carbohydrate metabolism in growing rice seedlings under arsenic toxicity. J Plant Physiol 161:867–872
Jomova K, Jenisova Z, Feszterova M, Baros S, Liska J, Hudecova D, Rhodes CJ, Valkoc M (2011) Arsenic: toxicity, oxidative stress and human disease. J Appl Toxicol 31:95–107
Keita SM, Vincent C, Schmit J, Belanger A (2000) Essential oil composition of Ocimum basilicum L., O. gratissimum L. and O. suave L. in the Republic of Guinea. Flavour Frag J 15:339–341
Langenau IEE (1948) The examination and analysis of essential oils, synthetics and isolates. In: Guenther E (ed) The essential oil. Huntington, vol I. Krieger Publishing Co., New York, pp 227–348
Lobinski R, Adams FC (1993) Recent advances in speciation analysis by capillary gas chromatography—microwave induced plasma atomic emission spectrometry. Trends Anal Chem 12:41–49
Martena MJ, Van Der Wielen JC, Rietjens IM, Klerx WN, De Groot HN, Konings EJ (2010) Monitoring of mercury, arsenic, and lead in traditional Asian herbal preparations on the Dutch market and estimation of associated risks. Food Addit Contam A 27:190–205
Meharg AA, Raab A (2004) Getting to the bottom of arsenic standards and guidelines. Environ Sci Technol 44:4395–4399
Melo JS, D’Souza SF (2004) Removal of chromium by mucilaginous seeds of Ocimum basilicum. Bioresour Technol 92:151–155
Mishra S, Srivastava S, Dwivedi S, Tripathi RD (2011) Investigation of biochemical responses of Bacopa monnieri L. upon erxposure to arsenate. Environ Toxicol. doi:10.1002/tox.20733
Mondal P, Majumder CB, Mohanty B (2006) Laboratory based approaches for arsenic remediation from contaminated water: recent developments. J Hazard Mater 137:464–479
Morales C, Cissudo RMS, Palazon J, Bonfill M (1993) Response of Digitalis purpurea plants to temporary salinity. J Plant Nutr 16:327–335
Mukherjee A, Sengupta MK, Hossain A, Ahamed S, Das B, Nayak B, Lodh D, Rahman MM, Chakraborti D (2006) Arsenic contamination in groundwater: a global perspective with emphasis on the Asian scenario. J Health Popul Nutr 24:142–163
Rai V, Kakkar P, Khatoon S, Rawat AKS, Mehrotra S (2001) Heavy metal accumulation in some herbal drugs. Pharm Biol 39:384–387
Rai V, Vajpayee P, Singh SN, Mehrotra S (2004) Effect of chromium accumulation on photosynthetic pigments, oxidative stress defense system, nitrate reduction, proline level and eugenol content of Ocimum tenuiflorum L. Plant Sci 167:1159–1169
Saper RB, Phillips RS, Sehgal A, Khouri N, Davis RB, Paquin J, Thuppil V, Kales SN (2008) Lead, mercury, and arsenic in US and Indian-manufactured Ayurvedic medicines sold via the Internet. J Am Med Assoc 300:915–923
Scora RW, Chang AC (1997) Essential oil quality and heavy metal concentrations of peppermint grown on a municipal sludge-amended soil. J Environ Qual 26:975–979
Sharma H (2011) Metal hyperaccumulation in plants: a review focusing on phytoremediation in plants. J Environ Sci Technol 4:118–138
Singh N, Ma LQ, Vu JC, Raj A (2009) Effects of arsenic on nitrate metabolism in arsenic hyperaccumulating and non-hyperaccumulating ferns. Environ Pollut 157:2300–2305
Stancheva I, Geneva M, Hristozkova M, Boychinova M, Markovska Y (2009) Essential oil variation of salvia officinalis (L.), grown on heavy metals polluted soil. Biotechnol Biotechnol Equip 23:373–376
Trease GE, Evans GE (1989) Text book of pharmacognosy, 2nd edn. Bailliera Tindall, London
Tripathi P, Dwivedi S, Mishra A, Kumar A, Dave R, Srivastava S, Shukla MK, Srivastava PK, Chakrabarty D, Trivedi PK, Tripathi RD (2012) Arsenic accumulation in native plants of West Bengal, India: prospects for phytoremediation but concerns with the use of medicinal plants. Environ Monit Assess 184:2617–2631
Tsai S, Chou H, The H, Chen CM, Chen CJ (2003) The effects of chronic arsenic exposure from drinking water on the neurobehavioral development in adolescence. Neurotoxicology 24:747–753
Zheljazkov VD, Warman PR (2003) Application of high Cu compost to Swiss chard and basil. Sci Total Environ 302:13–26
Zheljazkov VD, Craker LE, Xing B (2006) Effects of Cd, Pb, and Cu on growth and essential oil contents in dill, peppermint, and basil. Environ Exp Bot 58:9–16
Zheljazkov VD, Jeliazkova EA, Kovacheva N, Dzhurmanski A (2008) Metal uptake by medicinal plant species grown in soils contaminated by a smelter. Environ Exp Bot 64:207–216
Acknowledgments
The authors are thankful to the University of Lucknow, Lucknow for the facilities provided. FS and SKK are grateful to Council of Scientific and Industrial Research (CSIR), New Delhi, India for the award of Junior Research Fellowship (JRF).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by B. Zheng.
Rights and permissions
About this article
Cite this article
Siddiqui, F., Krishna, S.K., Tandon, P.K. et al. Arsenic accumulation in Ocimum spp. and its effect on growth and oil constituents. Acta Physiol Plant 35, 1071–1079 (2013). https://doi.org/10.1007/s11738-012-1145-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-012-1145-1