Abstract
The present study was performed to investigate the regulatory role of selenium (Se) in the antioxidant defense system, Na+ uptake, and essential oil (EO) production of Stachys byzantine (S. byzantine) under salt stress. For this purpose, a greenhouse study was conducted in a factorial experiment based on a randomized complete design with three replications. The studied factors included foliar-applied Se (0, 4, 8, and 16 mg L−1) and salinity (0, 30, 60, and 90 mM NaCl). Malondialdehyde, H2O2, electrolyte leakage, oxidized glutathione (GSSG), ascorbate peroxidase (APX), catalase (CAT), and superoxide dismutase (SOD) significantly decreased by increasing salinity. Conversely, the chlorophyll content and glutathione (GSH), as well as monodehydroascorbate reductase (MDHAR), dehydroascorbate reductase (DHAR), and glutathione reductase (GR) activities, represented noticeable decreases. However, Se supplementation alleviated the oxidative stress by activating some enzymes involved in the ascorbate–glutathione (AsA-GSH) cycle, including APX, DHAR, MDHAR, and GR activities, which further enhanced the activities of CAT and SOD. Exposition to Se also modulated the redox state by reducing GSSG while enhancing AsA and GSH contents when compared to non-Se supplemented salt-stressed S. byzantine plants. Mild salinity stress (30 mM NaCl), especially when combined with Se treatments, resulted in the highest production of the EO of S. byzantine. The findings of this study suggest the use of Se treatment as an efficient method for increasing the resistance of S. byzantine to salinity by reducing the damage to oxidative stress, activating antioxidant enzymes, modulating the redox state, and finally, improving the production of EOs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Increasing soil salinity has become an extremely destructive environmental agent that is rapidly spreading around the globe and restricting plant growth and production, the negative effects of which are exacerbated by water shortages (Gupta et al. 2018; Fariduddin et al. 2019). More than 45 million hectares of arable lands, which make up about 20% of the world’s land, are destroyed by salt stress, and 1.5 million hectares of agricultural lands are annually turned into barren lands due to salinity (Pitman and Lauchli 2002). Salinity stress negatively affects plant physiology by reducing soil osmotic potential, disrupts ionic balance and water potential, and causes oxidative stress, which in turn leads to the generation of reactive oxygen species (ROS) in crop cells (Wani et al. 2018). Accordingly, oxidative stress destroys lipids in the cell membrane and breaks down proteins, nucleic acids, and photosynthetic pigments within the cell (Abdel Latef et al. 2018; Zaid and Wani 2019). Thus, maintaining high antioxidant capacity and photosynthetic capacity should be considered for continuing plant growth close to normal growth conditions when exposed to salinity stress to increase plant resistance to salinity (Diao et al. 2014). Previous research (Feng et al. 2013) has reported success in inducing this resistance by the foliar application of some elements such as selenium (Se). Undoubtedly, Se is vital to animals and humans and seems to be beneficial for plants as well (Feng et al. 2013; Khademi Astaneh et al. 2018). Although the role of Se as a beneficial element in plants is still not well understood and the line between toxicity and usefulness of this element is narrow, the use of Se in low concentrations could be beneficial for plant cell growth and metabolism (Diao et al. 2014). It has been reported that Se may play a dual role in plants that at low concentrations it can protect plants from the negative effects of environmental stresses, but when applied at high concentrations, it has a toxic role for plants (Gupta and Gupta 2017; Hasanuzzaman et al. 2020a, b). The antioxidant properties of Se have been recently reported as a highly important antioxidant in plant defense systems when exposed to salinity and drought stresses (Habibi 2017; Jiang et al. 2017; Karimi et al. 2020). The application of Se in salt-stressed parsley (Petroselinum crispum L.) has been found to prevent the Na+ uptake by the plants, improving the efficiency of photosystem II and ultimately increasing the plant yield (Habibi 2017). Abul-Soud and Abd-Elrahman (2016) demonstrated that the foliar application of Se resulted in improving the growth and yield characteristics of plant crops compared to its soil application. However, the role of Se in improving photosynthesis and the antioxidant defense system of medicinal plants under salinity is still not extremely clear. Furthermore, there are no reports of Stachys byzantine defensive responses to saline conditions.
The genus Stachys, which belongs to the Lamiaceae family, includes 34 species in Iran, and Stachys byzantine (S. byzantine) is one of the most important and rare species of this genus (Mozaffarian 1996). Similar to other plant crops, medicinal plants such as S. byzantine, which are in charge of treating many human diseases, are also influenced by abiotic stresses and the accumulation of the EO in these plants is strongly correlated with environmental conditions (Selmar and Kleinwachter 2013; Wink 2015; Zaid et al. 2020a, b). Therefore, S. byzantine was selected as a rare medicinal plant while emphasizing whether the use of sodium selenite (Na2SeO3) as a successful strategy to increase crop yield and improve salt tolerance in a sustainable agricultural system can be used. Due to the novelty of this important and rare species of medicinal plant and rare species of medicinal plant, and insufficient information regarding Se application in medicinal plants under salinity stress, this study aimed to evaluate the application of Na2SeO3 and different salinity levels on photosynthesis, Na+ accumulation, oxidative stress, antioxidant system, and EO production of S. byzantine C. Koch.
2 Materials and Methods
2.1 Plant Material, Growth Conditions, and Treatments
Forty-five-day seedlings of S. byzantine C. Koch were purchased from a commercial nursery (ZARRINGIAH Company, Urmia, Iran). This research project was implemented as a greenhouse study in the Department of Agricultural Extension and Education, Higher Education Center, Shahid Bakeri Miyandoab, Urmia University, Urmia, Iran, at a temperature of 10/15 °C, night/day, and relative humidity of 70% under a soilless culture system. In February 2019, the seedlings were transmitted to plastic pots filled with 1 kg of perlite by planting two seedlings in each pot. The pots were arranged in a factorial experiment in the form of a completely randomized design with three replications and irrigated with 500 ml of Hoagland’s modified solution every day. Se treatments, including various levels (0, 4, 8, and 16 mg L−1) of Na2SeO3 selected based on the experiment of Karimi et al. (2020), were started immediately after the emergence of the fourth fully developed leaf in growing seedlings. Each pot containing two seedlings was sprayed with 100 ml of each of the prepared solutions according to the abovementioned concentrations. To ensure that the Se solution is sprayed with a high percentage of absorption by the leaf tissue, two drops of Twin 20 were added per 100 ml of solution. Na2SeO4 solutions were applied by a small hand-sprayer equipped with a pump two times a week for 42 days. It is noteworthy that spraying with distilled water was only intended for the foliar application of control plants without Se treatment. Salinity stress was imposed by supplementing the nutrient solution with various levels of NaCl (0, 30, 60, and 90 mM) after 10 days of spraying with Na2SeO4. The concentrations of NaCl were also selected based on the experiments of Karimi et al. (2020). After 30 days of salinity treatments (Fig. 1), fresh samples of the leaf were collected to measure photosynthesis, oxidative stress, and antioxidant enzymes. The leaves and roots of S. byzantine were desiccated at 70 °C and then completely crushed before measuring the concentration of Na+. The extraction and production calculation of the EO in S. byzantine were achieved using 120-day-old plants (from seed germination to the full bloom stage).
2.2 Chlorophyll Content, Fv/Fm Ratio, and Proline Content
To measure chlorophyll content, fully developed upper leaves were used according to the experimental method of Arnon (1949). A fluorometer (Walz, Effeltrich, Germany) was applied to measure chlorophyll fluorescence, and the method of Bates et al. (1973) was employed to estimate proline content.
2.3 Oxidative Stress Biomarkers Including Electrolyte Leakage, Malondialdehyde, and Hydrogen Peroxide
A conductivity meter was applied to assess electrolyte leakage (EL) according to Ozden et al. (2009), and the accumulations of malondialdehyde (MDA) and H2O2 content were evaluated according to Wang et al. (2009) and Velikova and Loreto (2005), respectively.
2.4 Estimation of Enzymatic Antioxidant Activities and the Ascorbate–Glutathione Cycle
Leaves, which were immediately separated from S. byzantine seedlings, were mixed in a mortar completely homogeneously by adding 1 mL of ice-cold 100 mM potassium phosphate buffer (pH 7.0) containing 1% of polyvinyl pyrrolidone. The homogeneous mixture was centrifuged at 11500 g for 30 min at 4 °C, and its supernatant was used to assay different enzyme activities.
AsA, GSH, and oxidized glutathione (GSSG) contents were measured according to the procedure described by Hasanuzzaman et al. (2017), followed by measuring ascorbate peroxidase (APX, 1.11.1.11) activity according to Nakano and Asada (1981). Moreover, monodehydroascorbate reductase (MDHAR, 1.6.5.4) activity was assessed according to the experimental procedure reported by Hossain et al. (2010). Additionally, the activities of dehydroascorbate reductase (DHAR, 1.8.5.1) and glutathione reductase (GR, 1.6.4.2) were determined using the methods of Nakano and Asada (1981) and Hasanuzzaman et al. (2011), respectively. Eventually, the activities of CAT (CAT: 1.11.1.6) and superoxide dismutase (SOD, EC1.15.1.1) were determined following the methods described by Hasanuzzaman et al. (2011) and Cavalcanti et al. (2004), respectively.
2.5 Determination of Na+ Concentration in Leaves and Roots
Dried samples in an oven at 60 °C for 48 h were used to determine the leaf and root concentrations of Na+. The root and leaf samples (1 g) were crushed using an electric grinder and then completely reduced to ashes at 560 °C. To digest the ashes, 10 mL of HCl (1 mol·L−1) was added, followed by determining the concentration of Na+ by an atomic absorption instrument (Model AAS- 6300- Shimadzu).
2.6 EO Content and Production Measurement
The EO of S. byzantine was extracted and calculated according to the experimental method of Fernandes et al. (2013), and the collected plant materials (the whole plant with flower) were left at 30 °C as long as the weight changes remained constant. After grinding the dried plant materials, 50 g was picked up and maintained in the freezer at − 20 °C until distillation initiation. A Clevenger-type apparatus was applied to extract the EO. Finally, anhydrous sodium sulfate was used and then weighed to remove the water from the EO.
2.7 Statistical Analysis
The analyzed data were represented by calculating the mean with the standard deviation (± SD) so that each data was the result of an average of three replications. The analysis of variance (ANOVA) of the data and the least significant difference test (LSD, P ≤ 0.05) for the comparison of means were performed by the statistical analysis system software, version 9. All evaluated traits in S. byzantine plants subjected to salt stress and Se were analyzed by the principal component analysis using the program Statgraphics Centurion, version 16.
3 Results
3.1 Chlorophyll Content, Fv/Fm Ratio, and Proline Content in Response to Salinity and Se Application
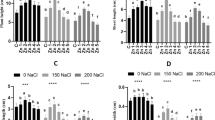
As shown in Table 1, the effects of salinity and Se on chlorophyll content, Fv/Fm ratio, and proline content of S. byzantine are significant. However, there was no significant effect of salinity × Se on chlorophyll and Fv/Fm except for proline content, on which the effect of salinity × Se was significant. Foliar applied Se significantly improved chlorophyll content and Fv/Fm ratio under both saline and non-saline conditions (Figs. 2A, B). When no salinity stress was imposed, the content of chlorophyll and the Fv/Fm ratio increased by 7.23, 22.67, and 23.97%, as well as 12.99, 18.29, and 12.99% at 2, 8, and 16 mg L−1 Se compared to the treatment without Se (0 mg L−1), respectively. In the most severe salinity stress (90 mM NaCl), the chlorophyll content and Fv/Fm ratio of stressed S. byzantine plants reduced 88.31 and 89.55% compared to those grown under normal conditions, respectively (Figs. 2A, B). However, Se supplementation noticeably mitigated the ruinous effects of salinity on chlorophyll content and Fv/Fm ratio; as in the most severe salinity stress (90 mM NaCl), 16 mg L−1 Se heightened the respective attributes by 62.70 and 66.67% over salinized plants. No significant variation in proline content was noted in non-stressed plants at any of the examined Se levels (Fig. 2C). Contrarily, the imposition of NaCl stress on S. byzantine seedlings significantly increased proline content by 28.71, 53.75, and 70.06% at 30, 60, and 90 mM NaCl, respectively, versus control plants (Fig. 2C). Interestingly, Se supplementation caused further increases in the proline accumulation of salt-affected plants. In other words, in the most severe salinity stress (90 mM NaCl), proline content increased by 18.26, 24.46, and 22.14% at 4, 8, and 16 mg L−1 Se in the most severe salinity stress (90 mM NaCl), respectively, relative to those of salt-affected plants (Fig. 2C).
Effect of different levels of salinity (S) and Se (Na2 SeO3) on chlorophyll content (A), Fv/Fm ratio (B), and proline content (C) of seedlings in S. byzantine C. Koch. S1, S2, S3, and S4 are NaCl concentrations at 0, 30, 60, and 90 mM, respectively. Bars within a chart followed by the same letter are not significantly different at the 5% probability level by the LSD test. Values represent the means of three replications ± standard deviations
3.2 Oxidative damage, EL, and ROS Accumulation Under Salinity and Se Application
The obtained data showed the significant impacts of salinity, Se, and salinity × Se on the EL of S. byzantine (Table 1). Under non-salinity stress conditions, EL, MDA, and H2O2 reduced by 11.61, 4.88, and 20.59%, as well as 12.74, 8.29, and 14.32% in seedlings treated with 4 mg L−1 Se and 8 mg L−1 Se, respectively. In addition, the abovementioned parameters decreased by 1.45, 10.73, and 12.66% in seedlings sprayed with 16 mg L−1 Se (Fig. 3A, B, C). Plant exposition to salinity caused an increase in EL, H2O2, and MDA levels depending on the applied dose with the utmost increase of 89.56, 76.73, and 75.65%, respectively, revealed in S. byzantine plants that were exposed to 90 mM NaCl compared with those of the control plants (Fig. 3A, B, C). It is noteworthy that foliar-applied Se significantly mitigated the destructive effect of salinity by 8.11, 17.14, and 16.97% reductions in EL, H2O2, and MDA, respectively, with the highest level (16 mg L−1) of Se under salt stress conditions induced by the highest level (90 mM) of NaCl in comparison with 90 mM salt-affected plants (Fig. 3A, B, C).
Effect of different levels of salinity (S) and Se (Na2 SeO3) on electrolyte leakage (A), MDA accumulation (B), and H2O2 generation (C) of seedlings in S. byzantine C. Koch. S1, S2, S3, and S4 are NaCl concentrations at 0, 30, 60, and 90 mM, respectively. Bars within a chart followed by the same letter are not significantly different at the 5% probability level by the LSD test. Values represent the means of three replications ± standard deviations
3.3 Non-enzymatic Antioxidant Defense System in Response to Salinity and Se
The amounts of ASA, GSH, GSSG, and GSH/GSSG ratios of S. byzantine were noticeably influenced by salinity and Se. However, the effect of salinity × Se was significant except for ASA which was not significant at all (Table 1).
Under non-saline conditions, no noticeable changes were observed in ASA, GSH, and GSSG contents as well as the GSH/GSSG ratio of S. byzantine in response to foliar Se application (Fig. 4A, B, C, D). In addition to significantly reducing the amount of ASA, GSH, and GSH/GSSG, salinity stress also significantly increased the amount of GSSG. At 90 mM NaCl, the contents of ASA and GSH, as well as the GSH/GSSG ratio, reduced by 43.75, 54.85, and 77.37%, respectively, while the amount of GSSG increased by 49.67%, relative to those of non-salt stressed plants (Figs. 4 A, B, C, D). Foliar application with Se significantly reduced the negative effect of salinity on ASA and GSH contents, while the superior impacts were recorded with 8 mg L−1 Se. Based on the findings, Se at 8 mg L−1 increased the ASA content, GSH content, and the GSH/GSSG ratio by 8.78, 18.27, and 34.6% while decreased the GSSG content by 45.4% (at 90 mM NaCl), respectively, in comparison with those of 90 mM NaCl plants (Figs. 4A, B, C, D).
Effect of different levels of salinity (S) and Se (Na2 SeO3) on ASA content (A), GSH content (B), GSSG content (C), and GSH/GSSG ratio (D) of seedlings in S. byzantine C. Koch. S1, S2, S3, and S4 are NaCl concentrations at 0, 30, 60, and 90 mM, respectively. Bars within a chart followed by the same letter are not significantly different at the 5% probability level by the LSD test. Values represent the means of three replications ± standard deviations
3.4 Enzymatic Antioxidant Defense System in Response to Salinity and Se
The data shown in Table 2 indicate the significant effects of salinity, Se, and salinity × Se on DHAR and MDHAR, GR, CAT, SOD, and APX activities of S. byzantine. When no salinity was imposed, no significant changes were found in DHAR and MDHAR, GR, CAT, SOD, and APX activities in S. byzantine in response to foliar Se application (Figs. 5A, B; Figs. 6A, B, C, D). Upon exposure of S. byzantine plants to salinity, the alterations in the activity of each of the studied enzymes in this experiment relied on the type of enzyme with the maximum decrease of 45.90, 64.12, and 70.98% in DHAR, MDHAR, and GR activities, respectively. On the other hand, the maximum increase of 90, 58.43, and 85.12% was recorded in CAT, SOD, and APX activities, respectively (at 90 Mm NaCl), relative to those of the non-stressed control S. byzantine plants (Figs. 5A, B; Figs. 6A, B, C, D). However, Se boosted the antioxidant defense system of salt-affected S. byzantine plants by further enhancing the activity of CAT, SOD, and APX and preventing the reduction of DHAR, MDHAR, and GR activities (Figs. 5A, B; Figs. 6A, B, C, D). The activities of DHAR, MDHAR, GR, CAT, SOD, and APX increased by 16.91, 18.40, 38.90, 35.67, 14.02, and 26.85% in Se-sprayed plants grown under the most severe salinity stress (90 mM NaCl) compared with those of 90 mM NaCl plants, respectively.
Effect of different levels of salinity (S) and Se (Na2 SeO3) on DHAR (A) and MDHAR (B) activities of seedlings in S. byzantine C. Koch. S1, S2, S3, and S4 are NaCl concentrations at 0, 30, 60, and 90 mM, respectively. Bars within a chart followed by the same letter are not significantly different at the 5% probability level by the LSD test. Values represent the means of three replications ± standard deviations
Effect of different levels of salinity (S) and Se (Na2 SeO3) on GR (A), CAT (B), SOD (C), and APX (D) activities of seedlings in S. byzantine C. Koch. S1, S2, S3, and S4 are NaCl concentrations at 0, 30, 60, and 90 mM, respectively. Bars within a chart followed by the same letter are not significantly different at the 5% probability level by the LSD test. Values represent the means of three replications ± standard deviations
3.5 Foliar-Applied Se Modulating the Accumulation of Na+ in the Root and Leaf Samples of S. byzantine Grown Under Saline Conditions
The content of Na+ in the roots and shoots of S. byzantine (Table 2) represented significant changes in response to salinity, Se, and salinity × Se. Under non-saline conditions, supplementing S. byzantine plants with Se treatments was not accompanied by significant alterations in the amounts of root and leaf Na+ (Figs. 7A, B). Salt-affected S. byzantine plants demonstrated increased Na+ since the accumulated Na+ in roots and leaves was boosted by 70.87 and 64.87% at 30 mM NaCl while 89.36 and 89.77% at 90 mM NaCl, respectively, relative to those of non-salt-stressed plants. Foliar-applied Se decreased the accumulation of Na+ in both roots and leaves at all NaCl doses. At 90 mM NaCl, Na+ accumulations in roots and leaves reduced by 16.33 and 17.47% with 4 mg L−1 Se application while 23.68 and 24.03% with 16 mg L−1 Se application relative to those of 90 mM NaCl plants, respectively (Figs. 7A, B).
Effect of different levels of salinity (S) and Se (Na2 SeO3) on Na+ concentration in roots (A) and leaves (B) of seedlings in S. byzantine C. Koch. S1, S2, S3, and S4 are NaCl concentrations at 0, 30, 60, and 90 mM, respectively. Bars within a chart followed by the same letter are not significantly different at the 5% probability level by the LSD test. Values represent the means of three replications ± standard deviations
3.6 EO Production of S. byzantine as Affected by Salt Stress and Se Application
EO production of S. byzantine represented noticeable changes in response to salinity and Se although the effect of salinity × Se showed no significant impact (Table 2). Under non-saline conditions, adding varying Se levels (4, 8, and 16 mg L−1) resulted in no considerable changes in the production of EO in S. byzantine (Figs. 8A, B). Although the production of EO in S. byzantine demonstrated a significant increase by imposing salt stress up to a concentration of 30 mM NaCl, a significant decrease in both of the abovementioned traits occurred with the continuation of increasing salinity (60 and 90 mM NaCl). The content and production of EO in S. byzantine increased by 32.8 and 32.23% at 30 mM NaCl while they decreased by 23.81 and 23.81% at 60 mM NaCl and then further decreased by 55.95 and 57.14% at 90 mM NaCl, respectively, relative to those of control plants. It is noteworthy that spraying salt-treated plants with Se further increased the content and production of EO in S. byzantine at a concentration of 30 mM NaCl and prevented their reduction at higher concentrations (60 and 90 mM NaCl) relative to those of non-Se treated plants. Nonetheless, there was no noticeable difference among various concentrations of Se under all NaCl concentrations (Figs. 8A, B).
Effect of different levels of salinity (S) and Se (Na2 SeO3) on EO content (A) and EO production (B) of seedlings in S. byzantine C. Koch. S1, S2, S3, and S4 are NaCl concentrations at 0, 30, 60, and 90 mM, respectively. Bars within a chart followed by the same letter are not significantly different at the 5% probability level by the LSD test. Values represent the means of three replications ± standard deviations
3.7 Pearson’s Correlation Matrix of Traits Measured in Response to Salt Stress and Se Application
According to Pearson’s correlation coefficients in Table 2, significant positive relationships were found between EO production (0.52, P = 0.001) and chlorophyll content, Fv/Fm ratio, and the activities of DHAR, GR, CAT, SOD, and APX (0.96, 0.92, 0.94, 0.96, 0.93, and 0.96, respectively, P = 0.001). Interestingly, there was a significant positive correlation between the EO production and root Na+ content (0.49, P = 0.001). Conversely, the relationship between EO production and leaf Na+ content was significantly negative (− 0.66, P = 0.001) and the findings showed a greater increase in EO production at low salinity (30 mM NaCl) and the beginning of a decreasing trend as salinity intensified (Figs. 8), which further justified this correlation (Table 2). A statistically significant correlation in the opposite direction was also found that was related to chlorophyll content, Fv/Fm ratio, and EO production with MDA accumulations (− 0.92, − 0.95, and − 0.60, respectively, P = 0.001), in addition to chlorophyll content, Fv/Fm ratio, and EO production with H2O2 generation (− 0.94, − 0.97, and − 0.63, respectively, P = 0.001). The correlation between proline accumulations and EL, as well as MDA (0.90 and 0.97, respectively, P = 0.001) and H2O2 generation (0.88 and 0.98, respectively, P = 0.001), was also noticeably positive (Table 2). The correlation between the EO production and the activity of antioxidant enzymes such as DHAR, GR, CAT, SOD, and APX (0.65, 0.66, 0.51, 0.58, and 0.49, respectively, P = 0.001) was significantly positive (Table 2), justifying the positive effect of Se on increasing salt stress resistance by activating enzymes and heightening the EO production (Table 3).
4 Discussion
The findings of our study revealed that salinity significantly reduced chlorophyll content and the Fv/Fm ratio of S. byzantine. Photosynthesis is one of the first processes in plants that are negatively affected by salinity (Munns and Tester 2008; Chutipaijit et al. 2011). It has been found that the destructive effect of salinity on photosynthesis is due to the reduced efficiency of photosystem II in CO2 absorption and metabolism (Najar et al. 2019). The ratio of Fv/Fm in a plant that grows under stress-free conditions is about 0.83 (Murchie and Lawson 2013). In our study, this rate was observed to be 0.67 in stress-free conditions while showing a significant decline with the imposition of salinity. However, in the present study, Se improved chlorophyll contents and enhanced the Fv/Fm ratio in S. byzantine plants under saline conditions. One of the mechanisms by which Se increases plant resistance to salinity stress is the effect of this micronutrient on increasing the rate of net photosynthetic rate while alleviating the damage to chloroplast ultrastructure under these stressful conditions (Jiang et al. 2017). Habibi (2017) and Mozafariyan et al. (2016) also attributed the increase in plant resistance to salinity to the reduced damage effect on Fv/Fm in plants due to the use of Se, which is consistent with our findings. The improved photosynthesis in response to Se has also been reported in tomato (Diao et al. 2014), maize plants (Jiang et al. 2017), and garlic (Khademi Astaneh et al. 2018) under salt stress, which further supported our findings regarding S. byzantine. Our obtained data on the increased generation of H2O2 and accumulation of MDA in salinized S. byzantine plants are in line with those of other previous studies (Yildiztugay et al. 2016; Jiang et al. 2017). The generation of ROS in plants is balanced under normal conditions while it results in a disruption between the production and scavenging of ROS due to damage to respiration, photorespiration, and photosynthesis under salt stress (Abdel Latef et al. 2017; Garcia-Caparros et al. 2019). However, our findings approved that Se treatment alleviated salt-induced oxidative damage by decreasing the amount of H2O2 and MDA in S. byzantine, which conforms to the findings of Djanaguiraman et al. (2010), Yildiztugay et al. (2016), and Karimi et al. (2020) on soybean, maize plants, and Vitis vinifera, respectively. They reported the positive impacts of Se on reducing H2O2 generation under salinity conditions. Salt stress significantly decreased enzymes that were involved in the ascorbate–glutathione (ASA-GSH) cycle, including MDHAR, DHAR, and GR in S. byzantine plants. The treatment of salinized S. byzantine plants with Se resulted in higher activities of antioxidant enzymes such as CAT, SOD, APX, DHAR, MDHAR, and GR, as well as the concentration of non-enzymatic antioxidants such as AsA and GSH contents. The AsA-GSH cycle contains AsA, GSH, and enzymes such as APX, MDHAR, DHAR, and GR, which play an important role in detoxifying ROS by decreasing H2O2 and MDA generation, along with the redox state leading to reduced oxidative damage (Hasanuzzaman et al. 2019). Our findings indicated the probable role of Se in improving the ASA-GSH cycle by heightening the activities of DHAR, MDHAR, GR, and APX. According to previous research (Yildiztugay et al. 2016; Karimi et al. 2020), Se is defined as an antioxidant that is beneficial to plants under stressed conditions by enhancing the activity of antioxidant enzymes. To the best of our knowledge, this is the first study reporting the positive role of Se in the redox state and ascorbate–glutathione cycle regarding the salt tolerance of S. byzantine. Under salt stress, a significant increase was observed in CAT, SOD, and APX activities with Se-treated S. byzantine plants relative to non-Se-treated plants. These findings are consistent with those of Jiang et al. (2017), Habibi and Sarvary (2015), Shekari et al. (2017), and Agbolade et al. (2019) on salt-stressed maize plants, salt-stressed lemon balm, salt-stressed dill plants, and salt-stressed wheat plants, implying that Se application further heightened the activities of the abovementioned enzymes. The application of Se significantly boosts the antioxidant defense system in the chloroplasts of plants under saline stress, thus improving photosynthesis (Jiang et al. 2017). It is well known that salinity causes lipid peroxidation and increases EL (Wu et al. 2013). In the present study, salinity led to noticeable damage to S. byzantine plants, followed by finding corresponding enhancements in EL in S. byzantine leaves under salinity, which is in line with the findings of Zhang et al. (2006). These findings further confirm that Se plays an important role in the maintenance of the cell membrane structure and cell integrity by reducing EL under salt stress. The current data on the chloroplast ultrastructure in Se-treated leave also confirm this observation (Jiang et al. 2017). A similar impact of Se on reducing EL was reported by Karimi et al. (2020) on salt-stressed Vitis vinifera leaves when treated with Se application, respectively. In justifying these data for the positive impact of Se on photosynthetic efficiency (Fv/Fm ratio), Diao et al. (2014) asserted that Se improves photosynthetic efficiency by regulating the antioxidant defense system of the plant under saline conditions. Lower lipid peroxidation caused by elevated activities of antioxidant enzymes under saline conditions was similarly reported in Se-treated maize under salt stress (Zhang et al. 2006). Moreover, previous research demonstrated that Se resulted in enhanced activity of antioxidant enzymes thus improving the tolerance of plants in response to environmental stresses (Diao et al. 2014). In our investigation, an elevated accumulation of proline was recorded with salinity although it further increased by Se treatment, which is also consistent with the findings of Bideshki et al. (2019) representing that exogenous Se increased proline production in potato plants. Habibi and Sarvary (2015) also concluded that the proline content of the lemon balm was higher in salt-stressed plants in response to Se. Proline acts as an osmoregulator in salt-affected plants (Abdel Latef et al. 2009). Further increases in proline accumulations in response to Se application in salt-affected plants could be related to the effect of Se on proline metabolism by modulating the activities of the γ-glutamyl kinase and proline oxidase leading to its greater synthesis while lesser degradation (Elkelish et al. 2019). Salt stress is often associated with a noticeable accumulation of excessive Na+ ions (Azooz et al. 2004; Jiang et al. 2017; Shekari et al. 2017), which further supported our findings regarding the NaCl toxicity-induced Na+ accumulation of different parts (leaves and roots) of S. byzantine plants. Our findings further revealed that Na+ accumulations in S. byzantine under salinity stress significantly reduced by Se treatment. Se increases the binding of Na+ to the cell wall, ultimately reducing the accumulation of Na+ ions in plant organs (Habibi 2017). However, the mechanism by which Se ameliorates salt stress and lowers Na+ accumulation remains unknown and needs further investigation. Furthermore, our findings approved that Se treatments decreased the accumulation of Na+ in the plant modulating important physiochemical processes in S. byzantine under salt stress. Our findings also showed a positive effect of 30 mM NaCl on increasing the EO production of S. byzantine. Medicinal plants produce more secondary metabolites (EO) to adapt to adverse conditions, which will cause more osmotic regulation and balance and increase plant resistance to stress (Petropoulos et al. 2008). This is a highly good justification for confirming our findings regarding increasing the EO production of S. byzantine plants exposed to salinity. Interestingly, our data demonstrated that Se treatments further increased the EO production of S. byzantine, especially when combined with lower levels (30 mM NaCl) of salinity. In the current investigation, Se treatment heightened the production of EO in S. byzantine plants, which is in line with the findings of previous reports (Misra et al. 2010; Khalid et al. 2017; Zaji et al. 2019) on the positive effects of Se on EO production. The increased production of EO can also be attributed to the positive effect of Se on CO2 assimilation in photosynthetic activity (Misra et al. 2010). Likewise, Shekari et al. (2017) demonstrated that salt-affected dill plants with Se supplementation resulted in an enhanced EO production, which corroborates with our findings on S. byzantine. Another proof for the positive effect of Se on increasing the EO production of medicinal plants might be related to the role of this element in enhancing the density of glandular trichomes and storage structures that are responsible for producing EO (Zaji et al. 2019). To the best of our knowledge, this study first provided information on the existence of a potential correlation between cellular Na+ ionic stresses, oxidative stress components of salinity tolerance, and the antioxidant defense system with the EO production of S. byzantine. Due to the positive correlation between the production of EO and the activity of antioxidant enzymes, as well as the negative correlation between the accumulations of Na+ ions in plant cells and increased oxidative stress, the positive effect of Se on improving EO production in S. byzantine might be attributed to the activation of the plant defense system, the reduction of oxidative stress, and the toxicity of Na+ ions in the plant. However, information on the mechanism of action of Se in increasing the EO production of medicinal plants remains extremely insufficient and should be well addressed in future research.
5 Conclusion
Based on the findings, selenium alleviated the negative consequences of salinity by boosting photosynthesis and preventing chlorophyll degradation, osmotic adjustment, activating the antioxidant defense system for alleviating oxidative damage, and reducing the Na+ accumulation of roots and leaves in S. byzantine plants under salt stress. The obtained data represented a positive correlation between the magnitude of the reduced toxicity of Na+ flux in the cells and salinity tolerance with the essential oil production of S. byzantine. Further increases in the essential oil production of selenium-supplemented S. byzantine plants under moderate saline conditions (30 mM NaCl) are new data. These results show new evidence on the positive effect of selenium on essential oil production of S. byzantine. However, further studies are needed to elucidate the functional mechanisms of selenium in essential oil production, especially in contrast to moderate salinity levels. Nonetheless, considering the implementation of these experiments under greenhouse conditions and some other influential factors in the field, field experiments should be conducted to complete those assumed in this study.
References
Abdel Latef AA, Alhmad MF, Abdelfattah KE (2017) The possible roles of priming with ZnO nanoparticles in mitigation of salinity stress in Lupine (Lupinus termis) plants. J Plant Growth Regul 36:60–70. https://doi.org/10.1007/s00344-016-9618-x
Abdel Latef AAH, Srivastava AK, El-sadek MSA, Kordrostami M, Tran LSP (2018) Titanium dioxide nanoparticles improve growth and enhance tolerance of broad bean plants under saline soil conditions. Land Degrad Dev 29:1065–1073. https://doi.org/10.1002/ldr.2780
Abdel Latef AAH, Shaddad MAK, Ismail MA, Abu Alhmad FM (2009) Benzyladenine can alleviate saline injury of two roselle (Hibiscus sabdariffa) cultivars via equilibration of cytosolutes including anthocyanins. Int J Agric Biol 11:151–157
Abul-Soud MA, Abd-Elrahman SH (2016) Foliar selenium application to improve the tolerance of eggplant grown under salt stress conditions. Int J Plant Soil Sci 9:1–10. https://doi.org/10.9734/IJPSS/2016/19992
Agbolade JO, David O, Ajiboye A, Kioko J, Jolayemi O, Olawuni I, Ojo M, Akomolafe G, Adekoya M, Komolafe R (2019) Morpho-physiological effect of selenium on salinity-stressed wheat (Triticum aestivum L.). J Biol Res 92:24–29. https://doi.org/10.4081/jbr.2019.7650
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta Vulgaris Plant Physiol 24:1–15. https://doi.org/10.1104/pp.24.1.1
Azooz MM, Shaddad MA, Abdel-Latef AA (2004) Leaf growth and K+/Na+ ratio as an indication of the salt tolerance of three sorghum cultivars grown under salinity stress and IAA treatment. Acta Agron Hung 52:287–296. https://doi.org/10.1556/AAgr.52.2004.3.10
Bates LS, Waldren EP, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207. https://doi.org/10.1007/BF00018060
Bideshki A, Arvin MJ, Aien A, Hasandokht MR, Khalighi A (2019) Interactive effects of foliar 24-Epibrassinolide and selenium applications on yield, reduce nitrate accumulation and selenium enrichment in potato tuber in field. Cogent Food Agric 5:1690315. https://doi.org/10.1080/23311932.2019.1690315
Cavalcanti FR, Oliveira JT, Martins-miranda AAS, Viegas RA, Silveira JAG (2004) Superoxide dismutase, catalase and peroxidase activities do not confer protection against oxidative damage in salt-stressed cowpea leaves. New Phytol 163:563–571. https://doi.org/10.1111/j.1469-8137.2004.01139.x
Chutipaijit S, Chaum S, Sompornpailin K (2011) High contents of proline and anthocyanin increase protective response to salinity in Oryza sativa L. spp. Indica Aust J Crop Sci 5:1191–1198
Diao M, Ma L, Wang J, Cui J, Fu A, Liu HY (2014) Selenium promotes the growth and photosynthesis of tomato seedlings under salt stress by enhancing chloroplast antioxidant defense system. J Plant Growth Regul 33:671–682. https://doi.org/10.1007/s00344-014-9416-2
Djanaguiraman M, Prasad PVV, Seppanen M (2010) Selenium protects sorghum leaves from oxidative damage under high temperature stress by enhancing antioxidant defense system. Plant Physiol Biochem 48:999–1007. https://doi.org/10.1016/j.plaphy.2010.09.009
Elkelish AA, Soliman MH, Alhaithloul HA, El-Esawi MA (2019) Selenium protects wheat seedlings against salt stress-mediated oxidative damage by up-regulating antioxidants and osmolytes metabolism. Plant Physiol Biochem 137:144–153. https://doi.org/10.1016/j.plaphy.2019.02.004
Fariduddin Q, Zaid A, Mohammad F (2019) Plant growth regulators and salt stress: mechanism of tolerance trade-off. In: Akhtar M (ed) Salt Stress, Microbes, and Plant Interactions: Causes and Solution. Springer, Singapore. https://doi.org/10.1007/978-981-13-8801-9_4
Feng R, Wei C, Tu S (2013) The roles of selenium in protecting plants against abiotic stresses. Environ Exp Bot 87:58–68. https://doi.org/10.1016/j.envexpbot.2012.09.002
Fernandes VF, de Almeida LB, Feijo EVS, Silva DC, Oliveira RA, Mielke MS, Costa LCB (2013) Light intensity on growth, leaf micromorphology and essential oil production of Ocimum gratissimum Braz J Pharmacogn 23:419–424. https://doi.org/10.1590/S0102-695X2013005000041
Garcia-Caparros P, Hasanuzzaman M, Lao MT (2019) Oxidative stress and antioxidant defense in plants under salinity. In: Hasanuzzaman, M, Fotopoulos V, Nahar K, Fujita M (eds) Reactive Oxygen, Nitrogen and Sulfur Species in Plants: Production, Metabolism, Signaling and Defense Mechanisms, John Wiley & Sons Ltd., pp 291- 309. https://doi.org/10.1002/9781119468677.ch12
Gupta J, Dubey RK, Kaur N, Choudhary OP (2018) Evaluation of subtropical ornamental trees for reclaiming salinity affected lands. J for Res 31:807–817. https://doi.org/10.1007/s11676-018-0851-y
Gupta M, Gupta S (2017) An overview of selenium uptake, metabolism, and toxicity in plants. Front Plant Sci 7:2074. https://doi.org/10.3389/fpls.2016.02074
Hasanuzzaman M, Borhannuddin Bhuyan MHM, Anee TI, Parvin K, Nahar K, Al Mahmud J, Fujita M (2019) Regulation of ascorbate-glutathione pathway in mitigating oxidative damage in plants under abiotic stress. Antioxidants 8:384. https://doi.org/10.3390/antiox8090384
Hasanuzzaman M, Bhuyan MHMB, Reza A, Hawrylak-Nowak B, Matraszek-Gawron R, Al Mahmud J, Nahar K, Fujita M (2020) Selenium in plants: boon or bane? Environ Exp Bot 178:104170. https://doi.org/10.1016/j.envexpbot.2020.104170
Hasanuzzaman M, Bhuyan MHMB, Reza A, Hawrylak-Nowak B, Matraszek-Gawron R, Nahar K, Fujita M (2020) Selenium toxicity in plants and environment: biogeochemistry and remediation possibilities. Plants 9:1711. https://doi.org/10.3390/plants9121711
Hasanuzzaman M, Hossain MA, Fujita M (2011) Nitric oxide modulates antioxidant defense and the methylglyoxal detoxification system and reduces salinity-induced damage of wheat seedlings. Plant Biotecnol Rep 5:353–365. https://doi.org/10.1007/s11816-011-0189-9
Hasanuzzaman M, Nahar K, Shahadat Hossain M, Anee TI, Parvin K, Fujita M (2017) Nitric oxide pretreatment enhances antioxidant defense and glyoxalase systems to confer PEG-induced oxidative stress in rapeseed. J Plant Interact 12:323–331. https://doi.org/10.1080/17429145.2017.1362052
Habibi G (2017) Selenium ameliorates salinity stress in Petroselinum crispum by modulation of photosynthesis and by reducing shoot Na accumulation. Russ J Plant Physl 64:368–374. https://doi.org/10.1134/S1021443717030086
Habibi G, Sarvary S (2015) The roles of selenium in protecting lemon balm against salt stress. Iran J Plant Physiol 5:1425–1433
Hossain MA, Hasanuzzaman M, Fujita M (2010) Up-regulation of antioxidant and glyoxalase systems by exogenous glycinebetaine and proline in mung bean confer tolerance to cadmium stress. Physiol Mol Biol Plants 16:259–272. https://doi.org/10.1007/s12298-010-0028-4
Jiang C, Zu C, Lu D, Zheng Q, Sen J, Wang H, Li D (2017) Effect of exogenous selenium supply on photosynthesis, Na+ accumulation and antioxidative capacity of maize (Zea mays L.) under salinity stress. Sci Rep 7:42039. https://doi.org/10.1038/srep42039
Karimi R, Ghabooli M, Rahimi J, Amerian M (2020) Effects of foliar selenium application on some physiological and phytochemical parameters of Vitis vinifera L. cv. Sultana under salt stress. J Plant Nutr 43:2226–2242. https://doi.org/10.1080/01904167.2020.1766072
Khademi Astaneh R, Bolannazar S, Zaare Nahandi F (2018) The effects of selenium on some physiological traits and K, Na concentration of garlic (Allium sativum L.) under NaCl stress. Inf Process Agric 5:156–161. https://doi.org/10.1016/j.inpa.2017.09.003
Khalid KA, Amer HM, Wahba HE, Hendawy SF, El-Razik TMA (2017) Selenium to improve growth characters, photosynthetic pigments and essential oil composition of chives varieties. Asian J Crop Sci 9:92–99. https://doi.org/10.3923/ajcs.2017.92.99
Misra A, Srivastava A, Srivastava N, Khan A (2010) Se-acquisition and reactive oxygen species role in growth, photosynthesis, photosynthetic pigments, and biochemical changes in essential oil(s) monoterpene of Geranium (Pelargonium graveolens L. Her’.ex. Ait.). Am- Eurasian J Sustain Agric 4:39–46
Mozafariyan M, Kamelmanesh MM, Hawrylak-Nowak B (2016) Ameliorative effect of selenium on tomato plants grown under salinity stress. Arch Agron Soil Sci 62:1368–1380. https://doi.org/10.1080/03650340.2016.1149816
Mozaffarian V (1996) A dictionary of Iranian plant names. Farhang Moaser, Tehran, Iran, p 522
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Ann Rev Plant Biol 59:651–681. https://doi.org/10.1146/annurev.arplant.59.032607.092911
Murchie EH, Lawson T (2013) Chlorophyll fluorescence analysis: a guide to good practice and understanding some new applications. J Exp Bot 64:3983–3998. https://doi.org/10.1093/jxb/ert208
Najar R, Aydi S, Sassi-Aydi S, Zarai A, Abdelly C (2019) Effect of salt stress on photosynthesis and chlorophyll fluorescence in Medicago truncatula Plant Biosyst 153:88–97. https://doi.org/10.1080/11263504.2018.1461701
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplast. Plant Cell Physiol 22:867–880. https://doi.org/10.1093/oxfordjournals.pcp.a076232
Ozden M, Demirel U, Kahraman A (2009) Effects of proline on antioxidant system in leaves of grapevine (Vitis vinifera L.) exposed to oxidative stress by H2O2 Sci Hortic 119:163–168. https://doi.org/10.1016/j.scienta.2008.07.031
Petropoulos SA, Dimitra D, Polissiou MG, Passam HC (2008) The effect of water deficit stress on the growth, yield and composition of essential oils of parsley. Sci Hortic 115:393–397. https://doi.org/10.1016/j.scienta.2007.10.008
Pitman MG, Lauchli A (2002) Global impact of salinity and agricultural ecosystems. In: Salinity: environment-plants molecules. Springer, Dordrecht p. 3–20. https://doi.org/10.1007/0-306-48155-3_1
Selmar D, Kleinwachter M (2013) Influencing the product quality by deliberately applying drought stress during the cultivation of medicinal plants. Ind Crop Prod 42:558–566. https://doi.org/10.1016/j.indcrop.2012.06.020
Shekari F, Abbasi A, Mustafavi SH (2017) Effect of silicon and selenium on enzymatic changes and productivity of dill in saline condition. J Saudi Soc Agric Sci 16:367–374. https://doi.org/10.1016/j.jssas.2015.11.006
Velikova V, Loreto F (2005) On the relationship between isoprene emission and thermo tolerance in Phragmites ausrralis leaves exposed to high temperatures and during the recovery from a heat stress. Plant, Cell Environ 28:318–327. https://doi.org/10.1111/j.1365-3040.2004.01314.x
Wang F, Zeng B, Sun Z, Zhu C (2009) Relationship between proline and Hg+2 – induced oxidative stress in tolerant rice mutant. Arch Environ Contam Toxicol 56:723–731. https://doi.org/10.1007/s00244-008-9226-2
Wani SH, Tripathi P, Zaid A, Challa GS, Kumar A, Kumar V, Upadhyay J, Joshi R, Bhatt M (2018) Transcriptional regulation of osmotic stress tolerance in wheat (Triticum aestivum L.). Plant Mol Biol 97:469–487. https://doi.org/10.1007/s11103-018-0761-6
Wink M (2015) Modes of action of herbal medicines and plant secondary metabolites. Medicines 2:251–286. https://doi.org/10.3390/medicines2030251
Wu GQ, Liang N, Rui JF, Zhang JJ (2013) Evaluation of salinity tolerance in seedlings of sugar beet (Beta vulgaris L.) cultivars using proline, soluble sugars and cation accumulation criteria. Acta Physiol Plant 35:2665–2674
Yildiztugay E, Ozfidan-Konakci C, Kucukoduk M, Tekis SA (2016) The impact of selenium application on enzymatic and non-enzymatic antioxidant systems in Zea mays roots treated with combined osmotic and heat stress. Arch Agron Soil Sci 63:261–275
Zaid A, Asgher M, Wani IA, Wani SH (2020a) Role of triacontanol in overcoming environmental stresses. In: Roychoudhury A, Tripathi DK (eds) Protective chemical agents in the amelioration of plant abiotic stress: biochemical and molecular perspectives, John Wiley & Sons Ltd., pp 491- 509. https://doi.org/10.1002/9781119552154.ch25
Zaid A, Mohammad F, Fariduddin O (2020b) Plant growth regulators improve growth, photosynthesis, mineral nutrient and antioxidant system under cadmium stress in menthol mint (Mentha arvensis L.). Physiol Mol Biol Plants 26:25–39. https://doi.org/10.1007/s12298-019-00715-y
Zaid A, Wani SH (2019) Reactive oxygen species generation, scavenging and signaling in plant defense responses. In: Jogaiah S, Abdelrahman M (eds) Bioactive Molecules in Plant Defense. Springer, Cham, pp 111–132. https://doi.org/10.1007/978-3-030-27165-7_7
Zaji B, Khavari-Nejad R, Saadatmand S, Nejadsatari T, Iranbakhsh A (2019) Combined application of 24-Epibrassinolide and selenium improves essential oil content and composition of dragonhead (Dracocephalum moldavica L.). TEOP 22:893–902. https://doi.org/10.1080/0972060X.2019.1668304
Zhang Y, Wang L, Liu Y, Zhang Q, Wei Q, Zhang W (2006) Nitric oxide enhances salt tolerance in maize seedlings through increasing activities of proton-pump, Na+/H+ antiport in the tonoplast. Planta 224:545–555. https://doi.org/10.1007/s00425-006-0242-z
Funding
This study was financially supported by Urmia University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sharifi, P., Amirnia, R., Torkian, M. et al. Protective Role of Exogenous Selenium on Salinity-Stressed Stachys byzantine Plants. J Soil Sci Plant Nutr 21, 2660–2672 (2021). https://doi.org/10.1007/s42729-021-00554-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42729-021-00554-5