Abstract
Bacterial mannitol 1-phosphate dehydrogenase (mtlD) gene was introduced into potato (Solanum tuberosum L.) by Agrobacterium tumefaciens-mediated transformation. Transgenic plants were selected on a medium containing 100 mg l−1 kanamycin and confirmed by polymerase chain reaction (PCR), Southern blotting, and RT-PCR analyses. All of the selected transformants accumulated mannitol, a sugar alcohol that is not found in wildtype potato. Experiments designed for testing salt tolerance revealed that there was enhanced NaCl tolerance of the transgenic lines both in vitro and in hydroponic culture. Compared to 0 mM NaCl, the shoot fresh weight of wildtype plants was reduced by 76.5% at 100 mM NaCl under hydroponic conditions. However, under the same condition, the shoot fresh weight of transgenic plants was reduced only by 17.3%, compared to 0 mM NaCl treatment. The improved tolerance of this transgenic line may be attributed to the induction and progressive accumulation of mannitol in the roots and shoots of the plants. In contrast to in vitro experiments, the mannitol content in the transgenic roots and shoots increased at 50 mM NaCl and decreased slightly at 75 and 100 mM NaCl, respectively. Overall, the amount of accumulated mannitol in the transgenic lines was too small to act as an osmolyte; thus, it might act as an osmoprotectant. However, the results demonstrated that mannitol had more contribution to osmotic adjustment in the roots (but not in shoots). Finally, we concluded that mtlD expression in transgenic potato plants can significantly increase the mannitol accumulation that contributes to the enhanced tolerance to NaCl stress. Furthermore, although this enhanced tolerance resulted mainly from an osmoprotectant action, an osmoregulatory effect could not be ruled out.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Abiotic stresses, such as drought and high salinity, are the most important environmental factors responsible for the induction of osmotic stress in plants and reductions in plant growth and crop productivity (Boyer 1982). More than 800 million hectares of land throughout the world are salt-affected, either by salinity alone (397 million ha) or by the associated condition of sodicity (434 million ha) (Munns 2005). It is predicted that increased salinization of arable lands will have damaging global effects; these could result in a 30% loss of land within the next 25 years and up to a 50% loss by the year 2050 (Wang et al. 2003). Therefore, research into the breeding of crop plants for drought and salinity stress tolerance should be given a high priority in plant genetic engineering programs.
To adapt to these stress conditions, plants have evolved an extensive variety of mechanisms, including osmotic adjustment, selective uptake, and compartmentalization of ions (Blumwald 2000).
In response to osmotic stress, plants usually accumulate low molecular weight compatible solutes; these act to maintain cell turgor and are thus the driving gradient for water uptake (osmoregulation) (Hare et al. 1998; Wang et al. 2003; Waditee et al. 2007). Moreover, it has been discovered that the compatible solutes can also function as oxygen free radical scavengers or chemical chaperones, and thus protect macromolecules, enzymes, and proteins (Hare et al. 1998; Yokoi et al. 2002; Wang et al. 2003). The most frequently encountered compatible osmolytes in higher plants include sugars (e.g., trehalose), polyols (e.g., mannitol, sorbitol), amino acids (e.g., proline), and tertiary (1,4,5,6-tetrahydro-2-methyl-4-carboxyl pyrimidine) and quaternary (e.g., glycine betaine) ammonium and sulfonium compounds (Yokoi et al. 2002; Wang et al. 2003; Almeida et al. 2007).
Many crops naturally lack the ability to synthesize high levels of osmoprotectants. Therefore, it has been suggested that metabolic engineering of osmoprotectant synthesis pathways could be one of the best strategies for enhancing the abiotic stress tolerance of plants (Bhatnagar-Mathur et al. 2008). Overexpression of sugar alcohols (mannitol, trehalose, myo-inositol, and sorbitol) has previously been used as a potential route for improving stress tolerance in plants (Wang et al. 2003).
Mannitol is the most abundant sugar alcohol in nature; it is known to be present in more than 100 species of higher plants, including many crops such as celery, olive, and carrot (Shen et al. 1997; Patonnier et al. 1999; Prabhavathi and Rajam 2007). The accumulation of mannitol is known to increase in plants in response to osmotic stress (Prabhavathi and Rajam 2007). It has been proposed that mannitol can improve tolerance to water deficits through osmoregulation, free radical scavenging, and stabilization of macromolecular structures (Stoop et al. 1996; Prabhavathi et al. 2002; Tang et al. 2005; Prabhavathi and Rajam 2007). It can thereby alleviate osmotic and salinity-induced stresses in plants.
When tobacco and Arabidopsis plants, which do not normally accumulate mannitol, were genetically transformed with the E. coli mannitol-1-phosphate dehydrogenase (mt1D) gene, they exhibited biosynthesis of mannitol and an increased salinity tolerance (Tarczynski et al. 1993; Thomas et al. 1995; Karakas et al. 1997). Increased resistance to oxidative stress was also found in transgenic tobacco plants in which mannitol biosynthesis was targeted to chloroplasts (Stoop et al. 1996). Abebe et al. (2003) reported an improved tolerance to water stress and salinity in transgenic wheat expressing the mtlD gene. Expression of the mtlD gene in eggplant resulted in an increased tolerance to osmotic stress that was induced by salt, drought, and chilling stresses (Prabhavathi et al. 2002). Similar results have been achieved by the genetic transformations of petunia (Chiang et al. 2005), loblolly pine (Tang et al. 2005), poplar (Hu et al. 2005), and rice (Huizhong et al. 2000) with mtlD gene.
Potato (Solanum tuberosum L.) is a moderately salt-sensitive plant (Rahnama and Ebrahimzadeh 2005), particularly in the early growth stages (Heuer and Nadler 1998). Moreover, it is very sensitive to water stress (Gopal and Iwama 2007). Typically, when soil salinity increases to 5.9 dS/m, potato yield is decreased by 50% (Kotuby-Amacher et al. 2000). Therefore, the development of salinity stress-tolerant potato cultivars could be considered as one of the promising approaches to improve yield stability under stressful conditions.
In this study, we report the successful transfer of E. coli mtlD gene to potato plant (cv. Marfona) under the control of the CaMV35S constitutive promoter. Expression of mtlD gene resulted in the synthesis of mannitol and a consequent improvement in salt stress tolerance of the transgenic potato plants.
Materials and methods
Vector construction
A plasmid containing the coding sequence of E. coli mtlD gene under the control of nos terminator at the 3′ end was received as a gift from the Late Professor Ray Wu, Cornell University, USA. The fragment containing mtlD coding sequence and nos terminator was excised with XbaI and EcoRI from the plasmid and replaced in gus-nos fragment in the binary vector pBI121 resulting a new binary vector, pBICaMVMTLD (Fig. 1), containing also a plant selectable marker gene, nptII. This plasmid was then introduced into Agrobacterium tumefaciens strain AGLO1 via a freeze–thaw method.
T-DNA region of the binary vector pBICaMVMTLD used for Agrobacterium tumefaciens-mediated transformation. RB, LB, right and left T-DNA borders, respectively; P-nos, nos promoter; nptII, neomycine phosphoteransferase II; T-nos, nos terminator; CaMV35S, cauliflower mosaic virus promoter; mtlD, mannitol-1-phosphate dehydrogenase; HindIII, XbaI, EcoRI, restriction enzymes
Overnight cultures of A. tumefaciens harboring pBICaMVMTLD in LB broth medium supplemented with 50 mg l−1 rifampicin and 50 mg l−1 kanamycin were used for plant transformation.
Plant materials and transformation
In vitro virus-free potato plants (Solanum tuberosum L. cv. Marfona) were micropropagated on a multiplication medium consisting of MS salts and vitamins (Murashige and Skoog 1962) supplemented with 30 g l−1 sucrose and 7 g l−1 agar. The plants were regularly propagated by sub-culturing of the auxiliary buds every 3–4 weeks, and they were grown at 25°C under lights of 40–60 μmol m−2 s−1 intensity and with a 16-h photoperiod.
For potato transformation, 5 mm internode segments were pre-cultured in callus induction media (MS salts and vitamins, 2 mg l−1 2,4-d, 5 mg l−1 gibberellic acid (GA3), 2 mg l−1 benzylamino purine (BAP), 1 mg l−1 thidiazoron (TDZ), 30 g l−1 sucrose, and 7 g l−1 agar). After 3 days, the internodes were immersed in an overnight-cultured A. tumefaciens (OD600: 0.8) for a period of 10 min. The explants were dried on sterilized filter papers and then co-cultured on callus induction medium. After another 3 days, the internode segments were transferred to the regeneration media (MS salts, 100 mg l−1 myo-inositol, 8 mg l−1 adenine sulfate, 8 mg l−1 thiamine HCl, 3 mg l−1 GA3, 2 mg l−1 BAP, 1 mg l−1 TDZ, 30 g l−1 sucrose, and 7 g l−1 agar) supplemented with cefotaxime (250 mg l−1) and kanamycin (100 mg l−1) for selection. After approximately 6–8 weeks, the shoots were regenerated on the cut edges of internodes (Ghasimi Hagh et al. 2009).
Single shoots, 2–3 cm in length, were excised and transferred to test tubes that contained multiplication medium supplemented with 250 mg l−1 cefotaxime and 100 mg l−1 kanamycin. Individual rooted shoots in the test tubes were labeled and propagated on multiplication media, as described above.
For NaCl treatments, potato micro-propagation was performed on liquid multiplication media.
All media, after adjusting the pH to 5.7, were autoclaved at 121°C for 20 min. All cultures were kept under cool white fluorescent lights with 40–60 μmol m−2 s−1 intensity at 22–24°C, with 16-h photoperiod.
Molecular analysis of transgenic plants
DNA extraction and PCR analysis
Genomic DNA was isolated from the leaves of the putative transgenic and wildtype (control) plants as described by Dellaporta et al. (1983). Initial screening of the transformants for the presence of the nptII and mtlD genes was performed by PCR of DNA extracted from 18 putative transgenic plants regenerated from separately kept calli, using standard protocols. For PCR analysis, the 5′-GAACAAGATGGATTGCACGC-3′ and 5′-AAGAACTCGTCAAGAAGGC-3′ primers were used to amplify a 500 bp fragment of the NPTII coding region, and the 5′-GTCATAGCTATCAGGATGT-3′ and 5′-CTTGATCAATACTGCACCACTT-3′ primers were used to amplify a 680 bp fragment of the MTLD coding region. The amplification reactions consisted of 94°C for 5 min (1 cycle), followed by 35 cycles (94°C for 1 min, 60°C for 1 min, and 72°C for 2 min); this was followed by an extension cycle of 7 min at 72°C. The PCR products were separated by electrophoresis on 1% agarose gel containing 0.1 μg l−1 ethidium bromide and visualized under UV light.
Reverse transcription PCR
The expression of mtlD gene at transcript level was confirmed using RT-PCR. Total RNA was extracted from five PCR positive plants following the protocol described by Chomczynski and Sacchi (1987) using Tripure isolation reagent (Roche, Germany), and was treated extensively with RNase free DNase in order to remove any contaminating genomic DNA. The DNA-free RNA was carefully quantified. Care was also taken to use equal amount of RNA from each sample for the one-step RT-PCR reaction according to the manufacturer’s guidelines (Titan One Tube RT-PCR Kit, Roche, Germany). The same mtlD primers pairs used in PCR reaction was also used for RT-PCR. The reaction mixture was incubated at 50°C for 30 min for reverse transcription. The amplification reactions consisted of 94°C for 5 min (1 cycle), followed by 35 cycles (95°C 1 min, 58°C 1 min, and 72°C 1 min) and finally an extension cycle of 5 min at 72°C. The PCR products were analyzed on 1% agarose gel as explained earlier.
Southern blot analysis
The integrity of integrated mtlD gene and its copy numbers were determined using Southern blotting analysis of one of the selected transgenic plants with highest level of transcript production as judged by semi quantitative RT-PCR, described above. Genomic DNA (30 μg) from the selected transgenic and a wildtype control plant were individually digested with EcoRI and XbaI + EcoRI. Digested DNA was separated on 0.8% agarose gel and then was blotted onto a positively charged nylon membrane (Roche, Germany) using the capillary transfer method (Sambrook and Russell 2001). The blot was probed with a 680 bp mtlD fragment (PCR product) labeled with DIG-dUTP using a PCR DIG Probe Synthesis Kit (Roche, Germany). Hybridization, series of stringency washing, and detection were carried out according to the instructions of the manufacturer.
Salt stress tolerance assessment
In vitro
For in vitro salt stress treatment, rooted cuttings from five transgenic (M1, M2, M8, M11, and M12) plants expressing the mtlD as judged by RT-PCR and one wildtype (control) plant were transplanted into test tubes (3 × 15 cm) containing 20 ml of basal MS medium supplemented with one of the five concentrations of NaCl (0, 50, 100, and 150 mM). One plant was established in each tube with five replications (five test tubes for each treatment). The growth conditions were as explained earlier. After 4 weeks, plant growth-related parameters, such as the fresh and dry weight of the shoots, the osmotic potential, and the mannitol content, were measured.
Hydroponics
The NaCl stress assay was performed for the best selected transgenic plant (M2) and a wildtype control in hydroponic culture. In vitro grown plants with well-developed root systems were cultured in dark plastic boxes that each contained 6.5 l MS medium. The root systems of the plantlets were inserted through small apertures in the lid, through the foam floating on the culture solution were immersed in the liquid medium. After a 1-week adaptation, the boxes were supplemented with five concentrations of NaCl (0, 50, 75, 100, 125, and 150 mM), respectively. Two boxes were used for each treatment. Each box contained four transgenic and four wildtype plants. The boxes were kept under conditions of 100 μmol m−2 s−1 light at 25 ± 2°C, under a 16-h photoperiod.
After 7 days, plant growth-related parameters, such as fresh and dry weights of the roots and shoots, root length, osmotic potential, and mannitol content, were analyzed. Fresh weight of shoots and roots and the root length were measured after 18 days. In all cases, the dry weights were measured after the plant samples were dried in an oven for 24 h at 70°C.
Measurements of osmotic potential
Plant shoots and roots were separately frozen in liquid nitrogen and stored at −70°C. A pestle was then used to grind the frozen shoots and roots in 1.5 ml test tubes in the presence of liquid nitrogen. The thawed pastes were then centrifuged at 20,000g for 10 min at 25°C. The supernatant fluid was used for osmotic potential analysis. A vapor pressure osmometer (VAPRO 5520, USA) was then used to determine the osmolality (c) of a 10 μl volume of the extracts (Mahdieh et al. 2008; Silveira et al. 2009). The osmotic potential (MPa) was calculated using the Van’t Hoff equation: ψs (MPa) = −cRT, where c is solute concentration in mol l−1 (mosmol), R is a constant (0.00831 MPa kg mol−1 K−1), and T is temperature in K.
Sugar analyses
Sugar alcohols were extracted from the dry matter based on the modified method of Ruperez and Toledano (2003). Powdered leaf samples (30 mg dry weight) were extracted with 1.5 ml of 80% (v/v) aqueous ethanol in 2 ml test tubes. The tubes were incubated overnight in a water bath at 80°C, before being centrifuged at 3,000g for 5 min at 4°C. The supernatants were removed to 15 ml screw-capped tubes and then evaporated and dried in an oven at 50°C for 2 h. The dried residue was dissolved in 10 ml distilled water, and 0.5 ml 5% ZnSO4 and 4.8% Ba(OH)2 were successively added to the solution to remove the protein. The suspension was then vortexed and centrifuged for 10 min at 3,000g. The supernatant was dried at 50°C. Immediately prior to conducting high-performance liquid chromatography (HPLC) analyses, the pellets were re-dissolved in Milli-Q water (1 ml) and filtered through 0.22 μm filters for aqueous solutions (Schleicher & Schuell, Germany). The sugar alcohol contents were analyzed via HPLC (Knauer), using a PA1 column (Eurokat H, 300 × 8.0 mm) in an isocratic run, with H2O (pH 2, with H2SO4) at a flow rate of 1 ml min−1 and detected by an RI detector. Mannitol content were identified by comparison of their retention times with those of authentic standards under analysis conditions and quantified by external standard method.
Statistical analysis
The data are given as the mean of at least three replicates. Data were analyzed by ANOVA and means were compared by Duncan test at 0.05 level of confidence. The standard deviation was plotted in all graphics.
Results
Transformation
The bacterial mtlD gene, under the control of a CaMV35S promoter (Fig. 1) was used for the genetic transformation of potato plants (c.v Marfona) by A. tumefaciens AGLO1. Twenty-one independently regenerated lines were developed from a total of 110 explants. Morphological and growth-related traits were carefully monitored during in vitro culture. Eighteen out of a total of 21 regenerated plants (85%) displayed normal phenotypes. The phenotypic appearances of the selected transgenic lines were undistinguishable from the wildtype control plants.
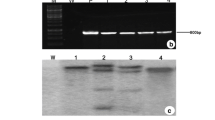
The putative transgenic plants were analyzed for the presence of the transgenes by PCR, Southern blotting, and RT-PCR analyses. All of the eighteen putative regenerated plants were PCR positive for both nptII and mtlD genes (Fig. 2a, b). Five of these plants were selected for further analysis.
Molecular analysis of mtlD transgenic potato plants. PCR analysis of transgenic plants using mtlD (a) and nptII (b) gene primers. c RT-PCR analysis of mtlD gene expression. d Southern blotting analysis of the integration of the mtlD gene, using the mtlD gene as a probe. M: 1 Kb ladder, M1–12: putative transgenic plants, P: plasmid, WT: wildtype plant, X: XbaI, E: EcoRI
All of the five PCR positive plants that were analyzed using RT-PCR confirmed the expression of mtlD gene at transcript level. This was absent in the control plant (Fig. 2c). There were some differences in the intensity of the RT-PCR bands. The most intense band was obtained for M2, indicating the highest level of expression of mtlD gene in this line. As a result, M2 was selected for southern analysis and the hydroponic culture experiments.
Further confirmation of the integration of the transgene into the genome of transgenic plants was achieved by Southern blot analysis (Fig. 2d). Southern analysis was carried out for one transgenic (M2) and one wildtype control plant. A single band of expected size at 1,750 bp was observed in the lane for DNA extracted from transgenic plant and double-digested with XbaI and EcoRI. This band was absent in the lane representing DNA extracted from control plant. This fragment corresponds with the entire coding sequence of mtlD and the terminator. Presence of this band in the same position in both lanes for plasmid and the transgenic plant indicates that at least on intact copy of the gene (coding sequence and terminator) has been integrated into the genome of this plant. For DNA that was digested with XbaI only, two bands were observed (about 1,750 and 2,900 bp). This indicated that two copies of the mtlD gene were integrated in the genome of the M2 line.
Salt stress tolerance
In vitro
The salt tolerance of the selected transgenic potato plants was initially studied in test tubes (in vitro salt stress analysis). Selected transgenic lines (M1, M2, M8, M11, and M12) and wildtype (WT) potato plants as controls were grown in 0, 50, 100, and 150 mM NaCl. After 4 weeks, the growth parameters were measured. There was no significant difference in the shoot’s fresh and dry weights of wildtype and transgenic plants under 0 mM NaCl. Although salt stress reduced the growth of both WT and transgenic plants (Fig. 3), the effect of NaCl stress was more severe on WT plants. The shoot fresh and dry weights in WT plants were reduced by 45.7 and 30.1%, respectively, but in the transgenic plants, the reduction in shoot fresh weight ranged from 0% (M2, M8, M11) to 15.1% (M1) and the shoot dry weight reduction ranged from 0 to 24.5%. Moreover, there was no observable difference between the appearance of all of transgenic plants at 0 and 50 mM NaCl. After 2 months and in response to increasing concentrations of NaCl, there was initial desiccation of the shoots of the transgenic plants; however, new shoots were subsequently regenerated from the lateral buds and grew on the little callus tissue. We observed this re-growth in all transgenic plants (but not in control plants) at all NaCl levels (Fig. 3a).
Effects of salt stress on wildtype (WT) and transgenic (M) lines grown under in vitro conditions. a Effect of 0 (a, e), 50 (b, f), 100 (c), and 150 (d) mM NaCl on transgenic (M) and wildtype (WT) potato plants. The re-grown plants shown in Mb, Mc, and Md. Shoot fresh (b) and dry (c) weights of WT and M plants after 4 weeks of growth in MS medium supplemented with 0, 50, 100, and 150 mM NaCl. Data are expressed as the mean ± SE of three replicates
The mannitol content of both stressed transgenic and wildtype plants were determined by HPLC analysis. A peak with a retention time (6.8 min) identical to that of mannitol was observed in all transgenic lines. In contrast, there was no peak that corresponded to the retention time for mannitol, even under stress conditions, in wildtype potato plants (Fig. 4), an indication of the absence of mannitol in wildtype plants.
Quantitative analysis showed that the mannitol content in different transgenic lines ranged from 12.1 to 28.27 μmol g−1 dw at 0 mM NaCl (Table 1). There was a reduction in the mannitol content with increasing concentrations of NaCl in the culture media.
Osmotic potential of shoots from stressed plants grown under in vitro conditions showed that there was no significant difference in the osmotic potential of WT and M (except in M12) lines at 0 mM NaCl. However, the osmotic potential in WT and transgenic lines was enhanced with increasing NaCl levels in the culture media (Table 2).
Hydroponics
In vitro analysis revealed that transgenic potato plants are more tolerant to NaCl stress in comparison with their wildtype counterpart. As a result, the evaluation of resistance of transgenic plants to salt stress under hydroponic conditions was encouraged. To achieve that, the most tolerant transgenic (M2) and one wildtype control plant were cultured in hydroponic conditions with different concentrations of NaCl. Plant growth parameters were analyzed 7 and 18 days after culture. Overall, the initial shoots and roots fresh and dry weights of the transgenic line (M2) were similar to those of the wildtype plants at 0 mM NaCl (Figs. 5, 6). However, with increasing NaCl levels, there were significant differences in plant growth between the M2 and wildtype plants. After 7 days of exposure to 100 mM NaCl, the shoot fresh weight of the wildtype plants decreased by 56.4%, whereas that of the M2 line declined by only 17.7% (Fig. 6). Although the root fresh weight of wildtype was reduced by 61.8%, there was no significant reduction in the root fresh weight of the M2 line at 100 mM NaCl. There was no significant reduction in the shoot and root fresh weights of M2 plants at 50 and 75 mM in comparison to those at 0 mM NaCl. Similar results were observed for the dry weights of wildtype and M2 plants that were exposed to salt stress. At 100 mM NaCl, the shoot and root dry weights of wildtype plants were reduced by 55.5 and 50.5%, respectively. However, at 100 mM NaCl, the shoot and root dry weights of the M2 line decreased by 28 and 9%, respectively. Moreover, after 7 days, 100 mM NaCl reduced the root length of wildtype plants by 33.3% of that under non stressed condition. However, there was no significant difference between the root lengths of M2 line plants under 125 mM NaCl and those that experienced 0 mM NaCl conditions.
NaCl stress tolerance of transgenic (M2, left) and wildtype (WT, right) potato lines after 7 days in hydroponic culture conditions. a 0 mM NaCl. b 75 mM NaCl. c Whole appearance of M2 and WT plants at 0 mM NaCl. d Whole appearance of M2 and WT plants at 75 mM NaCl. e Root length differences of M2 and WT plants after 18 days exposure to 75 mM NaCl
Fresh weight, dry weight and root length of wildtype plants (but not the transgenic ones) were more profoundly affected by NaCl stress after 18 days compared to those after 7 days (Figs. 5, 6). At 100 mM NaCl, the shoot and root fresh weights of wildtype plants were reduced by 76.5 and 38.6%, respectively. Their root length was reduced by 44.9% under the same conditions. However, at 100 mM NaCl concentration, the shoot fresh weight, root fresh weight, and root length were reduced in M2 plants by 17.3, 33, and 35.4%, respectively.
The mannitol contents of wildtype and M2 plants were measured under hydroponic culture conditions (Table 3). Although, there was no significant difference between the mannitol content of M2 roots and shoots in the absence of NaCl stress, the accumulation of mannitol in 50 mM NaCl was over 1.8-fold more in the shoots and 1.2-fold more in the roots in comparison with those at 0 mM NaCl. However, when the NaCl concentration was increased to 100 mM, there was no further increase in the mannitol content of M2 plant shoots. At 125 and 150 mM NaCl, there was a slight decrease in the mannitol content of M2 transgenic plant. The wildtype plants did not accumulate mannitol under any conditions.
Under hydroponic culture conditions, there was a significant reduction in osmotic potential of shoots with increasing NaCl levels, in both wildtype and M2 plants (Table 4). However, the osmotic potential in M2 roots reduced more than in wildtype roots. At 150 mM NaCl, the osmotic potential in the wildtype roots was reduced to −1.025 MPa, whereas there was a reduction of −1.159 MPa in M2 plants. There was no significant difference between the osmotic potential changes in the shoots of wildtype and M2 plants with increasing NaCl levels.
Discussion
Potato is relatively sensitive to salinity, particularly at early growth stages (Patel et al. 2001; Rahnama and Ebrahimzadeh 2005). Naturally, potato plants do not synthesize the osmoprotectant mannitol. Therefore, we hypothesized that the tolerance of potato plants to stress could be improved by introducing a gene for the synthesis of mannitol. Hence, the bacterial mannitol-1-phosphate dehydrogenase gene mtlD, under the control of the cauliflower mosaic virus 35s (CaMV35S) promoter, was used for Agrobacterium mediated transformation of potato plants. The MTLD enzyme catalyzes the biosynthesis of mannitol-1-phosphate from fructose 6-phosphate. Thereafter, plant nonspecific phosphatases can convert mannitol-1-phosphate to the osmolyte mannitol (Tarczynski et al. 1992; Zhifang and Loescher 2003).
In this study, 3 of 21 potato plants that were grown on regeneration media containing 100 mg l−1 kanamycin had abnormal phenotypes, such as stunted growth. Periclinal chimerism is a common phenomenon in tetraploid potatoes (Wilkinson 1994) that leads to genetic changes and phenotypic abnormalities. These types of genomic alterations have been reported in transgenic potato plants. Therefore, the phenotypic abnormalities in the transgenic lines may be due to a genomic change, ploidy chimerism, positional effects of the transgene, or regeneration events (Behnam et al. 2006; Celebi-Toprak et al. 2005).
PCR analyses showed that all of the eighteen regenerated plants that had normal phenotypes contained the mtlD gene. Therefore, there was no escape following selection at 100 mg l−1 kanamycin. Moreover, RT-PCR confirmed the expression of mtlD gene in the transgenic potato plants. On the other ands, Southern blotting analysis confirmed the integration of two copies of the mtlD gene in one selected putative transgenic line (M2). This transgenic line was then used for salt tolerance analysis under hydroponic conditions.
In vitro salt tolerance analysis demonstrated that there were significant differences between the fresh and dry weights of shoots from transgenic and wildtype lines. The transgenic potato plants were proved to be less affected by NaCl stress than the WT plants (Fig. 3b, c). This confirmed that, the introduction of the mtlD gene enabled all selected transgenic lines (M1, M2, M8, M11, and M12) to synthesize mannitol and increased their tolerance to salt stress. However, the mannitol content in individual lines was variable. Similar results have been reported for transgenic tobacco (Tarczynski et al. 1993; Ruperez and Toledano 2003), Arabidopsis (Thomas et al. 1995; Zhifang and Loescher 2003), petunia (Chiang et al. 2005), loblolly pine (Tang et al. 2005), and eggplant (Prabhavathi and Rajam 2007). Variations in the mannitol content may be due to copy number and to the positional effects of the transgene that may result in differential expression of the transgene in different lines (Hobbs et al. 1990; Celebi-Toprak et al. 2005; Tang et al. 2005; Waditee et al. 2007).
Although mannitol levels in all transgenic lines declined with increasing NaCl concentrations, the decrease in mannitol content of transgenic lines M1 and M2 was significantly less than the other lines (Table 1). On the other hand, there was no overall difference in the osmotic potentials of the shoot samples from wildtype and transgenic lines during exposure to in vitro salt stress. However, the osmotic potentials of the stressed plants were higher than those under non-stress conditions. These data suggest that the expression of the mtlD gene enhances tolerance to salt stress in transgenic lines by the production of mannitol. This also may suggest that this is not only the osmotic effect of mannitol, but also the other activities of mannitol such as osmoprotectant activity of this sugar alcohol that makes the difference in plant’s stress tolerance.
The results based on hydroponically grown plants revealed no significant differences between the growth of transgenic (M2) and wildtype plants at 0 mM NaCl. The growth of both M2 and wildtype lines was affected by increasing NaCl levels. Nevertheless, growth parameter analysis showed that the M2 line was significantly more tolerant to NaCl stress than the wildtype plants (Figs. 5, 6).
Wildtype plants were unable to survive 150 mM NaCl; after 1 week, they appeared dried-up and withered. In contrast, plants of the M2 line had a normal appearance and a standing growth under these conditions. Moreover, whereas after 3 weeks at 75 mM NaCl, there was only a slight reduction in the growth of M2 line plants, the growth of wildtype plants was completely inhibited, and they appeared desiccated. The improved tolerance of the transgenic line may be attributed to the induction and incremental increases in the mannitol content in the roots and shoots of the plants. In contrast to in vitro grown plants, the mannitol content of the transgenic line (M2) increased in the roots and shoots at 50 mM NaCl, but it slightly decreased after 75 and 100 mM NaCl. Moreover, as shown in Fig. 6, the root length of the wildtype and M2 plants at 150 mM NaCl decreased after 18 days in comparison with 7 days. This can be attributed to decomposition of the roots at that NaCl level.
It has been proposed that mannitol enhances tolerance to osmotic stress primarily through osmotic adjustment (Loester et al. 1992; Abebe et al. 2003). In the hydroponic culture, the osmotic potential in wildtype and M2 plants reduced with increasing NaCl levels. This reduction in osmotic potential could help the plants to uptake more water and grow normally under high osmotic pressure (Abebe et al. 2003). The present results suggest that mannitol may contribute to the osmotic adjustment of roots and shoot cells to some extent. Although our results revealed that the osmotic potential in the M2 roots increased more than in the wildtype roots with increasing NaCl levels, there was no significance difference in osmotic potential between the wildtype and M2 shoots (Table 4). Therefore, we suggest that the osmoregulatory function of mannitol in M2 plants is more important in the roots than in the shoots.
Moreover, from the results of HPLC analysis, we found that the mannitol content in the transgenic lines was not sufficient to act as an osmolyte. Thus, the tolerance of transgenic plants to NaCl-induced by lowering of the osmotic potential is probably limited. It is known that sugar alcohols, such as mannitol, can also act as free radical scavengers (antioxidant function) or chemical chaperones (osmoprotectant function) by directly stabilizing membranes and enzymes/proteins (Hare et al. 1998).
Earlier studies have demonstrated that mannitol accumulation in transgenic tobacco plants is not adequate to exert an osmoregulatory effect (Tarczynski et al. 1992, 1993; Thomas et al. 1995). Nevertheless, Karakas et al. (1997) estimated that mannitol contributes only 0.003–0.004 MPa to osmotic adjustment in transgenic tobacco under salt stress. However, when the biosynthesis of mannitol was targeted specifically to chloroplasts in transgenic tobacco plants, there was increased resistance to oxidative stress (Shen et al. 1997). The ectopic expression of the mtlD gene in transgenic wheat improved tolerance to salt and water stress both at the callus and whole plant levels (Abebe et al. 2003). However, these results also demonstrated that the amount of accumulated mannitol was too small to account for its effect as an osmolyte; therefore, the authors suggested that it might act as an oxygen free radical scavenger. Similar results have been reported for transgenic eggplants (Prabhavathi et al. 2002) and petunia (Chiang et al. 2005) that expressed the bacterial mtlD gene. Enhanced tolerance to salt stress has also been reported in trees such as loblolly pine (Tang et al. 2005) and poplar (Hu et al. 2005). Results from the latter study demonstrated that transgenic poplar plants are better able than wild-type plants to maintain cell membrane integrity under salt stress than wildtype plants; this confirmed the osmoprotection function of mannitol in plant cells.
In contrast to mtlD transgenic plants, salt stress increased the mannitol content of transgenic Arabidopsis plants that expressed celery mannose-6-phosphate reductase (M6PR) (Zhifang and Loescher 2003). In 50 mM NaCl, the M6PR transformants accumulated more than twice as much mannitol as did salt-free controls. In this study, the mannitol content assay in 4 weeks old transgenic plants did not reveal any changes with increasing NaCl levels under in vitro conditions. However, the mannitol content of the shoots and roots at 50 mM NaCl were 1.8- and 1.2-fold more, respectively, under salt stressed condition that resulted in increased mannitol levels in transgenic line (M2) plants grown under hydroponic cultures. Therefore, we suggest that the mannitol content of transgenic potato plants may be altered by differential expression of the mtlD gene that is due to either environmental factors (e.g., NaCl) or to the precise stage of plant development.
In conclusion, our results indicate that successful integration of the mtlD gene resulted in the synthesis of mannitol in transgenic potato plants, and that this enhanced the tolerance of transgenic lines to NaCl stress. Overall, the amount of accumulated mannitol in the transgenic lines was too small to act as an osmolyte; thus, we assumed that it might act as an osmoprotectant. However, the results demonstrated that mannitol has a greater contribution to the osmotic adjustment of transgenic roots than shoots. Moreover, it appears likely that NaCl may increase the mannitol content of transgenic plants via induction of the mtlD gene.
References
Abebe T, Guenzi AC, Martin B, Cushman JC (2003) Tolerance of mannitol-accumulating transgenic wheat to water stress and salinity. Plant Physiol 131:1748–1755
Almeida AM, Cardoso LA, Santos DM, Torné JM, Fevereiro PS (2007) Trehalose and its applications in plant biotechnology. In Vitro Cell Dev Biol Plant 43:167–177
Behnam B, Kikuchi A, Celebi-Toprak F, Yamanaka S, Kasuga M, Yamaguchi-Shinozaki K, Watanabe KN (2006) The Arabidopsis DREB1A gene driven by the stress-inducible rd29A promoter increases salt-stress tolerance in proportion to its copy number in tetrasomic tetraploid potato (Solanum tuberosum). Plant Biotechnol 23:169–177
Bhatnagar-Mathur P, Vadez V, Sharma KK (2008) Transgenic approaches for abiotic stress tolerance in plants: retrospect and prospects. Plant Cell Rep 27:411–424
Blumwald E (2000) Sodium transport and salt tolerance in plants. Curr Opin Cell Biol 12:431–434
Boyer JS (1982) Plant productivity and environment. Science 218:443–448
Celebi-Toprak F, Behnam B, Serrano G, Kasuga M, Yamaguchi-Shinozaki K, Naka H, Watanabe JA, Yamanaka S, Watanabe KN (2005) Tolerance to salt stress in transgenic tetrasomic tetraploid potato, Solanum tuberosum cv. Desiree appears to be induced by DREB1A gene and rd29A promoter of Arabidopsis thaliana. Breed Sci 55:311–320
Chiang YJ, Stushnoff C, McSay AE (2005) Overexpression of mannitol-1-phosphate dehydrogenase increase mannitol accumulation and adds protection against chilling injury in petunia. J Am Soc Hort Sci 130:605–610
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate–phenol–chloroform extraction. Anal Biochem 162:156–159
Dellaporta SL, Wood J, Hicks JB (1983) A plant DNA minipreparation: version II. Plant Mol Biol Rep 1:19–21
Ghasimi Hagh Z, Rahnama H, Panahandeh J, Baghban Kohneh Rouz B, Arab Jafari KM, Mahna N (2009) Green-tissue-specific, C4-PEPC-promoter-driven expression of Cry1Ab makes transgenic potato plants resistant to tuber moth (Phthorimaea operculella, Zeller). Plant Cell Rep 28:1869–1879
Gopal J, Iwama K (2007) In vitro screening of potato against water-stress mediated through sorbitol and polyethylene glycol. Plant Cell Rep 26:693–700
Hare PD, Cress WA, Van Staden J (1998) Dissecting the roles of osmolyte accumulation during stress. Plant Cell Environ 21:535–553
Heuer B, Nadler A (1998) Physiological response of potato plants to soil salinity and water deficit. Plant Sci 137:43–51
Hobbs SLA, Kpodar P, Delong CMO (1990) The effect of T-DNA copy number, position and methylation on reporter gene expression in tobacco transformants. Plant Mol Biol 15:851–864
Hu L, Lu H, Liu Q, Chen X, Jiang X (2005) Overexpression of mtlD gene in transgenic Populus tomentosa improves salt tolerance through accumulation of mannitol. Tree Physiol 25:1273–1281
Huizhong W, Danian H, Ruifang LU, Junjun LIU, Qian Q, Xuexian P (2000) Salt tolerance of transgenic rice (Oryza sativa L.) with mtlD gene and gutD gene. Chinese Sci Bull 45:1685–1689
Karakas B, Ozias-Akins P, Stushnoff C, Suefferheld M, Rieger M (1997) Salinity and drought tolerance of mannitol-accumulating transgenic tobacco. Plant Cell Environ 20:609–616
Kotuby-Amacher J, Koeing R, Kitchen B (2000) Salinity and plant tolerance. Electronic Publishing AG-SO-03
Loester WH, Tyson RH, Everard JD, Redgwell RJ, Bieleski RL (1992) Mannitol synthesis in higher plants: evidence for the role and characterization of a NADPH-dependent mannose-6-phosphate reductase. Plant Physiol 98:1396–1402
Mahdieh M, Mostajeran A, Horie T, Katsuhara M (2008) Drought stress alters water relations and expression of PIP-type aquaporin genes in Nicotiana tabacum Plants. Plant Cell Physiol 49:801–813
Munns R (2005) Genes and salt tolerance: bringing them together. New Phytol 167:645–663
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissues cultures. Physiol Plant 15:473–479
Patel RM, Prasher SO, Donnelly D, Bonnell RB (2001) Effect of initial soil salinity and subirrigation water salinity on potato tuber yield and size. Agric Water Manage 46:231–239
Patonnier MP, Peltier JP, Marigo G (1999) Drought-induced increase in xylem malate and mannitol concentrations and closure of Fraxinus excelsior L. stomata. J Exp Bot 50:1223–1229
Prabhavathi V, Rajam MV (2007) Mannitol-accumulating transgenic eggplants exhibit enhanced resistance to fungal wilts. Plant Sci 173:50–54
Prabhavathi V, Yadav JS, Kumar PA, Rajam MV (2002) Abiotic stress tolerance in transgenic eggplant (Solanum melongena L.) by introduction of bacterial mannitol phosphodehydrogenase gene. Mol Breed 9:137–147
Rahnama H, Ebrahimzadeh H (2005) The effect of NaCl on antioxidant enzyme activities in potato seedlings. Biol Plant 49:93–97
Ruperez P, Toledano G (2003) Celery by-products as a source of mannitol. Eur Food Res Technol 216:224–226
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Shen B, Jensen RG, Bohnert HJ (1997) Increased resistance to oxidative stress in transgenic plants by targeting mannitol biosynthesis to chloroplasts. Plant Physiol 113:1177–1183
Silveira JAG, Araújo AM, Lima JPM, Viégas RA (2009) Roots and leaves display contrasting osmotic adjustment mechanisms in response to NaCl-salinity in Atriplex nummularia. Environ Exp Bot 66:1–8
Stoop JMH, Williamson JD, Pharr DM (1996) Mannitol metabolism in plants: a method for coping with stress. Trends Plant Sci 1:139–144
Tang W, Peng X, Newton RJ (2005) Enhanced tolerance to salt stress in transgenic loblolly pine simultaneously expressing two genes encoding mannitol-1-phosphate dehydrogenase and lucitol-6-phosphate dehydrogenase. Plant Physiol Biochem 43:139–146
Tarczynski MC, Jensen RG, Bohnert HJ (1992) Expression of a bacterial mtlD gene in transgenic tobacco leads to production and accumulation of mannitol. Proc Natl Acad Sci USA 89:2600–2604
Tarczynski MC, Jensen RG, Bohnert HJ (1993) Stress protection of transgenic tobacco by production of the osmolyte mannitol. Science 259:508–510
Thomas JC, Sepahi M, Arendall B, Bohnert HJ (1995) Enhancement of seed germination in high salinity by engineering mannitol expression in Arabidopsis thaliana. Plant Cell Environ 18:801–806
Waditee R, Bhuiyan NH, Hirata E, Hibino T, Tanaka Y, Shikata M, Takabe T (2007) Metabolic engineering for betaine accumulation in microbes and plants. J Biol Chem 282:34185–34193
Wang W, Vinocur B, Altman A (2003) Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta 218:1–14
Wilkinson MJ (1994) Genome evolution on potatoes. In: Bradshaw JE, MacKay GR (eds) Potato genetics. CAB International, Wallingford, pp 56–57
Yokoi S, Bressan RA, Hasegawa PM (2002) Salt stress tolerance of plants. JIRCAS Working Report 25–33
Zhifang G, Loescher WH (2003) Expression of a celery mannose 6-phosphate reductase in Arabidopsis thaliana enhances salt tolerance and induces biosynthesis of both mannitol and a glucosyl-mannitol dimer. Plant Cell Environ 26:275–283
Acknowledgments
We thank Mrs. Z. Ghasimi Hagh and Mr. M. R. Shams from ABRII, Karaj, Iran, for their technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by T. Moriguchi.
Rights and permissions
About this article
Cite this article
Rahnama, H., Vakilian, H., Fahimi, H. et al. Enhanced salt stress tolerance in transgenic potato plants (Solanum tuberosum L.) expressing a bacterial mtlD gene. Acta Physiol Plant 33, 1521–1532 (2011). https://doi.org/10.1007/s11738-010-0690-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-010-0690-8