Abstract
To understand the response patterns to soil drying and the water use properties of commonly reforested trees in the semiarid Loess Plateau region of China, a glasshouse experiment was carried out with the seedlings of four species, i.e., Robinia pseudoacacia, Armeniaca sibirica, Syringa oblata, and Quercus liaotungensis. Severe water stress induced by withholding water resulted in permanent wilting of most of the seedlings pot-cultured with sandy soil in 8–12 days. Predawn and midday leaf water potentials and gas exchange characteristics (e.g., stomatal conductance) in the seedlings did not show marked changes until the volumetric soil water content decreased to about 0.05. As the soil water content decreased further, these physiological parameters rapidly declined, approaching their minimal levels at the stage of permanent wilting. The response of each parameter to soil water content changes was fitted with a non-linear saturation curve. Though the results suggested that the general pattern of responses to soil drying was identical among the species, quantitative differences in drought tolerance and water use properties were detected. Leaf stomatal conductance in R. pseudoacacia and A. sibirica showed earlier responses to reduced predawn leaf water potentials. However, water use characteristics and specific leaf area indicated that these two species consumed more water and may not be as drought tolerant as S. oblata and Q. liaotungensis. These results may provide important information to compare the reforestation species with respect to soil drying.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Reforestation has long been considered to be of great importance for the conservation of soil and water as well as environmental restoration in vegetation-degraded areas (Tian et al. 2003). The Loess Plateau in China is one of the areas with dry climates and extremely dissected topography. This region poses a major challenge for environmental restoration because of aridity and severe soil erosion. A better understanding of drought-response mechanisms and the ecophysiological properties of commonly applied species in the region is needed to address large-scale reforestation (Cheng and Wan 2002; Tian et al. 2003).
The environmental characteristics, soil water conditions in particular, substantially dominate the growth and distribution of vegetation (Wu and Yang 1998; Wu 1980). Species growing in arid and semiarid regions must be able to respond to ecological conditions of the region that are characterized by frequent soil water deficit. Ecophysiological properties of trees are commonly considered when their capacity for growth and stress tolerance is evaluated (Kozlowski and Pallardy 1997; Larcher 2003). Several physiological strategies of plants have been documented in response to drying soil and air. These include the decrease in water potentials of aboveground organs, leaf stomatal closure, osmotic adjustment, activation of anti-oxidative enzymes, and even defoliation (Hara et al. 2008; Ingram and Bartels 1996; Kozlowski 1976; Monneveux and Belhassen 1996; Rouhi et al. 2007). It has been reported that observable growth inhibition appears when soil water content reduces to nearly the permanent wilting point, an important parameter that is highly texture-dependent (Sinclair et al. 1998; Veihmeyer and Hendrickson 1950).
The response of physiological characteristics to soil drying has been less studied in tree species than in the annual crop species (Sinclair et al. 2005). Several studies revealed that the general pattern of response to soil drying is virtually the same in tree and crop species, except for a variation in the thresholds for significant changes in the physiological properties (Gollan et al. 1985; Sadras and Milroy 1996; Sinclair et al. 2005). The response patterns of plant dehydration processes can be divided into two or three stages. Physiological characteristics, e.g., leaf gas exchange, do not show substantial changes in the initial stage of stress, whereas a distinct decline (almost linear) of the final stage appears after a threshold of water availability (Sadras and Milroy 1996; Sinclair et al. 2005; Sinclair and Ludlow 1986).
Extractable soil water content was frequently used as a parameter to discuss the threshold for the decrease in growth and transpiration of plants. With rigorous definitions, the fractions of available soil water and transpirable soil water were widely documented (Sadras and Milroy 1996; Sinclair et al. 1998; Wahbi and Sinclair 2007). However, this parameter is highly texture-dependent with respect to the actual soil water content. As the determination of the upper limit of the extractable soil water is subjected to high variation, it has also been suggested that the physiological performance in response to soil water condition be expressed as a function of the volumetric water content of the soil, especially in the case of sandy soils (Ritchie 1981; Savage et al. 1996; Sinclair et al. 1998). Thus, the present study was designed to comparatively investigate the response patterns of leaf water potential and gas exchange parameters to decreasing soil water content in four semiarid reforestation species. Robinia pseudoacacia is an exotic species and widely planted in the Loess Plateau, whereas the other three species are indigenous to the region. We also examined the water use differences among the species, so that proper information could be provided for the selection and management of reforestation species in the region.
Materials and methods
Plant material and experimental protocol
About 1.5-year-old seedlings of four tree species were used for the glasshouse experiment at Arid Land Research Center, Tottori University, Japan. One is Robinia pseudoacacia L., which is exotic to the Loess Plateau of China but widely planted in the region; the other three are Armeniaca sibirica L., Syringa oblata Lindl., and Quercus liaotungensis Koidz., which are native to this region. The seeds were collected from the northern Shaanxi province of China in the autumn of 2003. The seeds of Q. liaotungensis were sown on a germination tank filled with vermiculite and kept at relatively low temperatures (5–10°C) for the winter season. The seeds of the other three species were sown on the germination tanks in the early spring of 2004. The seedlings were subsequently transplanted and cultured in 7 × 7 × 19.5-cm paper pots containing sand and peat (2/1, v/v) under normal conditions for about 16 months, during which they were irrigated regularly to maintain a normal growth. During this period, the seedlings did not experience soil drought and all species were under the same soil and atmospheric conditions. A diluted nutrient solution of general composition (Hyponex®, Hyponex, Japan) was applied once a month for the growing season. An insecticide (Kadan®, Fumakilla Co. Ltd., Japan) was sprayed several times on the R. pseudoacacia seedlings to ward off aphids, which were observed on the leaves occasionally.
Polyvinyl pots of 15.8 cm internal diameter and 19.5 cm height were prepared for the stress treatment. Each pot was heaped up with 5 kg of dry sand (3.5 dm3), and a hole covered with a piece of net near the bottom for the outflow of excess water. On 20 July 2005, one month before the treatment, 20 seedlings of each species were selected based on their uniformity of size and development. Each seedling was carefully washed with clean water to remove the original soil from the roots, and was weighed on an electronic balance. Subsequently, it was transplanted to a prepared polyvinyl pot and watered to approximately the field capacity, with a little leaching being observed from the bottom hole. The soil water content for each seedling was monitored by weighing the pot, assuming that the change in the seedling weight during the subsequent 1.5-month consecutive measurements was negligible, when compared with that in the soil water content. During the one-month acclimation period of the seedlings to the new pots with sandy soil, watering was carried out every evening to replenish the amount lost over the previous 24 h.
Drought stress treatment was induced by withholding water supply from 20 August 2005, by which time the prepared seedlings were growing normally. A total of 15 seedlings for each species were subjected to soil drying treatment and 5 seedlings were watered regularly and kept as a control group. The treated seedlings were totally dehydrated on days 8–12 of water withholding. Therefore, the measurements were carried out until day 12.
Measurements of physiological parameters
Leaf water potential, leaf gas exchange, and soil water content were measured at 2-day intervals after commencing the drought treatment. For gas exchange measurements, a well-expanded leaf was selected from each seedling and measured at 8:00–9:00 h with a portable photosynthesis system (LI-6400, Li-Cor Inc., Lincoln, NE, USA) under approximately optimal conditions. The leaf air-vapor pressure deficit during the measurements was observed to be 1–4 KPa, and the ambient CO2 concentration was 370–380 μmol mol−1. The light intensity inside the leaf chamber was controlled at photosynthetic active radiation (PAR) of 1,500 μmol m−2 s−1.
For the measurement of leaf water potential, 5−6 seedlings were sampled each time for each species, except at the last stage of the treatment when 4 or 3 were measured, as the other seedlings were dehydrated. Predawn (Ψpd) and midday (Ψmd) leaf water potentials were measured at about 4:00 and 12:00 h of local time, respectively, using a pressure chamber (DIK-7001, Daiki Rika Kogyo Co. Ltd., Tokyo, Japan). For each R. pseudoacacia seedling, a lamina containing 5 leaflets was measured. For the other three species, a single leaf was used for each measurement. The data for leaf water potential measurements were recorded as 6 MPa when they were beyond the measuring limit of 5 MPa, i.e., at the final stage of the treatment and the last measurement for the same seedling. The pots were weighed on an electronic balance just after the measurements of predawn leaf water potentials and the soil volumetric water contents were calculated. The control seedlings were not measured for leaf water potentials, as the data measured at the beginning of drought treatment for treated seedlings could be referenced as control.
Determination of specific leaf area
For the determination of specific leaf area (SLA), 30 mature leaves were randomly taken from the control seedlings of each species (in all directions, at about 20-cm height), kept in paper bags, and brought to the laboratory. After making full-scale copy images, the leaves were oven dried at 80°C for 24 h and weighed. The leaf images were then measured for surface area with an area meter on Microsoft Windows (LIA32 ver. 0.376, K. Yamamoto, Nagoya Univ., Japan), and SLA was calculated as the ratio of leaf area to dry leaf weight.
Biomass determination and water consumption analysis
For determination of water use, each seedling, including any dropped leaves, was collected at the end of the experiment. After washing the roots with clean water to remove the attached soil from the roots, we divided the seedlings into leaves and other materials including stems and roots. We gathered the materials separately, and oven dried them at 80°C for 24 h to calculate the dry weight. The total leaf area for each seedling was calculated from the dry weight and SLA. The total water consumption during the initial 6 days of treatment, during which the seedling did not show signs of permanent wilting and drastic decline of physiological parameters, was calculated from the pot weight difference. The water use for each seedling was calculated on the basis of both leaf area and biomass.
Data analysis
The data for gas exchange measurements were double-normalized, so that the relative stomatal conductance, net photosynthesis, and transpiration rates were obtained. The data were first processed by dividing the data for each drying pot by the mean of the well-watered pots (control seedlings) measured on the same day. This was expected to eliminate the error caused by different environmental conditions during the measurement days. To minimize the error from the differences among the seedlings, e.g., variation in total leaf area, the relative data for each seedling was further divided by the value for the same seedling obtained under non-limited conditions. This was determined by averaging the data of the early experimental period (6 days). Consequently, normalized stomatal conductance, net photosynthesis, and transpiration rates were set to a value of 1.0 for all the seedlings during the early stage of the experiment.
As the pace of total water loss varied with the individual seedlings during the drought treatment, we did not calculate the average for the data of the replicated seedlings in each measuring day. Physiological parameters should be correlated with the soil water conditions as such, rather than the treated days. Therefore, the effects of water stress on the parameters of leaf water potential and gas exchange were expressed by dotted graphs with the variable of volumetric soil water content (instead of treated days). Non-linear saturation regressions were fitted as suggested by Sinclair and Ludlow (1986), and all the regressions were found to be statistically significant (P < 0.001). SigmaPlot 10.0 software for Windows (Systat Software Inc., USA) was used for the analyses and graph making.
Results
Leaf water potential change during the soil drying course
For all the four investigated species, the predawn and midday leaf water potentials did not show marked changes until the soil water content decreased to <0.05 (Fig. 1). With the progressing water stress, the leaf water potentials decreased. However, steady declines only lasted for a short period before they vastly decreased to the extent of permanent wilting. The four species followed the same pattern and a non-linear saturation curve was plotted on each dataset. The predawn leaf water potentials during the early stage did not show large variation among the species, except that the data for A. sibirica were slightly lower (−0.6 MPa) than those of the other species. Particularly, A. sibirica showed the lowest midday leaf water potentials (−2.3 MPa), followed by R. pseudoacacia (−1.7 MPa). However, these two species showed rather steep declines when compared with Q. liaotungensis and S. oblata at the late stage. These differences were also shown in the regression equations where the absolute values for coefficients of θ were higher in R. pseudoacacia and A. sibirica than in Q. liaotungensis and S. oblata.
Change in the gas exchange parameters
The responses of leaf stomatal conductance, photosynthesis, and transpiration were essentially identical in the four species examined, and hence, only the changes in the stomatal conductance were presented (Fig. 2). Both R. pseudoacacia and S. oblata were slightly more sensitive to soil drying than the other two species, as illustrated by the relatively lower coefficient value for θ in the regression equations of the former species.
To further investigate the variation among the species, we carried out another kind of regression analysis so that the curved part of a curve was highlighted. The response pattern of the physiological parameters (e.g., stomatal conductance) was linearly fitted with a plateau line of 1.0 normalized data and a decline line (Sinclair et al. 2005; Wahbi and Sinclair 2007). The datasets obtained at low θ values where normalized stomatal conductance was <0.8 were included in the linear decline regression for the severely stressed phase. The intersection of the linear decline with the plateau value of 1.0 was calculated to present the onset of drying-induced response in gas exchange (Fig. 3). These results are consistent with those suggested by regressions presented in Fig. 2.
Normalized stomatal conductance vs. volumetric soil water content in which linear regressions were applied. The datasets obtained at low θ values, where normalized stomatal conductance was <0.8, were included in the linear decline regression. All the linear regression curves are statistically significant (P < 0.001). Note the difference among the species in θ value for the intersection of the linear decline with the plateau value of 1.0
Based on the regression equations given in Figs. 1 and 2, we calculated the normalized stomatal conductance for each species at the conditions when predawn leaf water potentials reduced to twice the quantity measured at the non-stressed early phase (Fig. 4). Consequently, we observed that R. pseudoacacia and A. sibirica showed stomatal closure earlier than the other two species in response to decreasing leaf water potentials.
Specific leaf area and water use features
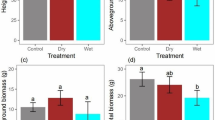
The average daily water consumption at the early stage and SLA for the four species are shown in Fig. 5. In general, R. pseudoacacia and A. sibirica were observed to have a high water consumption feature, in contrast to S. oblata and Q. liaotungensis. The water use based on leaf area was the highest for R. pseudoacacia among the four species. On the other hand, S. oblata and Q. liaotungensis were observed to have a smaller SLA and physically tough leaves.
Water consumption characteristics and specific leaf area (SLA) for the seedlings of each species. Water use was calculated on the basis of leaf area and biomass, respectively. Error bars represent standard errors (n = 15). Data for the calculation of SLA were obtained from the sum of 30 leaves in each species
Discussion
Seedlings consume much water during the growing season. The depletion dynamics of soil water in response to discontinuance of watering varies with species and size. In our study, R. pseudoacacia seedlings demonstrated more rapid water loss. This can be attributed to both their large number of leaves and higher rate of transpiration than the other species examined. In this study, we focused on the response patterns of physiological characteristics during the soil drying period. The differences in leaf numbers of the seedlings among the species were neglected, because the data were normalized. However, as the structure of rooting systems may result in variations in absorption of soil water, the extractable water content in each pot during the drying treatment could not be determined. We used the parameter of volumetric soil water content in this experiment and showed a pattern of physiological responses similar to that demonstrated by other researchers (Sinclair et al. 2005; Sinclair and Ludlow 1986). The threshold values of volumetric soil water contents at which the physiological indices began to decline significantly were lower than those documented earlier (Sinclair et al. 1998). This should be reasonable considering the differences in soil texture and root structure among different studies.
It is widely documented that stomatal conductance responds to both soil moisture and air conditions. However, whether it is dominated by the available soil water or meteorological conditions is still controversial. The reports suggesting that soil water deficit caused stomatal closure support the control of stomatal mechanism by the signal of abscisic acid produced in roots (Bates and Hall 1981; Gollan et al. 1985, 1986; Turner et al. 1985; Zhang et al. 1987). On the contrary, there have been reports suggesting a relationship between the leaf water potential and stomatal conductance independent of soil and root conditions (Fort et al. 1997; Fuchs and Livingston 1996; Saliendra et al. 1995; Whitehead et al. 1996). It is difficult to reject either of the above-mentioned explanations, as soil drying was observed to cause declines in both leaf water potential and gas exchange in our study, along with quantitative variations among the species. The regression curves and coefficient values for θ in the equations indicated that the reduction of leaf water potentials in R. pseudoacacia and A. sibirica lagged behind the other two species examined (Fig. 1). This might be related to their well-developed rooting systems that could absorb the maximum available water inside the pots. However, Fig. 4 suggests early closure of stomata in R. pseudoacacia and A. sibirica, relative to the decreasing predawn leaf water potentials, whereas S. oblata and Q. liaotungensis were observed to maintain leaf stomatal conductance and photosynthesis at lower water potentials, though the investigation of gas exchange was only conducted in the morning hours.
Plants may adapt to water stress through various strategies. The SLA that represents the light-intercepting area of a leaf per unit dry mass is one of the indices related to many important physiological characteristics (Reich et al. 1999). Leaves with small SLA are observed to be physically tough, and lower SLA may be correlated with the increasing aridity (Reich et al. 1999; Zhang et al. 2005). High rates of photosynthesis and transpiration were recorded for R. pseudoacacia and A. sibirica, which have higher SLA. Furthermore, water utilization of these two species was observed to be less economical (Fig. 5). In addition, with the properties of rapid growth, relatively thin leaflets, and high rate of photosynthesis, R. pseudoacacia has been observed to accommodate drought conditions with abscission of leaves at a later stage (Hara et al. 2008). There might be a positive correlation between SLA and water consumption.
For proper reforestation and development of sustainable agriculture, water resources must be managed in semiarid regions. Both reforestation and agriculture can deplete water resources as a result of transpiration by planted trees (Thomas 1991; White 1978). While the results of our comparative investigation suggest that the general pattern of response to soil drying is similar among the species, quantitative differences in drought tolerance and water use properties among the species are presumed to provide important information for reforestation practice. It is suggested that the commonly planted exotic species of R. pseudoacacia is the most sensitive to soil drying in the four investigated species, and should be reconsidered with caution.
References
Bates LM, Hall AE (1981) Stomatal closure with soil water depletion not associated with changes in bulk leaf water status. Oecologia 50:62–65
Cheng JM, Wan HE (2002) Vegetation construction and soil and water conservation in the Loess Plateau of China. China Forestry Publishing House, Beijing. (In Chinese)
Fort C, Fauveau ML, Muller F, Label P, Granier A, Dreyer E (1997) Stomatal conductance, growth and root signaling in young oak seedlings subjected to partial soil drying. Tree Physiol 17:281–289
Fuchs EE, Livingston NJ (1996) Hydraulic control of stomatal conductance in Douglas fir [Pseudotsuga menziesii (Mirb) Franco] and alder [Alnus rubra (Bong)] seedlings. Plant Cell Environ 19:1091–1098
Gollan T, Turner NC, Schulze ED (1985) The responses of stomata and leaf gas exchange to vapour pressure deficits and soil water content III. In the sclerophyllous woody species Nerium oleander. Oecologia 65:356–362
Gollan T, Passioura JB, Munns R (1986) Soil water status affects the stomatal conductance of fully turgid wheat and sunflower leaves. Aust J Plant Physiol 13:459–464
Hara Y, Zhang W, Du S, Tamai S, Yamanaka N (2008) Water relations of 4 afforestation species in the Loess Plateau, China. J Jpn Forest Soc 90:247–252 (In Japanese)
Ingram J, Bartels D (1996) The molecular basis of dehydration tolerance in plants. Ann Rev Plant Physiol Plant Mol Biol 47:377–403
Kozlowski TT (ed) (1976) Water deficits and plant growth. Academic Press, New York
Kozlowski TT, Pallardy SG (1997) Physiology of woody plants, 2nd edn. Academic Press, San Diego
Larcher W (2003) Physiological plant ecology, 4th edn. Springer, Berlin
Monneveux P, Belhassen E (1996) The diversity of drought adaptation in the wide. Plant Growth Regul 20:85–92
Reich PB et al (1999) Generality of leaf trait relationships: A test across six biomes. Ecology 80:1955–1969
Ritchie JT (1981) Water dynamics in the soil–plant-atmosphere system. Plant Soil 58:81–96
Rouhi V, Samson R, Lemeur R, Van Damme P (2007) Photosynthetic gas exchange characteristics in three different almond species during drought stress and subsequent recovery. Environ Exp Bot 59:117–129
Sadras VO, Milroy SP (1996) Soil–water thresholds for the responses of leaf expansion and gas exchange: a review. Field Crops Research 47:253–266
Saliendra NZ, Sperry JS, Comstock JP (1995) Influence of leaf water status on stomatal response to humidity, hydraulic conductance, and soil drought in Betula occidentalis. Planta 196:357–366
Savage MJ, Ritchie JT, Bland WL, Dugas WA (1996) Lower limit of soil water availability. Agron J 88:644–651
Sinclair TR, Ludlow MM (1986) Influence of soil water supply on the plant water balance of four tropical grain legumes. Aust J Plant Physiol 13:329–341
Sinclair TR, Hammond LC, Harrison J (1998) Extractable soil water and transpiration rate of soybean on sandy soils. Agron J 90:363–368
Sinclair TR, Holbrook NM, Zwieniecki MA (2005) Daily transpiration rates of woody species on drying soil. Tree Physiol 25:1469–1472
Thomas GW (1991) Arid land development: striking a balance between economic and ecological issues. In: Baishay A, Dregne HE (eds) Desert development, Part 1, desert agriculture, ecology, and biology. Harwood Academic Press, Chur, Switzerland, pp 1–18
Tian JL, Liang YM, Liu PL (eds) (2003) Investigation into the ecological agriculture construction in hilly-gully region of the Loess Plateau. Yellowriver Water Conservancy Press, Zhengzhou (In Chinese)
Turner NC, Schulze ED, Gollan T (1985) The responses of stomata and leaf gas exchange to vapour pressure deficits and soil water content II. In the mesophytic herbaceous species Helianthus annuus. Oecologia 65:348–355
Veihmeyer FJ, Hendrickson AH (1950) Soil moisture in relation to plant growth. Ann Rev Plant Physiol 1:285–304
Wahbi A, Sinclair TR (2007) Transpiration response of Arabidopsis, maize, and soybean to drying of artificial and mineral soil. Environ Exp Bot 59:188–192
White GF (1978) Environmental effects of arid land irrigation in developing countries. In: Man and the Biosphere Program Technical Notes 8. United Nations Educational, Scientific and Cultural Organization, Geneva
Whitehead D et al (1996) Response of transpiration and photosynthesis to a transient change in illuminated foliage area for a Pinus radiata D Don tree. Plant Cell Environment 19:949–957
Wu ZY (ed) (1980) Vegetation in China. (China’s) Science Press, Beijing. (In Chinese)
Wu QX, Yang WZ (eds) (1998) Forest and Grassland Vegetation Construction and Its Sustainable Development in Loess Plateau. (China’s) Science Press, Beijing. (In Chinese)
Zhang JH, Schurr U, Davies WJ (1987) Control of stomatal behaviour by abscisic acid which apparently originates in the roots. J Exp Bot 38:1174–1181
Zhang X, Wu N, Li C (2005) Physiological and growth responses of Populus davidiana ecotypes to different soil water contents. J Arid Environ 60:567–579
Acknowledgments
This research has been supported by the Knowledge Innovation Project of Chinese Academy of Sciences (kzcx2-yw-BR-02, kzcx2-XB2-05) and the Core University Exchange Program of Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Zwiazek.
Rights and permissions
About this article
Cite this article
Yan, MJ., Yamanaka, N., Yamamoto, F. et al. Responses of leaf gas exchange, water relations, and water consumption in seedlings of four semiarid tree species to soil drying. Acta Physiol Plant 32, 183–189 (2010). https://doi.org/10.1007/s11738-009-0397-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-009-0397-x