Abstract
We subjected seedlings from three tree species from the semi-arid “Caatinga” biome to water deficiency and rehydration. The species were Bauhinia monandra K. and Hymenaea courbaril L., (both Fabaceae), and Tabebuia aurea (Bignoniaceae). Seedlings were kept under water restriction until photosynthesis decreased to values around zero. Plants were rehydrated and photosynthesis measured until its values reached values of well-watered plants. We measured leaf water potential, maximum quantum yield, and chlorophyll index on (1) the first day of the experiment; (2) when photosynthesis decreased to around zero; and (3) after photosynthesis recovery. We then determined biomass and leaf area. To avoid water deficiency B. monandra and T. aurea (but not H. courbaril) reduced their leaf area resulting in lower biomass accumulation. The chlorophyll index was also not affected in H. courbaril, but it was lower for the other two species under stress. Maximum quantum yield was equally decreased in all the tree species as a mechanism to decrease light damage of photosynthetic apparatus. Drought differentially affected the vegetative growths of B. monandra, T. aurea, and H. courbaril when time and intensity were considered, affecting leaf area status leading to the leaf biomass decrease. Decreases in soil moisture led to decreased gas exchange. However, leaves were positively acclimated using chlorophyll strategies by lowering the light harvest in photosystems, which protect photosynthetic reaction centers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Global climate change will intensify water scarcity in many areas. Drought stress is especially common in semi-arid trees, which frequently employ morpho-physiological drought-tolerance strategies (Husen et al. 2014). The “Caatinga”, a dry climate tropical forest, located in the northeastern region of Brazil, is subject to very irregular rainfall and high temperatures leading to frequent droughts (Sampaio 1995; Souza et al. 2010). Thus, soil-water availability in “Caatinga” is a crucial factor driving plant growth and survival in this environment. Clearly, rapid and efficient morpho-physiological responses of species are crucial for tolerance of water deficit and, by extension, the use of drought-tolerant trees is one of the potentially most effective strategies to restore degraded areas of the “Caatinga”.
Depending on the species exposed to water stress, stomatal conductance can dramatically decrease after roots produce and transfer chemical signals to the leaves (Schachtman and Goodger 2008). Consequently, biomass accumulation is impaired since stomatal resistance limits photosynthesis (Kauser et al. 2006). Thus, decreases in intercellular CO2 concentration lead to the reduction of CO2 diffusion to chloroplast, which negatively affects net CO2 assimilation rates (Chaves et al. 2002; Tang et al. 2002). The reduction of leaf expansion under stressful conditions may also be beneficial to plants under drought stress, limiting transpiration rates but reducing biomass production by plant (Mahajan and Tuteja 2005). The relationship between water use and carbon gain at the leaf level thus largely determines plant performance and competitive advantage of tolerant species to water stress (Craven et al. 2013). Certainly, opening and closing controls of stomata by plants particularly result in specific performance of water-use efficiency by different plants species.
Photosynthetic limitations in response to water stress and high radiation may cause damage to the thylakoid membranes of chloroplasts, negatively affecting the process of electron transfer in photosystem II (Pospisil 2009; Santos et al. 2013). As a result, a decrease in quantum yield efficiency commonly occurs during the hottest hours of the day under condition of moderately excessive light—a phenomenon known as dynamic photoinhibition (Casaroli et al. 2007). In this case, transformation of excess light energy into heat and its dissipation represent a protective mechanism (Santos et al. 2013). However, the consequences of periodic light stress might be further exacerbated if plants are submitted to scarce rainfall. The average “Caatinga” rainfall is around 500–750 mm year−1, usually concentrated during 3–5 months in a year (Machado et al. 2006). In contrast to dynamic photoinhibition, the plant photosystem cannot recover overnight from excessive solar radiation, significantly decreasing efficiency of photosynthesis and indicating chronic photoinhibition (Casaroli et al. 2007).
We investigate the responses of three “Caatinga” tree species—Hymenaea courbaril L. (Fabaceae), Tabebuia aurea (M) B. & H. f. ex S. M. (Bignoniaceae), and Bauhinia monandra (Fabaceae)—to drought stress, focusing on photosynthetic activity and dissipation of energy, the water-use efficiency and the biomass allocation for different parts of trees, and the ability to recover after water stress. Based on our preliminary observations, we assumed that drought stress would alter the tree’s growth and photosynthesis. Some “Caatinga” plant species have specific ecophysiological capacities to assimilate carbon and maintain productivity and biological functions even in dry and warm season.
Materials and methods
Species
The species were selected based on field observations that indicated that they had high capacity to grow under scarce rainfall and survive under drought conditions. Hymenaea courbaril L. (Fabaceae) has wide geographical distribution especially in nuclei of seasonally dry forest in Brazil (Souza et al. 2014). Tabebuia aurea (M) B. & H. f. ex S. M. (Bignoniaceae) has a wide distribution in tropical and subtropical regions of American Continent (Lorenzi 1992). Bauhinia monandra (Fabaceae) is commonly found in West of Africa and India, but it is grown and traditionally used for diabetes treatment in Brazil (Dalziel 1995).
Experimental site and plant material
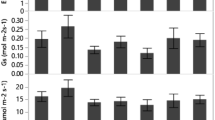
We collected seeds of Tabebuia aurea (Bignoniaceae), Hymenaea courbaril (Fabaceae), and Bauhinia monandra (Fabaceae) from the “Caatinga” biome (9°21′49″, 37°14′54″). Selected seeds were germinated in soil in plastic trays under natural, ambient temperature and light conditions in the nursery. After 6 months, we transferred 22 seedlings of each species into 22-L plastic pots (one plant per pot) containing 20 kg of soil as substrate. Young plants were grown between October 2013 and April 2014 under greenhouse conditions (9°28′02″, 35°49′43″). We irrigated the seedlings daily during growing period. Average of the maximum temperature at greenhouse during the experiment was around 36 °C, while the minimum temperature was around 25 °C. The averages of relative humidity (RH) and radiation were about 67 % and 95 W (m2)−1, respectively (Fig. 1).
Water deficiency treatments
The seedlings were allowed to undergo three phases of water stress and recovery: (1) Irrigated plants: plants receiving daily irrigation; (2) Drought-stressed plants: plants subjected to water deficit by suppressing irrigation until photosynthesis reached zero; and (3) re-watered plants: drought-stressed plants were re-watered until carbon assimilation rates reached those values of irrigated plants. All seedlings were irrigated to 100 % field capacity before drought treatment was begun. Then, one group of plants was subjected to a water stress. From a total of 66 plants, 33 plants (11 from each species) were submitted to drought stress by suppressing irrigation. Photosynthesis was assessed daily until its values reached zero. Plants were then re-watered, and when photosynthesis reached values of continuously irrigated plants, we assumed that plants were recovered from water stress. Leaf water status, chlorophyll index (SPAD), and chlorophyll fluorescence were measured on (1) the first day (all plants well irrigated); (2) during the water-stress period; and (3) at the end of experiment when plants had completely recovered from stress. Total dry mass and leaf area were also obtained at the end of the experiment. Soil-water potential was determined using a WP4 Dewpoint potential meter (Decagon devices Inc. Pullman, WA, USA) (Table 1).
Monitoring gas exchange
Gas-exchange analysis was carried out daily using infrared CO2 gas analyzer (LICOR, Li-6400xt). Leaf gas exchange was measured in a mature leaf from the middle of plant at 1000 µmol PPFD m−2 s−1 from 10:00 to 11:30 AM. The water-use efficiency was calculated as A/g s (Osmond et al. 1980). We used 36 plants in our study for performing gas-exchange analysis because of feasibility constraints of using 66 plants.
Leaf dry mass and leaf area
The total leaf biomass was obtained at the end of the experiment, after drought-stressed plants had completely recovered from water deficit stress. Dry mass was measured after drying leaves for 3 days at 70 °C. Leaf area was determined using an area meter (LICOR, LI-3100).
Leaf water status, SPAD, and chlorophyll a fluorescence
We measured water tension in the xylem, SPAD, and maximum quantum yield at three time periods: (1) before exposing the plants to drought stress; (2) at the point of maximum stress; and (3) after the water-stressed plants had recovered. Predawn leaf water potential was measured before sunrise during 03:30–04:00 AM using a Scholander pressure chamber (Soilmoisture Equipment).The SPAD index was estimated using a hand-held chlorophyll content meter (SPAD-502, Minolta, USA). Maximum quantum efficiency of PSII (F v /F m ) was determined after 20 min of leaf dark adaptation period using the saturation pulse of actinic light (8000 µmol m−2 s−1) of modulated fluorometer (PAM 2500).
Statistical analysis
We used a completely randomized design with three species, two treatments (control well-watered plants and drought stressed by withholding irrigation), and 11 replications for each treatment (except for gas-exchange analysis where we used six replications). Data recorded were according to the Normal distribution as verified by Lilliefors test and were subjected to analysis of variance (ANOVA) to determine any differences among drought treatments. Means were compared by Tukey’s test. Analysis of variance (ANOVA) for repeated measures was applied, and results from treatments showing significant overall changes were subjected to post hoc Tukey’s test and regression analysis. Statistical analyses were performed by means of version 7.0 of statistical software packages (StatSoft Inc., Tulsa, OK, USA).
Results
Gas exchange
For all species, irrigated plants showed different responses compared with those plants submitted to water deficiency and rehydration. Drought stress drastically decreased photosynthesis, but with different intensities between species (Fig. 2). Bauhinia monandra and T. aurea were more sensitive to water deficiency (fifth day) than H. courbaril (fifteenth day). Photosynthesis decreased to around zero after 13 days in B. monandra, 20 days in T. aurea, and 25 days in H. courbaril. Nevertheless, after rehydration, the recovery of photosynthesis occurred after 4, 7, and 8 days in B. monandra, H. courbaril, and T. aurea, respectively. The treated seedlings showed recoveries of g s and E, but WUE was not statistically different (Tables 2 and 3).
Leaf dry mass and area
Growth was affected by water stress in two of the three species. We observed that in B. monandra and T. aurea, leaf dry mass and leaf area decreased significantly after a short period of water deficiency, 13 and 20 days, respectively (Table 4). Water-stress-sensitive species lost leaves as a strategy to tolerate drought, although this negatively affected leaf area and biomass. This result was not observed for T. aurea (data not shown). H. courbaril grown during 25 days under water deficiency showed no decrease in total leaf area or dry mass in comparison with control plants.
Leaf water status, SPAD, and chlorophyll a fluorescence
Plants’ leaf water potential decreased significantly at critical periods compared with control, with a reduction of around 77 % in the predawn and 52 % at noon for B. monandra, and 86 % in the predawn and 69 % at noon in T. aurea (Fig. 3). Tabebuia aurea presented the lowest values 20 days after the beginning of the experiment compared to 13 days for B. monandra. The average water potentials were around −2.5 and −4.0 MPa in leaves of B. monandra and T. aurea, respectively. Stressed plants showed a different rehydration response, achieving similar values to those of the irrigated plants at the end of the experiment. Leaves of H. courbaril have a short petiole, and determination of leaf water potential was therefore not feasible.
Seedlings of H. courbaril maintained their chlorophyll content index unchanged under water stress. In contrast, in B. monandra and T. aurea, the SPAD index decreased with water deficiency, but recovered when plants were rehydrated (Table 5). The predawn maximum quantum efficiency of PSII (F v /F m ) varied during the water-stress period in all species. There was a small reduction of F v /F m from 8 to 12 % in the driest conditions, recovering to control values by the end of the experiment.
Discussion
Bahuinia monandra, T. aurea, and H. courbaril are potentially important tree species in the “Caatinga”, since they may be useful for restoration of degraded and deforested areas. Our data suggest that drought-induced changes in physiological parameters depend on to the species considered, even when all of those species show clear adaptations to living in semi-arid environments. Decrease in dry mass and total leaf area in young plants subjected to water stress revealed that water deficiency adversely affects vegetative growth of B. monandra and T. aurea in comparison with irrigated plants, but not in the case of H. courbaril.
Besides effects on growth, the physiological responses to water stress of the three experimental species can be perceived through alterations of gas exchange, chlorophyll index, and chlorophyll a fluorescence. It is noteworthy that plants of “Caatinga” are well adapted to periodic low water availability during about 9 months in the year. To survive under these drastic conditions, they have developed a range of different physiological and morphological adaptations.
Dombroski et al. (2011) demonstrated that, in the middle of the dry season, predawn and mid-day leaf water potentials were different in adults of six “Caatinga” tree species (Mimosa caesalpiniifolia Benth., Caesalpinia pyramidalis Tul., Auxemma oncocalyx (Allemão) Taub., Caesalpinia ferrea Mart. ex Tul. var. ferrea, Calliandra spinosa Ducke, and Tabebuia caraiba (Mart.)). The leaf water potentials at predawn were around −3.0 MPa for M. caesalpiniifolia and −2.5 MPa for C. pyramidalis and A. oncocalyx. C. spinosa, C. ferrea, and T. caraiba showed lower values of −1.5, −1.0, and −0.5 MPa, respectively. As observed in our study, T. aurea showed values of leaves’ water potentials around −4.0 MPa showing superior drought tolerance compared to plants studied by Dombroski et al. (2011). However, B. monandra showed leaf water potential values similar to those of Caesalpinia pyramidalis and Auxemma oncocalyx. It is noteworthy that plants studied by Dombroski et al. (2011) were adult individuals growing in natural environmental conditions, and it is therefore possible that they could acquire water from deep in the soil.
We observed that B. monandra not only rapidly decreased photosynthesis in response to reduction of stomatal conductance, but also rapidly lost leaves (data not shown). Such a leaf-loss strategy was not observed in T. aurea and H. courbaril, although these two latter species had reduced their growth. Under natural conditions in the “Caatinga”, some plants lose their leaves in the dry season (Sampaio 1995), presumably as an adaptation to prevent excessive water loss. Such a strategy has been observed for young plants of Swietenia macrophylla King subjected to drought in greenhouse conditions (Cordeiro et al. 2009). Likewise, Acacia tortilis (Forsk) Hayne ssp. raddiana (Savi) Brenan grown under water-stressed conditions showed a marked decrease in leaf water potential, leaf number, dry mass, shoot length, and total leaf area (Kebbas et al. 2015).
Low levels of photosynthesis, as the consequence of drought stress, led to a decrease in plant growth in our experimental seedlings. In this case, the decrease in dry mass is strongly affected by the leaf loss, which negatively affected carbon assimilation. Although a reduction in photosynthesis limits plant growth, higher level of transpiration are unlikely to be suitable for plants growing in semi-arid areas. The reduction in photosynthesis as a consequence of limited stomatal conductance is thought to be one of the main mechanisms of tolerance to water deficiency in plants from the “Caatinga” (Souza et al. 2010). Stomata are known to respond both to soil and atmosphere moisture, and the efficiency of the stomatal conductance response is crucial to avoid water-stress damage (Tominaga et al. 2014). Plants can respond to water scarcity over different time periods, as observed in B. monandra, T. aurea, and H. courbaril.
Photosynthesis was found to rapidly recover in all the three studied species, although B. monandra showed to recover most rapidly. Similar to our results, after water deficiency, another species of the same genus, Bauhinia forficata Link rapidly recover photosynthetic rates when soil-water availability was reestablished (Sanches and Silva 2013). According to Sanches and Silva, photosynthetic activity is strongly dependent on the soil-water availability, as was also observed in our work. Similarly, Khaya ivorensis A. Chev. (African Mahogany) (Albuquerque et al. 2013) and T. aurea (Oliveira et al. 2011) under water deficiency also quickly recovered pre-stress levels of photosynthesis after rewatering. Our results reinforce the dependence of photosynthesis of the tree species studied here on water availability.
Hymenaea courbaril retained its chlorophyll content, while the values of chlorophyll significantly decreased in B. monandra and T. aurea. Bauhinia monandra seedlings lost their leaves at higher leaves’ water potentials compared with T. aurea leading to reduction of gas exchange. In addition, chlorophyll fluorescence showed the effect of water stress on H. courbaril photosynthesis. Negative effects of photoinhibition can be caused by light intensity alone, but a combination of this factor plus high temperature and water deficiency and CO2 supply can cause even more drastic impacts (Ribeiro et al. 2008). Values of F v/F m decreased under drought conditions, but seedlings recovered after rehydration, indicating reduction of photo damage in PS-II reaction centers.
Hymenaea courbaril had a reduced value of F v/F m, but not of chlorophyll content as in B. monandra and T. aurea. It was anticipated that changes in chlorophyll content would be seen shortly after changes in photosynthesis, since stomatal apertures would be reduced. Such a result suggests that H. courbaril employs distinct drought-tolerance strategies to maintain ecological function during water stress. Nevertheless, in the case of H. courbaril, Fv/Fm reduction can be related to photochemical events occurring after light harvesting by PS-II reaction centers. Chlorophyll reduction together with Fv/Fm were adopted as an acclimation strategy by the other two tree species, but reduced photosynthesis levels (probably associated with metabolic damage) were observed under water-stress conditions. In H. courbaril, a reduction of photosynthesis preceded changes in leaf area (data not shown) and plant growth, and chlorophyll loss preceded loss of leaves.
Stomatal limitations induced by water deficiency decrease gas exchange, but light harvesting does not. Hence, further damage to the membranes of photosynthetic proteins are reduced by lower level of chlorophyll light-harvesting systems, limiting the formation of oxygen radicals (Kranner et al. 2002; Husen et al. 2014). Bauhinia monandra and T. aurea completely recovered chlorophyll content by the end of the experiment.
In conclusion, drought differentially affected the vegetative growths of seedlings of the drought-resistant “Caatinga” trees: B. monandra, T. aurea, and H. courbaril. A reduction of soil moisture decreased the gas exchange, but leaves were positively acclimated by reducing chlorophyll content (B. monandra and T. aurea) and harvesting of light thereby protecting the photosynthetic reaction. However, H. courbaril did not reduce chlorophyll contents, and further studies are needed to understand the strategies of growth of this species under drought conditions.
References
Albuquerque M, Moraes F, Santos R et al (2013) Ecofisiologia de plantas jovens de mogno-africano submetidas a deficit hídrico e reidratação. Pesq agropec bras 48:9–16
Casaroli D, Fagans E, Simon J et al (2007) Solar radiation and physiologics aspects in soybean—a review. Rev da FZVA Uruguaiana 14:102–120
Chaves M, Pereira J, Maroco J et al (2002) How plants cope with water stress in the field. Photosynthesis and growth. Ann Bot 89:907–916
Cordeiro YEM, Pinheiro HS, Santos Filho BG, Corrêa SS, Silva JRR, Dias Filho MB (2009) Physiological and morphological responses of Young mahogany (Swietenia machophylla King) plants to drought. For Ecol Manag 258:1449–1455
Craven D, Hall J, Ashton M, Berlyn G (2013) Water-use efficiency and whole-plant performance of nine tropical tree species at two sites with contrasting water availability in Panama. Trees 27:639–653
Dalziel J (1995) The useful plants of West Africa Crown Agents for Oversea Governments and Administration. London
Dombroski J, Praxedes S, Freitas R, Pontes F (2011) Water relations of Caatinga trees in the dry season. S Afr J Bot 77:430–434
Husen A, Iqbal M, Aref IM (2014) Growth, water status, and leaf characteristics of Brassica carinata under drought and rehydration conditions. Braz J Bot 37:217–227
Kauser R, Habib-Ur-Rehman A, Muhammad A (2006) Chlorophyll fluorescence: a potential indicator for rapid assessment of water stress tolerance in Canola (Brassica napus L.). Pak J Bot 38:1501–1509
Kebbas S, Lutts S, Aid F (2015) Effect of drought on the photosynthesis of Acacia tortilis subsp. Raddiana at the young seedling stage. Photosynthetica 53:288–298
Kranner I, Beckett R, Wornik S et al (2002) Revival of a resurrection plant correlates with its antioxidant status. Plant J 31:13–24
Lorenzi H (1992) Árvores brasileiras: manual de identificação e cultivo de plantas arbóreas nativas do Brazil. Editora Plamtarum, Nova Odessa
Machado I, Lopes A, Sazima M (2006) Plant sexual systems and a review of the breeding system studies in the Caatinga, a Brazilian tropical dry forest. Ann Bot 97:277–287
Mahajan S, Tuteja N (2005) Cold, salinity and drought stresses: an overview. Arch Biochem Biophys 444:139–158
Oliveira A, Gualtieri S, Bocchese R (2011) Gas exchange of potted Tabebuia aurea plants under hydric stress. Acta Sci Agron 33:641–647
Osmond CB, Winter K, Powles SL (1980) Adaptative significance of carbon dioxide cycling during photosynthesis in water stressed plants. In: Turner NC, Krammer PJ (eds) Adaptation of plants to water and high temperature stress. Wiley, New York, pp 139–155
Pospisil P (2009) Production of oxygen reactive species by photosystem II. Biochim Biophys Acta 1:1151–1160
Ribeiro R, Santos M, Machado E, Oliveira R (2008) Photochemical heat-shock response in common bean leaves as affected by previous water deficit. Russ J Plant Physiol 55:350–358
Sampaio EV de SB (1995) Overview of the Brazilian Caatinga. In: Mooney H, Bullock S, Ernesto M (eds) Seasonally dry tropical forest. University Press, Cambridge, pp 35–63
Sanches RFE, Silva EA (2013) Changes in leaf water potential and photosynthesis of Bauhinia forficata Link under water deficit and after rehydration. Hoehnea 40:181–190
Santos CM, Verissimo V, Wanderley Filho HCL, Ferreira VM, Cavalcante PGS, Rolim EV, Endres L (2013) Seasonal variations of photosynthesis, gas exchange, quantum efficiency of photosystem II and biochemical responses of Jatropha curcas L. grown in semi-humid and semi-arid areas subject to water stress. Ind Crops Prod 41:203–213
Schachtman D, Goodger J (2008) Chemical root to shoot signaling under drought. Trends Plant Sci 13:281–287
Souza B, Meiado M, Rodrigues B, Santos M (2010) Water relations and chlorophyll fluorescence responses of two leguminous trees from the Caatinga to different watering regimes. Acta Physiol Plant 32:235–244
Souza I, Funch L, Quiroz L (2014) Morphological analyses suggest a new taxonomic circumscription for Hymenaea courbaril L. (Leguminoae, Caesalpinoideae). PhytoKeys 38:101–118
Tang A, Kawamitsu Y, Kanechi M, Boyer J (2002) Photosynthetic oxygen evolution at low water potential in leaf discs lacking an epidermis. Ann Bot 89:861–870
Tominaga J, Inafuku S, Coetzee T, Kawamitsu Y (2014) Diurnal regulation of photosynthesis in Jatropha curcas under drought during summer in a semi-arid region. Biomass Bioenergy 67:279–287
Acknowledgments
The authors, J. S. Pinheiro and L. Endres, are grateful to the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for the scholarships and grants. The authors thank Richard Ladle for proofreading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Da Silva-Pinheiro, J., Lins, L., Souza, F.C. et al. Drought-stress tolerance in three semi-arid species used to recover logged areas. Braz. J. Bot 39, 1031–1038 (2016). https://doi.org/10.1007/s40415-016-0309-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40415-016-0309-4