Abstract
Iron (Fe) deficiency chlorosis (FDC) in plant is associated with high bicarbonate concentration in calcareous soil and irrigation water, which leads to new leaf yellowing and lessens crop yield and quality. However, little is known about whether the chlorosis under bicarbonate stress resulted from blocking root–shoot Fe translocation or root Fe absorption. Moreover, the molecular response of chlorotic leaf under bicarbonate stress has been rarely reported on. The purpose of this study was to investigate the effect of bicarbonate on Fe acquisition, Fe translocation as well as Fe accumulation in roots, normal leaf (NL) and chlorotic leaf (CL) of Medicago lupulina. Seeds were grown with and without Fe and NaHCO3 (Fe and Bic) in the nutrient solution for 10 d. Fe content, gene expression and enzymatic activity in different tissues were determined. A factorial statistical design with two factors (Fe and Bic) and two levels of each factor was adopted: + Fe, −Fe, + Fe + Bic and −Fe + Bic. Results indicated that bicarbonate stress increased the expression of genes MlHA1, MlFRO1 and MlIRT1 related to Fe acquisition and promoted the Fe absorption from solution. Furthermore, the presence of bicarbonate stress inhibited the expression of MlMATE66 in roots, prevented the Fe translocation from roots to developing leaf, brought about Fe accumulation in roots and reduced the Fe content in new leaf. Generally, according to our results, bicarbonate could prevent Fe translocation from roots into developing leaf, decrease Fe bioavailability and induce chlorosis in M. lupulina.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Iron (Fe) is the fourth abundant element in the Earth’s crust accounting for 5% (Souri et al. 2018; Mengel et al. 2001), while serving as an essential mineral microelement in plant growth and development. The alkaline and calcareous soil representing about one-third of the Earth’s soil contains bicarbonate ion (HCO3−) (Chen and Barak 1982), which is the most important inducing factor of Fe-deficient chlorosis in plant. It can inhibit Fe uptake by roots and Fe translocation into shoots and leaves (Shahsavandi et al. 2020) and then cause losses in the production of crops (Tato et al. 2013).
Fe plays a fundamental role in plant metabolism, for instance, electron transfer for photosynthesis or respiration (El-Gioushy et al. 2021; Roschzttardtz et al. 2013). Fe is also a key element in photosynthesis and carbon dioxide assimilation to ensure electron flow through the PS II-b6f-PSI complexes (Briat et al. 2015). Moreover, numerous enzymes in most organisms (including plants) cannot achieve their biological functions without Fe (Connorton et al. 2017).
When plants are under bicarbonate stress, many morphological and physiological responses are induced in roots to increase iron transport and uptake (García et al. 2014; Wang et al. 2020). Bicarbonate is widely reported to affect the ferric reductase oxidase (FRO) and H+-ATPase (HA) activity, including inhibition or promotion. Lucena et al. (Lucena et al. 2007) reported that bicarbonate inhibited the expression of FRO and HA in some species such as Cucumis sativus, Pisum sativum and Lycopersicon esculentum. In contrast, the above genes were induced by bicarbonate and enhanced FRO and HA in Citrus limon roots (Martínez-Cuenca et al. 2013). Robinson et al. (Robinson et al. 1999) reported that the AtFRO2 is expressed mainly in roots in Arabidopsis thaliana. In contrast to A. thaliana, LeFRO1 in L. esculentum and PsFRO1 in P. sativum are expressed in both roots and leaves (Waters et al. 2002). In roots epidermal cells, Fe3+ is reduced to Fe2+ by FRO, which is transported into roots by iron-regulated transporter 1 (IRT1) (Mora-Córdova et al. 2022). The expression of IRT1 in roots had been reported on the previous study under bicarbonate stress (García et al. 2014; Lucena et al. 2007; Martínez-Cuenca et al. 2013; Hsieh and Waters 2016). The zinc/iron-regulated transporter-like protein (ZIP) family is involved in metal transport (Curie et al. 2000; López-Millán et al. 2004), which can transport divalent cations such as Fe2+, Zn2+ and Mn2+. A couple of ZIP family transcription factor genes, such as HD-ZIP (Ariel et al. 2007) and bZIP (Liu et al. 2014), were identified as alkali stress-responsive genes (Cao et al. 2016). Although a number of previous studies have been reported about the roles of related genes in plant bicarbonate stress, little is known regarding their roles in bicarbonate stress. Therefore, it is urgent to understand the basic mechanism of plant responses to bicarbonate stress.
The previous study focused on the Fe uptake from soil and physiological responses to bicarbonate stress in roots. However, the effect of carbonate on Fe transport from roots to leaves and the effect of bicarbonate on Fe homeostasis in cells are still poorly understood. Fe is translocated into leaves from roots by carrier complexes on the xylem (Hell and Stephan 2003), which is usually a citrate and iron transport protein (ITP) (Kruger et al. 2002; Yokosho et al. 2009). It is proved that the multidrug and toxin extrusion (MATE) transporter involved in the transport of citrate, which translocates Fe from roots to shoot in Oryza sativa and A. thaliana (Yokosho et al. 2009; Durrett et al. 2007). In legume species, the citrate transporter gene (MtMATE66 and LjMATE1) is identified in Medicago truncatula and Lotus japonicus (Wang et al. 2017; Takanashi et al. 2013). Function analysis showed that MtMATE66 is primarily expressed in root epidermal cells and involved in chlorotic symptom under Fe deficiency (Wang et al. 2017). The iron transporter protein in plants is the natural resistance-associated macrophage protein (NRAMP) family (Curie et al. 2000) and Yellow Stripe-Like (YSL) protein family (Curie et al. 2001). In A. thaliana, AtYSL genes (AtYSL1, AtYSL2, and AtYSL3) have been identified, and YSL1 and YSL3 protein transported iron between vascular tissues (Waters et al. 2006). NRAMP3 transported Fe from cotyledons to roots, and its expression was inhibited by bicarbonate in Citrus (Martínez-Cuenca et al. 2013). However, whether iron deficiency chlorosis results from blocking iron translocation from roots under bicarbonate stress is still unclear. Furthermore, ferritin is ubiquitous iron storage proteins in the plastids and plays an important role in iron homeostasis (Harrison and Arosio 1996; Petit et al. 2001). The previous report showed that the transcriptional level of soybean ferritin gene is regulated in response to iron overload (Lescure et al. 1991). Santos et al. (Santos et al. 2015) found that ferritin was highly expressed in “Fe-inefficient” plant roots, which can resist Fe deficiency chlorosis. However, the relationship between the expression of ferritin and bicarbonate stress remains unknown.
Although the above cited literature had reported the expression of genes related to iron uptake (FRO1 and IRT1) under Fe deficiency or bicarbonate stress, the expression profiles of gene related to iron translocation and accumulation are unclear under bicarbonate stress. At the same time, does bicarbonate stress lead to the inability of plants to absorb iron from the external environment, or does the iron absorbed by plant roots fail to transfer from plant roots to leaves? These issues are also unclear. Thus, this paper aimed to test whether bicarbonate impairs Fe mobilization from roots to leaves or affects the expression of storage-related genes in M. lupulina roots or chlorotic leaf. To this end, the effect of bicarbonate on the expression ZIP family, MATE66 and ferritin was studied in roots, normal leaf and chlorotic leaf. In addition, the influence of Fe uptake-related genes (FRO, HA and IRT) and enzyme activity (FRO) was also determined in the present study.
Materials and methods
Plant growth, iron deficiency and bicarbonate treatment
The seeds of Medicago lupulina were collected from Wanfenglin Scenic Spot, Xingyi City, Guizhou Province, China (104° 56′ 2.868″ E, 25° 0.3672′ 0″ N). The average altitude is 1200 m. M. lupulina was cultured in the Key Laboratory of National Forestry and Grassland Administration on Biodiversity Conservation in Karst Mountainous Areas of Southwestern China (106° 38′ 34.62″ E, 26° 23′ 11.148″ N). The M. lupulina seeds were soaked in concentrated sulfuric acid for 7 min and then washed three times with distilled water. The seeds were planted in a plastic pot containing perlite with 1/4 MS solution in hydroponics. They were cultured in a chamber with 16/8 h light/dark at 22 °C/20 °C, irradiance of 350 µmol m−2 s−1 and air humidity of 60–70%. After seeding growth for 30 d, the solution with 10 Mμ Fe (+ Fe), without Fe (–Fe), + Fe with 10 mM NaHCO3 or KHCO3 (+ Fe + Bic), and –Fe with 10 mM NaHCO3 or KHCO3 (–Fe + Bic) were replaced. All treatments were carried out in three replications. Root, normal leaf and chlorotic leaf (Fig. 2A) were collected at 10 d for gene expression analysis by RT-PCR. Root and shoot were collected at 10 d for determination of biomass and Fe content.
The measurement of biomass and SPAD
The shoot and root were dried at 65 °C to a constant weight and weighed for biomass. SPAD values in all treatments were measured by a Minolta SPAD chlorophyll meter.
Determination of enzyme activity
FRO activity was measured using whole roots of individual M. lupulina; after treatment for 10 days, roots were rinsed in deionized water and submerged in 20 mL assay solution (1 mM MES buffer, pH 5.5, 150 µm Fe (III)–EDTA and 200 µM ferrozine). Ferrozine-Fe (II) was measured by absorbance at 562 nm (subtracting blanks of assay solution with no plants) (Hsieh and Waters 2016).
Identification of genes-related Fe uptake and phylogenetic analysis
These genes were obtained from M. truncatula (http://www.medicagogenome.org, Mt4.0v2) and NCBI database to identify the genes related to iron uptake. The identified sequences of M. truncatula were used as query sequences to identify corresponding genes from M. lupulina transcriptome database by a local BLAST program. These sequences are listed in Supplementary Table 1. In addition, A. thaliana isoforms were also obtained from the Arabidopsis Information Resource (http://www.arabidopsis.org/). Among the identified genes, the ZIP transporter is a multi-gene family. Thus, they were subjected to phylogenetic analysis. Trees were constructed using the neighbor-joining method as implemented in the ClustalW program. The following parameters were set during the construction of the phylogenetic tree, for example, substitution, Poisson model, complete deletion, replication and bootstrap analysis with 1000 replications.
Determination of Fe contents
The shoot and root were collected in all treated plant. The samples were dried at 65 °C. The dried samples were digested for 2–5 h in 50% perchloric acid and concentrated sulfuric acid. Then, the digested solution was filtered through filter paper and adjusted to 50 mL volumes with addition of deionized water. An inductively coupled plasma-optical emission spectrometer was applied to measure the Fe contents (ICP-OES, Thermo Elemental-IRIS Advantage, USA).
Gene expression analysis using qRT-PCR
The total RNA of the samples was extracted using an OmniPlant RNA Kit (Kangwei, Beijing, China) and was reverse-transcribed using the TransScript® All-in-One First-Strand cDNA Synthesis SuperMix for qPCR Kit (Transgen, Beijing, China) following the manufacturer’s protocol. Actin was used as the reference for gene expression normalization. The gene-specific primers are listed in Supplementary Table 1. RT-PCR was carried out using a Rotor-Gene Q real-time PCR system (Qiagen, Germany) and 10 μL 2 × TransStart Top/Tip Green qPCR SuperMix (Transgen, Beijing, China) according to the manufacturer’s instructions. The relative expression levels of the genes normalized to the expression level of actin were calculated from cycle threshold values using the 2 − ΔΔCt method (Livak and Schmittgen 2001). All expression levels in each treatment were log2-transformed. (transcript level).
Statistical analysis
One-way analysis of variance (ANOVA), performed using SPSS (version 18.0) was used to determine differences among groups. Data were presented as means ± standard deviation (SD), with values followed by P < 0.05 considered statistically significant. The results were visualized using sigmaplot version 12.5.
Results
Physiological responses to bicarbonate stress in M. lupulina
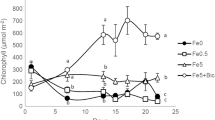
The growth of M. lupulina was not significantly affected in Fe-deficient plant for 10 d compared with Fe-supplied plant. For example, shoot biomass (Fig. 1C), root biomass (Fig. 1D) and chlorophyll content (Fig. 1E) were not significantly changed. In the presence of bicarbonate, shoot biomass was decreased. Still, it did not show a significant change (Fig. 1C). In contrast, root biomass was significantly inhibited (Fig. 1A, D). Furthermore, chlorophyll content was significantly decreased in bicarbonate-supplied plant (Fig. 1E). Chlorosis symptom was observed in new leaf of M. lupulina (Fig. 1B). To investigate the effect of cation on the growth of M. lupulina, sodium bicarbonate (NaHCO3) and potassium bicarbonate (KHCO3) were compared in the present study. Statistical analysis showed no significant difference in biomass and chlorophyll content (Fig. 1).
Identification of genes related to iron uptake in M. lupulina
One ferric reductase gene (MtIRO), one iron transporter gene (MtIRT1), one H+-ATPase gene (MtHA1), six ZIP family genes (MtZIP), three ferritin genes (MtFer1-3) and one citrate transporter gene (MtMATE66) were screened from M. truncatula (Table 1). The identified sequences of M. truncatula were used as query sequences to identify iron uptake genes from M. lupulina transcriptome database by a local BLAST program. The results showed that 11 genes related to iron uptake and translocation were screened. However, the homologous genes of MtZIP4 and MtZIP6 in M. lupulina were not found. The identification ratio of these genes to the corresponding homologous genes in M. truncatula was higher than 75% (Table 1). The phylogenetic tree analysis of ZIP family in A. thaliana, M. truncatula and M. lupulina showed that the members of ZIP gene family in M. lupulina were orthologous genes with M. truncatula (Sup Fig. 1).
FRO activity and gene expression profiles under bicarbonate stress
The activity of FRO exudate from roots of Fe-deficient plants was slightly higher than that of Fe-supplied plant. Meanwhile, application of bicarbonate (KHCO3 and NaHCO3) significantly increased the FRO activity from M. lupulina roots; for example, the FRO activity with NaHCO3 and KHCO3 increased 2.1 and 1.87 times than that without bicarbonate under Fe-deficiency, respectively (Fig. 2B). The same results were also shown in Fe-supplied plants (Fig. 2B). In Fe-deficient plants, the expression levels of MlFRO1 in roots or leaves, but no significant changes were found between normal leaf (NL) and chlorotic leaf (CL) (Fig. 2C). Interestingly, bicarbonate significantly enhanced MlFRO1 expression in CL (Fig. 2C). In contrast to MlFRO1, the expression of MlHA1 showed no significant changes between CL and NL in bicarbonate-supplied plants, but its expression in roots was sharply increased under bicarbonate supply, especially in Fe-deficient and bicarbonate-treated plants (Fig. 2D).
FRO activity B and gene expression levels of MlFRO1 C and MlHA1 D of M. lupulina under iron deficiency and bicarbonate treatment. A indicates sampling from different tissues, root (R), normal leaf (NL) and chlorotic leaf (CL). Data are mean ± SD (n = 3). Uppercases on bar represent the differences between tissues under the same treatment (p < 0.05). Lowercases on bar represent the differences between different treatments in the same tissue (p < 0.05)
Iron transporter responses to bicarbonate stress
Five genes related to iron transporter were identified based on the M. lupulina transcriptome database, including one iron transporter (MlIRT1) and four metal transporter family (MlZIP) (Table 1). The expression of MlIRT1 in roots, NL and CL was significantly up-regulated under Fe-deficiency compared with Fe supply. In addition, its expression level was further enhanced by bicarbonate (Fig. 3A). In the MlZIP family, the expression profiles of MlZIP3 (Fig. 3C) and MlZIP7 (Fig. 3E) were similar to that of MlIRT1 in roots and leaves under Fe-deficient and bicarbonate-treated plants. Furthermore, in contrast, the expression of MlZIP1 (Fig. 3B) and MlZIP5 (Fig. 3D) was suppressed in Fe-deficient or bicarbonate-supplied plants.
The expression profiles of iron transporter gene of MlIRT1 A, MlZIP1 B, MlZIP3 C, MlZIP5 D and MlZIP7 E in M. lupulina under iron deficiency and bicarbonate treatment. Data are mean ± SD (n = 3). Uppercases on bar represent the differences between tissues under the same treatment (p < 0.05). Lowercases on bar represent the differences between different treatments in the same tissue (p < 0.05)
Effects of bicarbonate stress on Fe translocation
Fe usually combines a citrate in plants to form a complex, which is translocated from roots to leaves. The present study identified the gene encoding citrate transporter (MtMATE66) in M. lupulina. Its expression was significantly down-regulated in bicarbonate-supplied root, compared with Fe supply or Fe deficiency, indicating Fe translocation was inhibited by bicarbonate. Furthermore, MlMATE66 in CL was significantly higher than in NL (4A). To analyze whether bicarbonate hinders the combination of ferritin to Fe, three genes encoding ferritin (MlFer1, MlFer2 and MlFer3) were down-regulated in roots, CL and NL under Fe deficiency and bicarbonate stress (Fig. 4B, C and D), indicating Fe in roots does not combine with ferritin. Fe content analysis showed that Fe was lower in both Fe-deficient and bicarbonate-supplied plants (Fig. 5A). Further analysis for proportion of Fe content in different tissues to total Fe content showed that Fe content in bicarbonate-supplied roots was higher than that in Fe-deficient roots and Fe supply (Fig. 5B), suggesting bicarbonate blocks Fe translocation from roots.
The expression level of citrate transporter gene of MlMATE66 A, MlFer1 B, MlFer2 C and MlFer3 D in M. lupulina under iron deficiency and bicarbonate treatment. Data are mean ± SD (n = 3). Uppercases on bar represent the differences between tissues under the same treatment (p < 0.05). Lowercases on bar represent the differences between different treatments in the same tissue (p < 0.05)
Fe content A and percentage of total iron B in root, stem and leaf of M. lupulina under iron deficiency and bicarbonate treatment. Data are mean ± SD (n = 3). Uppercases on bar represent the differences between tissues under the same treatment (p < 0.05). Lowercases on bar represent the differences between different treatments in the same tissue (p < 0.05)
Discussion
It is well known that chlorosis occurs in calcareous soil, but its physiological and molecular basis has not been fully revealed. Therefore, the physiological and molecular responses to bicarbonate stress were studied in this work.
Bicarbonate-induced chlorosis in M. lupulina
After the seedlings were subjected to Fe deficiency, Fe in old leaf might translocate to new leaf by MtMATE66, which was up-regulated in chlorotic leaf (Fig. 4A). Therefore, chlorophyll content in Fe-deficient plant showed no changes when compared with Fe-supplied plants (Fig. 1). The inhibition of root growth in bicarbonate-supplied plants is consistent with previous reports on other crops (Alhendawi et al. 1997; Mengel et al. 1984). The inhibition was considered that bicarbonate affects cell elongation in roots (Lee and Woolhouse 1969) and was induced by accumulation of organic acid in roots, such as malate, succinate and citrate (Guo-hui 2012; Yang et al. 1994; Hajiboland et al. 2005). In addition, the new leaf exhibited chlorotic symptoms and lower chlorophyll content with bicarbonate in both Fe-deficient and Fe-supplied plants (Fig. 1A, B and E), indicating that bicarbonate induced chlorotic symptoms of new leaf.
Bicarbonate enhanced FRO activity and proton exudation
Bicarbonate stress increases pH in soil and changes the redox state of iron in soil (Mengel et al. 1984; Konrad Mengel 1994). Furthermore, it also directly affects the physiological and biochemical reaction in roots. Bicarbonate treatment significantly increased FRO activity in both Fe-supplied plants and Fe-deficient plants, indicating that bicarbonate enhanced FRO activity in M. lupulina roots for reduction of Fe3+ (Fig. 2B). The results conflict with the reports of Hsieh (Hsieh and Waters 2016). In his report, Fe-deficient plants were designed as those with 0.5 M Fe. However, in this study, 0 M Fe was designed as an iron deficiency condition. Together, these results suggested that bicarbonate increased or decreased FRO activity depending on the Fe concentration in the environment (García et al. 2014; Lucena et al. 2007; Romera et al. 1997; Waters et al. 2007). In Fe-deficient plants, the expression of MlFRO1 in roots and leaves was up-regulated, but their expression in roots was higher than that in leaves. It is suggested that the expression of MlFRO1 is consistent with its role in the reduction of rhizosphere Fe3+ (Waters et al. 2002). The higher expression of MlFRO1 in chlorotic leaf with bicarbonate may increase the FRO activity in leaf apoplast, indicating a partial reduction of Fe3+ in leaf cells (Waters et al. 2002). Fe deficiency and bicarbonate supply induced significant MlHA1 expression levels in roots, especially in bicarbonate-supplied plants (Fig. 2D), indicating H+ was secreted from roots for acidification of rhizosphere soil with bicarbonate (Martínez-Cuenca et al. 2013). However, the expression of MlHA1 in NL and CL showed no significant changes under bicarbonate treatment. It suggested that bicarbonate did not change apoplast pH in leaves (Nikolic and Römheld 2002).
Fe uptake response to bicarbonate stress
When plants are subjected to Fe deficiency and bicarbonate, the secretion of FRO and H+ from roots increases Fe2+ availability, then Fe2+ is uptaken by root systems depending on iron transporter and metal transporter. Currently, the expression of MlIRT1 in roots was higher than that in leaves with Fe deficiency or bicarbonate, showing that MlIRT1 was mainly attributed to Fe transportation into roots from soil (Vert et al. 2002). In addition, higher MlIRT1 expression levels in CL with bicarbonate indicated that the MlIRT1 functions as Fe transporter in new leaf with bicarbonate supply. ZIP is a metal transporter family, which transports a variety of metal ions, such as Fe2+, Zn2+ and Mn2+ (López-Millán et al. 2004). Phylogenetic tree analysis showed that MlZIP1, MlZIP3, MlZIP5 and MlZIP7 were homologous with MtZIP1, MtZIP3, MtZIP5 and MtZIP7, respectively (Fig. S1). In the present study, RT-PCR analysis showed that the expression of MlZIP1 and MlZIP5 was down-regulated in Fe-deficient and bicarbonate-supplied roots and leaves (Fig. 3B and D). It is suggested that they are not involved in Fe transport. In fact, AtZIP1 functions as a transporter of Zn2+ and responds to zinc deficiency in A. thaliana (Grotz et al. 1998), which is an ortholog MtZIP1. In addition, MtZIP5 is down-regulated under Mn deficiency (López-Millán et al. 2004). López-Millán et al. (López-Millán et al. 2004) suggested that MtZIP3 was induced in roots and leaves in response to Zn2+ deficiency. Although the expression of MlZIP3 and MlZIP7 was up-regulated in Fe-deficient and bicarbonate-supplied plants (Fig. 3C and E), MtZIP3 was reported to be up-regulated under Zn2+ deficiency (López-Millán et al. 2004) and MtZIP7 mainly contributed to the transportation of Mn2+ (López-Millán et al. 2004). Therefore, this study did not clearly indicate whether MlZIP3 and MlZIP7 are involved in Fe transport.
Bicarbonate blocked Fe translocation from root
Fe is usually combined with ligands or transporters to form a complex transporter, which is translocated into leaf tissue through the xylem. Currently, the NRAMP and YSL genes are not identified from the M. lupulina transcriptome data (Data are not shown). RT-PCR analysis showed that MlMATE66 was mainly expressed in roots (Fig. 4A). The results were consistent with those reported by Yokosho (Yokosho et al. 2009). The expression of MlMATE66 was significantly down-regulated in the roots of bicarbonate-treated plants and up-regulated in chlorotic leaf. It is suggested that bicarbonate blocks the Fe translocation from roots, which results in Fe accumulation in roots (Fig. 5B). Of course, higher expression of MlMATE66 in chlorotic leaves with bicarbonate was involved in Fe translocation between leaf tissue. Three ferritin genes (MlFer1-3) were identified in M. lupulina and RT-PCR analysis showed that all of them were down-regulated in bicarbonate-supplied plants, especially in chlorotic leaf (Fig. 4B, C, D). It is shown that Fe did not combine with ferritin in the roots.
Conclusions
In conclusion, bicarbonate stress reduces the iron (Fe) availability in the root environment, which enhances the expression of genes related to Fe acquisition, such as MlHA1, MlFRO1 and MlIRT1, resulting in Fe uptake from soil. More importantly, bicarbonate stress inhibits the expression of MlMATE66 in roots and prevents the Fe translocation from roots to developing leaf, resulting in Fe accumulation in roots and reducing Fe content in new leaf. Therefore, chlorotic symptoms of iron deficiency induced by bicarbonate are triggered in the root (Fig. 6). Additionally, the functions of MlZIP3 and MlZIP7 under bicarbonate stress remain unclear in the present study. Our studies broaden our knowledge level about iron acquisition-related genes and further reveal the mechanism of iron deficiency chlorosis grown in calcareous soil (enrichment bicarbonate).
Schematic diagram of iron uptake and translocation in M. lupulina under bicarbonate stress. A and B indicate the normal growth condition and bicarbonate stress, respectively. Hollow circle represents HCO3−. Red circle represents Fe2+. Blue circle represents citrate transporter. Green circle represents ferritin. Genes with red represents up-regulation and genes with green represent down-regulation
Data availability
The raw data used in the present study are available within the article and Supplementary File.
References
Alhendawi RA, Römheld V, Kirkby EA, Marschner H (1997) Influence of increasing bicarbonate concentrations on plant growth, organic acid accumulation in roots and iron uptake by barley, sorghum, and maize. J Plant Nutr 20:1731–1753
Ariel FD, Manavella PA, Dezar CA, Chan RL (2007) The true story of the HD-Zip family. Trends Plant Sci 12(9):419–426
Briat JF, Dubos C, Gaymard F (2015) Iron nutrition, biomass production, and plant product quality. Trends Plant Sci 20(1):33–40
Cao L, Yu Y, DuanMu H, Chen C, Duan X et al (2016) A novel Glycine soja homeodomain-leucine zipper (HD-Zip) I gene, Gshdz4, positively regulates bicarbonate tolerance and responds to osmotic stress in Arabidopsis. BMC Plant Biol 16(1):184
Chen Y, Barak P (1982) Iron nutrition of plants in calcareous soils. In: Brady NC (ed) Advances in Agronomy. Academic Press, pp 217–240
Connorton JM, Balk J, Rodríguez-Celma J (2017) Iron homeostasis in plants-a brief overview. Metallomics 9(7):813–823
Curie C, Alonso JM, Le Jean M, Ecker JR, Briat JF (2000) Involvement of NRAMP1 from Arabidopsis thaliana in iron transport. Biochem J 347(Pt 3):749–55
Curie C, Panaviene Z, Loulergue C, Dellaporta SL, Briat JF et al (2001) Maize yellow stripe1 encodes a membrane protein directly involved in Fe(III) uptake. Nature 409(6818):346–349
Durrett TP, Gassmann W, Rogers EE (2007) The FRD3-mediated efflux of citrate into the root vasculature is necessary for efficient iron translocation. Plant Physiol 144(1):197–205
El-Gioushy SF, Ding Z, Bahloul AME, Gawish MS, Abou El Ghit HM et al (2021) Foliar application of nano, chelated, and conventional iron forms enhanced growth, nutritional status, fruiting aspects, and fruit quality of washington navel orange trees (Citrus sinensis L. Osbeck). Plants 10(12):2577
García MJ, García-Mateo MJ, Lucena C, Romera FJ, Rojas CL et al (2014) Hypoxia and bicarbonate could limit the expression of iron acquisition genes in Strategy I plants by affecting ethylene synthesis and signaling in different ways. Physiol Plant 150(1):95–106
Grotz N, Fox T, Connolly E, Park W, Guerinot ML et al (1998) Identification of a family of zinc transporter genes from Arabidopsis that respond to zinc deficiency. Proc Natl Acad Sci USA 95(12):7220–7224
Guo-hui Y (2012) Alkali stress induced the accumulation and secretion of organic acids in wheat. Afr J Agric Res 7
Hajiboland R, Yang X, Römheld V, Neumann G (2005) Effect of bicarbonate on elongation and distribution of organic acids in root and root zone of Zn-efficient and Zn-inefficient rice (Oryza sativa L.) genotypes. Environ Exp Bot 54:163–173
Harrison PM, Arosio P (1996) The ferritins: molecular properties, iron storage function and cellular regulation. Biochim Biophys Acta 1275(3):161–203
Hell R, Stephan UW (2003) Iron uptake, trafficking and homeostasis in plants. Planta 216(4):541–551
Hsieh EJ, Waters BM (2016) Alkaline stress and iron deficiency regulate iron uptake and riboflavin synthesis gene expression differently in root and leaf tissue: implications for iron deficiency chlorosis. J Exp Bot 67(19):5671–5685
Kruger C, Berkowitz O, Stephan UW, Hell R (2002) A metal-binding member of the late embryogenesis abundant protein family transports iron in the phloem of Ricinus communis L. J Biol Chem 277(28):25062–25069
Lee JA, Woolhouse HW (1969) A comparative study of bicarbonate inhibition of root growth in calcicole and calcifuge grasses. New Phytol 68(1):1–11
Lescure AM, Proudhon D, Pesey H, Ragland M, Theil EC et al (1991) Ferritin gene transcription is regulated by iron in soybean cell cultures. Proc Natl Acad Sci USA 88(18):8222–8226
Liu C, Mao B, Ou S, Wang W, Liu L et al (2014) OsbZIP71, a bZIP transcription factor, confers salinity and drought tolerance in rice. Plant Mol Biol 84(1–2):19–36
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25(4):402–408
López-Millán AF, Ellis DR, Grusak MA (2004) Identification and characterization of several new members of the ZIP family of metal ion transporters in Medicago truncatula. Plant Mol Biol 54(4):583–596
Lucena C, Romera FJ, Rojas CL, García MJ, Alcántara E et al (2007) Bicarbonate blocks the expression of several genes involved in the physiological responses to Fe deficiency of Strategy I plants. Funct Plant Biol 34(11):1002–1009
Martínez-Cuenca MR, Legaz F, Forner-Giner M, Primo-Millo E, Iglesias DJ (2013) Bicarbonate blocks iron translocation from cotyledons inducing iron stress responses in Citrus roots. J Plant Physiol 170(10):899–905
Mengel K (1994) Iron availability in plant tissues – iron chlorosis on calcareous soils. Plant Soil 165(2):275–283
Mengel K, Breininger MT, Bübl W (1984) Bicarbonate, the most important factor inducing iron chlorosis in vine grapes on calcareous soil. Plant Soil 81(3):333–344
Mengel K, Kirkby E, Kosegarten H, Thomas A (2001) Principles of plant nutrition. Springer Netherlands, Dordrecht, pp 585–597
Mora-Córdova CP, Tolrà R, Padilla R, Poschenrieder C, Simard M-H et al (2022) Rhizosphere acidification as the main trait characterizing the differential in vitro tolerance to iron chlorosis in interspecific pyrus hybrids (Article). Horticulturae 2022 v.8 no.6 (no. 6)
Nikolic M, Römheld V (2002) Does high bicarbonate supply to roots change availability of iron in the leaf apoplast? Plant Soil 241(1):67–74
Petit JM, Briat JF, Lobréaux S (2001) Structure and differential expression of the four members of the Arabidopsis thaliana ferritin gene family. Biochem J 359(Pt 3):575–582
Robinson NJ, Procter CM, Connolly EL, Guerinot ML (1999) A ferric-chelate reductase for iron uptake from soils. Nature 397(6721):694–697
Romera FJ, Alcántara E, de la Guardia MD (1997) Influence of bicarbonate and metal ions on the development of root Fe(III) reducing capacity by Fe-deficient cucumber (Cucumis sativus) plants. Physiol Plant 101:143–148
Roschzttardtz H, Conéjéro G, Divol F, Alcon C, Verdeil JL et al (2013) New insights into Fe localization in plant tissues. Front Plant Sci 4:350
Santos CS, Roriz M, Carvalho SM, Vasconcelos MW (2015) Iron partitioning at an early growth stage impacts iron deficiency responses in soybean plants (Glycine max L.). Front Plant Sci 6:325
Shahsavandi F, Eshghi S, Gharaghani A, Ghasemi-Fasaei R, Jafarinia M (2020) Effects of bicarbonate induced iron chlorosis on photosynthesis apparatus in grapevine. Sci Hortic 270:109427
Souri MK, Naiji MR, Aslani M (2018) Effect of Fe-glycine aminochelate on pod quality and iron concentrations of bean (Phaseolus vulgaris L.) under lime soil conditions. Commun Soil Sci Plant Anal 49:215–224
Takanashi K, Yokosho K, Saeki K, Sugiyama A, Sato S et al (2013) LjMATE1: a citrate transporter responsible for iron supply to the nodule infection zone of Lotus japonicus. Plant Cell Physiol 54(4):585–594
Tato L, De Nisi P, Donnini S, Zocchi G (2013) Low iron availability and phenolic metabolism in a wild plant species (Parietaria judaica L.). Plant Physiol Biochem 72:145–153
Vert G, Grotz N, Dédaldéchamp F, Gaymard F, Guerinot ML et al (2002) IRT1, an Arabidopsis transporter essential for iron uptake from the soil and for plant growth. Plant Cell 14(6):1223–1233
Wang J, Hou Q, Li P, Yang L, Sun X et al (2017) Diverse functions of multidrug and toxin extrusion (MATE) transporters in citric acid efflux and metal homeostasis in Medicago truncatula. Plant J 90(1):79–95
Wang N, Dong X, Chen Y, Ma B, Yao C et al (2020) Direct and bicarbonate-induced iron deficiency differently affect iron translocation in kiwifruit roots. Plants (Basel) 9(11):1578
Waters BM, Blevins DG, Eide DJ (2002) Characterization of FRO1, a pea ferric-chelate reductase involved in root iron acquisition. Plant Physiol 129(1):85–94
Waters BM, Chu HH, Didonato RJ, Roberts LA, Eisley RB et al (2006) Mutations in Arabidopsis yellow stripe-like1 and yellow stripe-like3 reveal their roles in metal ion homeostasis and loading of metal ions in seeds. Plant Physiol 141(4):1446–1458
Waters BM, Lucena C, Romera FJ, Jester GG, Wynn AN et al (2007) Ethylene involvement in the regulation of the H(+)-ATPase CsHA1 gene and of the new isolated ferric reductase CsFRO1 and iron transporter CsIRT1 genes in cucumber plants. Plant Physiol Biochem 45(5):293–301
Yang X, Römheld V, Marschner H (1994) Effect of bicarbonate on root growth and accumulation of organic acids in Zn-inefficient and Zn-efficient rice cultivars (Oryza sativa L.). Plant Soil 164(1):1–7
Yokosho K, Yamaji N, Ueno D, Mitani N, Ma JF (2009) OsFRDL1 is a citrate transporter required for efficient translocation of iron in rice. Plant Physiol 149(1):297–305
Acknowledgements
The authors would like to thank the Natural Science Foundation of China and the Karst Science Research Center of Guizhou Province of China (U1812401) and the Science and Technology Support Plant Project of China ([2021]224) for their support in this research.
Funding
This research was supported by the Guizhou Provincial Science and Technology Projects (QIANKEHEJICHU-ZK [2023] 268), the Natural Science Foundation of China and the Karst Science Research Center of Guizhou Province, China (U1812401), the Science and Technology Support Plant Project, China ([2021]224), Higher Education Science and Research Youth Project of Guizhou Education Department (Qianjiaoji [2022]130), the Guizhou Provincial Science and Technology Projects, China (QIANKEHEJICHU-ZK [2021] Key 038), Innovation and entrepreneurship training plan for national and provincial college students, Guizhou Normal University, China (S202110663037), Science and Technology Fund Project of Guizhou Province (No.[2020]4Y028), Guizhou forestry scientific research project, Qianlinkehe [2022] No. 28.
Author information
Authors and Affiliations
Contributions
Ximin ZHANG conceived and designed the experiments. Lunxian LIU performed the experiments on RT-PCR. Zhimeng SU performed the experiments on biomass and enzyme activity. Ming TANG designed the primers. Jing TANG identified the genes. Ximin ZHANG, Lunxian LIU, Meifeng CHEN and Xiaorong XU wrote the paper. Yin YI and Jiyi GONG revised the paper. All authors have read and approved the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare that they have no conflicts of interest.
Additional information
Communicated by V. P. Singh.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, L., Chen, M., Xu, X. et al. Bicarbonate affects the expression of iron acquisition and translocation genes involved in chlorosis in Medicago lupulina. Acta Physiol Plant 46, 55 (2024). https://doi.org/10.1007/s11738-024-03685-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-024-03685-1