Abstract
An efficient in vitro regeneration protocol and field performance of a multipurpose bamboo species Dendrocalamus hamiltonii Nees et Arn. Ex Munro has been demonstrated using single node cuttings taken from the lateral branches of a 20-year-old bush. Axillary buds on the nodal explant sprouted within 10 days of culture on Murashige and Skoog (MS) medium without any plant growth substance. High-frequency proliferation was induced on the propagules (small clusters with 3–5 multiple shoots and rhizomatous portions). Subsequent removal of the shoots (about 1.5 cm) from the rhizomatous portion of propagules (shoot cut) influenced the plantlet formation capacity. A multiplication of about 20-folds was achieved on MS medium supplemented with 8 μM BAP and 1 μM NAA. Rooting efficiency was also markedly enhanced (>90%) when the propagules, following shoot cut, were placed on to MS medium supplemented with 100 μM IBA for 10 days and then transferred to IBA-free medium. This is the first report from this species where 20-fold increment in multiplication was observed at the end of second subculture followed by >90% rooting. The hardened plants, established in the field, exhibited normal growth; their physiological performance has been monitored at 6-month intervals. The rate of photosynthesis increased from 3.55 μmol CO2 m−2 s−1 (hardened, ready for field transfer) to 5.44 μmol m−2 s−1 (6 months of field transfer); following a year of plantation net photosynthesis recorded was 14.0 μmol CO2 m−2 s−1 while after 1.5 years it was 12.76 μmol CO2 m−2 s−1. These values were compared with those observed for the mother bush. Genetic fidelity of these regenerants was established by RAPD analysis advocating clonal propagation of this species through nodal segment culture and its commercial cultivation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bamboos are one of the most important non-timber forest products and form the backbone of rural economy of several south-east Asian countries. Nowadays, it has become an integral component in social forestry programmes. One of the most important contributions of bamboo to the modern society is in the production of paper; besides its extensive use in house building, making furniture, agricultural implements, mat boards, baskets, handicrafts and numerous traditional uses is well known and provides employment opportunities (Rao et al. 1990). In view of these reasons, bamboo resources of the country are decreasing. Therefore, rapid and large-scale production of clonal planting material using tissue-culture technique followed by field plantation and evaluation would compensate the loss.
Dendrocalamus hamiltonii Nees et Arn. Ex Munro, a multipurpose, fast-growing species with strong culms and used in this region of Central Himalaya for various above-mentioned purposes including its leaves for good quality fodder for cattle during winter months (Negi et al. 1980), holds considerable promise. The use of tissue-culture technique for multiplication of D. hamiltonii has been reported earlier (Chambers et al. 1991; Bag 2001; Godbole et al. 2002; Sood et al.1992, 2002a, b). Although multiple shoot formation has been reported, rooting has been inconsistent with up to 30% rooting (Sood et al. 2002a, b). In addition, evaluation following field plantation of in vitro-propagated plants based on the physiological characters and assessment of genetic fidelity has not been reported in this species.
In the present communication, we describe an efficient in vitro propagation protocol for D. hamiltonii, using nodal explants of a selected 20-year-old field grown elite bamboo with the following objectives: (1) enhancing shoot multiplication, (2) improving rooting of microshoots, (3) evaluation of physiological characters of field grown plants and (4) assessment of genetic fidelity of in vitro-raised and field grown plants.
Materials and methods
In vitro propagation
Single node cuttings taken from the third internode of 1-year-old lateral branches of a 20-year-old vegetatively propagated plant (mother bush) of D. hamiltonii growing at the Institute nursery at Kosi (79°38′10′′E and 29°28′15′′N; 1,150 m) were collected and used for developing in vitro cultures as described earlier (Bag 2001). Nodal segments (about 3.0 cm in length) were soaked in distilled water containing Labolene (Liquid detergent; 0.2% v/v; Qualigens, India) for about 2–3 min; these were washed with distilled water three to four times, disinfected with 0.2% mercuric chloride (w/v, BDH, India; 5 min), rinsed again with sterilized distilled water (4×), both the ends trimmed and segments (approx. 20 mm) were cultured on Murashige and Skoog (1962) (MS) medium (20 ml) containing 2% sucrose (w/v) without any plant growth substance (PGS) and placed vertically in test tubes (15 × 1.5 cm). The pH of the medium was adjusted to 5.8 and gelled with 0.2% phytagel (w/v, Sigma) before autoclaving (0.103 MPa, 121°C, 20 min). All the cultures were maintained at 25 ± 1°C in 16/8 h day/night (42 and 60 μmol m−2 s−1 light intensity inside and outside of the cultured flasks).
After 3–4 weeks of incubation of nodal explants, the sprouted buds (20–25 mm long) were excised from the mother stumps and placed on MS medium supplemented with different concentrations of NAA (1.0–5.0 μM) and BAP (2.0–12.0 μM) used in various combinations. In some of these combinations, the buds produced small clusters with three to five healthy multiple shoots (2.5–3.0 cm) and a rhizomatous part, hereafter called ‘propagules’. The shoots were individually cut about 1.5 cm above the base and discarded while the ‘propagules’ containing a rhizomatous portion, now referred to as ‘shoot cut’, was separated and further transferred for multiplication (Table 1). Subsequently, the propagules (with shoot cut) were separated again and placed in half MS media containing IBA at different concentrations (10–200 μM) for induction of roots. Data were recorded following 7 days treatment in IBA containing medium and subsequently placing the plantlets on IBA-free MS medium for 2 weeks.
The propagules (with shoot cut) were also placed separately on MS medium supplemented with 8.0 μM BAP and 1.0 μM NAA, to examine the influence of lights [photosynthetically active radiation (PAR) and white cool fluorescent light (CFL)] and gelling agents (Phytagel 0.2%, w/v and agar 0.8%, w/v), if any, on multiplication of propagules and subsequent rooting (using above method), after 4 weeks of incubation.
Well-rooted plants were removed from the flasks, washed thoroughly with water, transferred to soil in plastic cups (6 × 6 × 7 cm; containing 250 g of autoclaved soil) and placed under stable greenhouse conditions (25°C, r.h. 65%; 18/6 h day/night) for 15 days. After another 15 days, the plants were transferred to polythene bags (16 × 10 cm) containing equal proportion of soil and farm yard manure (1:1, v/v; 1 kg) and placed inside a polyhouse/nethouse for hardening. After 1 month of hardening (now 2-month-old plants), 20 plants were randomly selected for field plantation (pit size: 50 × 50 × 50 cm; distance: plant to plant 4 m) in a nearby area and their growth performance was monitored at 6-month interval.
Morphological and physiological evaluation of field grown plants
Plant height was determined (on 10 plants) and the measurements were taken from the ground level to the top of the plant. Leaf characteristics were determined using an automatic leaf area meter (LI-3000A; LICOR, USA); ten leaves per plant were selected and the average area (cm2), length (cm) and width (cm) were measured on per leaf basis. The number of culms was counted manually on per plant basis and mean (±standard deviation) values provided.
CO2 and water vapor exchange (photosynthetic) measurements were carried out (3 leaves per plant; 5 randomly selected plants were taken) from the lateral branches of third internode with the help of an open-portable gas-exchange system (LI-6400; LI-COR, USA) equipped with red light emitting source fixed on the top of the leaf chamber. Different parameters viz. rate of photosynthesis (Pn), transpiration (E), the ratio of intercellular and ambient CO2 (Ci/Ca), water use efficiency (WUE) and a ratio of Ci/gs were recorded. All these measurements were carried out during day time between 10.00 a.m. and 2.00 p.m. at different growth stages of in vitro-raised plants and mother plant, at every 6-month interval in the month of September (autumn/fall) and March (spring/flush). The measurements were carried out at cuvette air temperature of 25°C, relative humidity of 50–55%, irradiance of 1,000 μmol m−2 s−1 at the leaf level and at a gas flow rate of 500 μmol s−1.
Molecular analyses
DNA isolation
Young bamboo leaves collected from mother bush (20-year-old), and from in vitro-raised plants [just after transfer to soil (0 day), after transfer to the polyhouse/nethouse (15-day-old), ready for field transfer (2-month-old and fully hardened plants) and 6 and 18 months after field plantation)] were collected. DNA was extracted using N-cetyl-N,N,N-trimethylammonium bromide (CTAB) as described by Doyle and Doyle (1987) with modifications. Briefly, fresh leaf material (500 mg) was washed and then ground in liquid nitrogen. Then, 10 ml of preheated extraction buffer [2% CTAB (w/v), 0.2% β-mercaptoethanol (v/v), 100 mM Tris–HCl (pH 8.0), 20 mM ethylene diamine tetraacetic acid (EDTA) and 1.4 mM NaCl] were added to the powdered material. After incubating the homogenate for 1 h (at 65°C), an equal volume of chloroform : isoamyl alcohol (24:1) was added and centrifuged at 10,000 rpm for 20 min. DNA was precipitated with 1/10 volume of 3 M sodium acetate and an equal volume of isopropanol followed by centrifugation at 10,000 rpm for 10 min. The DNA pellet was washed with 70% ethanol, air-dried and then re-suspended in 200–300 μl Tris–EDTA (TE; 1 mM). Quantification was performed by visualizing under UV light, after electrophoresis on 0.8% agarose gel stained with ethidium bromide. The re-suspended DNA was then diluted in sterile distilled water to 5 ng/μl concentration for use in amplification reactions.
RAPD fingerprinting
Random decamer oligonucleotides purchased from Operon Technologies Inc. (Alameda, CA, USA) was used as single primers for the amplification of RAPD fragments. The total genomic DNA from the mother bush and in vitro-raised plants at different growth stages were amplified with 80 random primers. Polymerase chain reactions (PCRs) were carried out in a final volume of 25 μl containing 20 ng template DNA, 200 μM each deoxynucleotide triphosphate, 20 ng of decanucleotide primers, 1.5 mM MgCl2, 10 mM Tris–HCl, 50 mM KCl, 0.1% Triton X-100 and 0.5U Taq DNA polymerase (M/s Bangalore Genei, India). Amplification was achieved in a Thermocycler (Biometra; Germany) programmed for a preliminary 5 min denaturation step at 94°C, followed by 40 cycles of denaturation at 94°C for 30 s, annealing at 36°C for 1 min and extension at 72°C for 1 min, finally at 72°C for 10 min (Nayak et al. 2003).
Amplification products were separated by electrophoresis on 1.5% agarose gels run in 1X TAE (Tris acetate EDTA) buffer, stained with ethidium bromide and visualized under UV light. Gel photographs were scanned through Gel Doc System (Alpha Imager™ IS-2200, USA). PCRs were repeated at least twice to establish reproducibility of results.
Statistical analysis
Standard deviation was calculated following the method of Snedecor and Cochran (1967).
Results and discussion
In vitro propagation
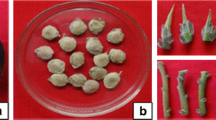
After culture on MS medium without any PGSs (Fig. 1a and b), the sprouted buds were subjected to MS medium containing BAP and NAA at different concentrations, differentiation was observed and multiple shoots were formed along with the increased rhizomatous portions (Fig. 1c). Out of the various combinations tried the medium containing 8.0 μM BAP and 1.0 μM NAA resulted in profuse shoot formation. Shoot portions (about 1.5 cm from the base) were removed and ‘propagules’ were cultured for multiplication (Table 1). Once again, the medium containing 8.0 μM BAP and 1.0 μM NAA resulted in enhanced multiplication (Table 1; Fig. 1d). Thus, starting with one explant more than 20 propagules could be obtained. There has been a fivefold increase in multiplication following ‘shoot cut’ compared with control, i.e. ‘without shoot cut’ (Table 2). Following this protocol 20% higher number of plantlets could be regenerated when compared with an earlier study (Bag 2001) where a combination of 5.0 μM BAP and 2.0 μM NAA supplemented to MS medium was used. This is the first report from this species wherein following in vitro ‘shoot cut’ method higher rate of multiplication (1:20) is obtained at the end of second subculture (Table 2; Fig. 1d).
Various stages during the development of in vitro propagation protocol of D. hamiltonii. a A 20-year-old mother bush of D. hamiltonii, the source of explants; bar 100 cm, b establishment of explant in MS medium without PGSs; bar 1 cm, c sprouted buds cultured on MS medium supplemented with 6.0 μM BAP and 1.0 μM NAA during first subculture, bar 1 cm, d shoot multiplication on MS medium supplemented with 8.0 μM BAP and 1.0 μM NAA during second subculture; bar 1.5 cm, e rooting in propagules following shoot cut; bar 1.5 cm; inset: profuse rooting in the propagules, f transfer of in vitro-raised plants to soil; bar 6 cm, g hardening of in vitro-raised plants in a polyhouse; bar 8 cm, h well-developed root system of hardened plants ready for field plantation; bar 25 cm, i in vitro-raised plants 6 months after field plantation; bar 75 cm
A short treatment of IBA for only 7 days, resulted in root initiation and IBA at 100 μM resulted in more than 90% rooting (Table 3; Fig. 1e); the number of roots per plantlet was more than six with length of longest root being 50 mm (data not shown). In an earlier study using IBA or NAA (0.5, 1.0 mg/l) up to 30% rooting was observed in 4–6 weeks (Sood et al. 2002b). When hardening (Fig. 1f, g) under greenhouse and polyhouse/nethouse condition, the plants formed well developed and profuse root system (Fig. 1h) with a survival rate of 85%.
Light and/or gelling agents have been reported to influence in vitro shoot multiplication, rooting and subsequent establishment (Kumar et al. 2002, 2003). Therefore, their effect was examined in this investigation and it indicated a gradual increase in average multiplication (values in parentheses represent increment in folds), in increasing order, i.e. white CFL + agar (2.55), PAR + agar (3.32), PAR + phytagel (5.59) and white CFL + phytagel (6.35). Thus, the later combination is recommended for faster multiplication of this species as it has been found to be 2.5 times more than the combination of white CFL + agar, which is commonly used in tissue culture.
Morphological and physiological evaluation of field grown plants
Data related to plant height, number of culms and leaf characteristics were recorded. Plant height was found to significantly increase with time and almost sixfold increment was observed within 1 year and 6 months. Although not much difference in the number of culms was recorded, all the leaf characteristics (leaf length, width, area and so on) improved with increase in plant height (data not shown).
CO2 and water vapor exchange parameters indicated that with leaf maturation, the photosynthetic characteristics of the in vitro-raised plants also increased markedly. The rate of photosynthesis increased from 3.55 CO2 μmol m−2 s−1 (hardened plants, ready for field transfer) to 5.44 μmol CO2 m−2 s−1 (6 months of field transfer; Fig. 1i); after a year of plantation, the rate of net photosynthesis was 14.0 μmol CO2 m−2 s−1, while after 1.5 years it was 12.76 CO2 μmol m−2s−1. These values are comparable to those observed for the mother bush (Fig. 2). Transpiration rate also increased simultaneously with the age of the plant (Fig. 2). WUE also showed a similar pattern like net photosynthesis. It is apparent that WUE of 18 months old plant (after field transfer) is comparable to that of the mother bush, i.e. 0.516 and 0.529, respectively. A similar trend was also observed for Ci/Ca ratio of 18-month-old field transferred plants and the mother bush with values 0.497 and 0.617, respectively (Fig. 2). During this span of time (1.5 years), the plant survival rate was over 70%. The observed slight decrease in the net photosynthesis after 1.5 years of field transfer is possibly due to recording of data on newly formed leaves during spring/flush.
Molecular analysis
Field evaluation of tissue-culture-raised plants and their performance (morphological and physiological evaluation) is important for long-term assessment and commercial applications. Furthermore, checking the genetic fidelity of such plants is necessary for the production of genetically uniform regenerants. In recent years, DNA profiling through RAPD techniques has been used for the analysis of diversity and identification of duplicates within large germplasm population (Virk et al. 1995), phylogenetic relationships (Millan et al. 1996) and for assessing genetic fidelity of tissue-culture-raised plants (Isabel et al. 1993; Rani et al. 1995). The need for assessing genetic stability in bamboo tissue culture using molecular techniques has also been highlighted (Gielis et al. 2002).
In the present investigation, during the primer-screening step for RAPD analysis, six primers produced clear and score able amplification products in all the samples from D. hamiltonii plants (Table 4). When amplified, these six-random primers produced a total of 33 fragments ranging in size from 0.3 to 2.6 kb (Table 4). The number of fragments produced by a primer ranged from 4 (OPC15) to 7 (OPA5 and OPA11; Table 4). The pattern of RAPD fragments produced by the random primer OPA5 is shown in Fig. 3. All amplification products were found to be monomorphic across the in vitro-propagated plants and the corresponding mother plant. In reaction on the template of DNA isolated from plants at different stages of growth with primers OPA3, OPA4, OPA5, OPA19 and OPA15, any variation was not observed in the mother plant and in vitro-raised plants. This indicates the presence of genetic fidelity among the regenerants of D. hamiltonii obtained in this study. In a similar study, the clonal fidelity of in vitro-raised plants of Bambusa balcooa and B. tulda was confirmed by RAPD analysis (Das and Pal, 2005); these workers advocated the use of axillary meristem culture for true-to-type or clonal propagation. In this investigation, preliminary test was carried out using RAPD during various stages of growth and development of in vitro-raised plants, up to 1.5 years after field plantation, to check the presence of somaclonal variation, if any, which may occur in plants propagated in vitro, and reported in many species, for example, Coffea arabica (Rani et al. 2000), Musa paradisiaca (Gimenez et al. 2001), Elaeis guineensis (Rival et al. 1998) and so on.
RAPD pattern of mother bush and in vitro-raised D. hamiltonii plants generated by primer OPA5, where M weight marker, MP mother plant, 1–2 plants just after transfer to soil (0 day), 3–4 plants after transfer to the polyhouse/nethouse (15-day-old), 5–6 fully hardened plants ready for field transfer (2-month-old), 7–8 plants 6 months after field plantation and 9–10 plants 18 months after field plantation
As in many other reports, nodal segments taken from precocious branches (Ramanayake and Yakanadawala 1997) were used as explant in this study, where axillary buds were used for further multiplication. The approach taken in the present investigation ensures clonal propagation of true-to-type plants. The efforts made in this study have enhanced in vitro multiplication (by 5-fold) when compared with control. More than 90% rooting was recorded in this study when compared with the earlier reports (Sood et al. 2002a, b) wherein only up to 30% rooting was demonstrated. The hardening and field performance of in vitro-propagated plants is particularly important aspect that needs utmost attention. Substantial numbers of in vitro-propagated plants do not survive after transfer from controlled in vitro conditions to ex vitro environment of the green house and later in the open field. Our result demonstrated more than 70% plant survival in the field and is comparable with the earlier report (Sood et al. 2002a).
In conclusion, this study describes an effective regeneration, multiplication, rooting and field establishment protocol for in vitro propagation of D. hamiltonii. These features are necessary for the adoption of in vitro propagation technology for large-scale multiplication of this species. Tissue-culture methods allows the production of a large number of plantlets identical with the mother plant is less labor intensive and cheaper once the methods have been standardized and, hence, offers distinct advantages over conventional methods for multiplication of elite bamboo clones (Prutpongse and Gavinlertvatana 1992; Saxena and Dhawan 1999; Gielis et al. 2002; Godbole et al. 2002; Sood et al. 2002a, b; Das and Pal 2005).
The outcome of the work would help in refining the multiplication and rooting steps for the development of technology packages for mass-scale propagation and cultivation of D. hamiltonii; this will not only help in large-scale multiplication of this useful multipurpose bamboo species for the restoration of degraded land, but also result in deriving economic benefits by the local communities.
References
Bag N (2001) Mass propagation of tea, maggar bamboo and dev ringal. D Phil Thesis, HNB Garhwal University, Srinagar, India, 168 p
Chambers SM, Heuch JHR, Pirre C (1991) Micropropagation and in vitro flowering of the bamboo Dendrocalamus hamiltonii Munro. Plant Cell Tissue Organ Cult 27:45–48. doi:10.1007/BF00048205
Das M, Pal A (2005) Clonal propagation and production of genetically uniform regenerants from axillary meristem of adult bamboo. J Plant Biochem Biotechnol 14:185–188
Doyle JJ, Doyle JL (1987) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochemical Bull 19:11–15
Gielis J, Peeters H, Gillis K, Oprimes J, Debergh PC (2002) Tissue culture strategies for genetic improvement of bamboo. Acta Hortic 552:195–203
Gimenez C, Garcia ED, Enrech NXD, Blanca I (2001) Somaclonal variation in banana: cytogenetic and molecular characterization of somaclonal variant CIEN BTA-03. Vitro Cell Dev Biol Plant 37:217–222
Godbole S, Sood A, Thakur R, Sharma M, Ahuja PS (2002) Somatic embryogenesis and its conversion into plantlets in a multipurpose bamboo, Dendrocalamus hamiltonii Nees et Arn Ex Munro. Curr Sci 83:885–889
Isabel NL, Tremblay MM, Tremblay FM, Bousquet J (1993) RAPD as an aid to evaluate the genetic integrity of somatic embryogenesis derived population of Picea mariana (Mill). Theor Appl Genet 86:81–87. doi:10.1007/BF00223811
Kumar A, Nandi SK, Bag N, Palni LMS (2002) Tissue culture studies in two important orchid taxa: Rhynchostylis retusa (L) Bl. and Cymbidium elegans Lindl. In: Nandi SK, Palni LMS, Kumar A (eds) Role of plant tissue culture in biodiversity conservation and economic development. Gyanodaya Prakashan, Nainital, pp 113–124
Kumar A, Palni LMS, Nandi SK (2003) The effect of light and gelling agent on micropropagation of Rosa damascena Mill. and Rhynchostylis retusa (L.) Bl. J Hortic Sci Biotechnol 78(6):786–792
Millan T, Osuna F, Cobos S, Torres AM, Cubero JJ (1996) Using RAPDs to study phylogenetic relationships in Rosa. Theor Appl Genet 92:273–277. doi:10.1007/BF00223385
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassay with tobacco tissue culture. Physiol Plant 15:473–497. doi:10.1111/j.1399-3054.1962.tb08052.x
Nayak S, Rout GR, Das P (2003) Evaluation of the genetic variability in bamboo using RAPD markers. Plant Soil Environ 49(1):24–28
Negi SS, Pal RN, Enrich C (1980) Tree fodders in Himachal Pradesh. German Agency for Technical Cooperation (GTZ), Eschborn (FRG), India
Prutpongse P, Gavinlertvatana P (1992) In vitro micropropagation of 54 species from 15 genera of bamboo. Hortic Sci 27:453–454
Ramanayake SMSD, Yakanadawala K (1997) Micropropagation of the giant bamboo (Dendrocalamus giganteus Munro) from nodal explants of field grown culms. Plant Sci 129:213–223. doi:10.1016/S0168-9452(97)00185-4
Rani V, Parida A, Raina SN (1995) Random amplified polymorphic DNA (RAPD) markers for genetic analysis in micropropagated plants of Populus deltoides Marsh. Plant Cell Rep 14:459–462. doi:10.1007/BF00234055
Rani V, Singh KP, Shiran M, Goel S, Devarumath RM, Sreenath HL, Raina SN (2000) Evidence for new nuclear and mitochondrial genome organizations among high frequency somatic embryogenesis-derived plants of allotetraploid Coffea arabica L. (Rubiaceae). Plant Cell Rep 19:1013–1020. doi:10.1007/s002990000228
Rao IVR, Yusoff AM, Rao AN, Shastry CB (1990) Propagation of bamboo and rattan through tissue culture. The IDRC Bamboo and Ratan Research Network, Canada, pp 1–60
Rival A, Bertrand L, Beale T, Combes M-C, Trouslot P, Leshermes P (1998) Suitability of RAPD analysis for detection of somaclonal variation in oil palm (Elaeis guineensis Jacq.). Plant Breed 117:73–76. doi:10.1111/j.1439-0523.1998.tb01451.x
Saxena S, Dhawan B (1999) Regeneration and large-scale propagation of bamboo (Dendrocalamus strictus Nees) through somatic embryogenesis. Plant Cell Rep 18:438–443. doi:10.1007/s002990050600
Snedecor GW, Cochran WG (1967) Statistical methods. Oxford and IBH Publishing Company, New Delhi. 593 p
Sood A, Sharma OP, Palni LMS (1992) Improved methods of propagation of maggar (Dendrocalamus hamiltonii Nees et Arn. Ex Munro) using single node cutting taken from juvenile culms of elite seedlings. J Am Bamboo Soc 9:17–24
Sood A, Ahuja PS, Sharma M, Sharma OP, Godbole S (2002a) In vitro protocols and field performance of elites of an important bamboo Dendrocalamus hamiltonii Nees et Arn. Ex Munro. Plant Cell Tissue Organ Cult 71:55–63. doi:10.1023/A:1016582732531
Sood A, Palni LMS, Sharma M, Chand G, Sharma OP (2002b) Micropropagation of Dendrocalamus hamiltonii Munro (Maggar Bamboo) using explants taken from seed raised and field-tested plus plants. J Plant Biol 29(2):125–132
Virk PS, Ford-Llyod BV, Jackson MT, Newbury HJ (1995) Use of RAPD for the study of diversity within plant germplasm collections. Heredity 74:170–179. doi:10.1038/hdy.1995.25
Acknowledgments
Drs. L.M.S. Palni and N. Bag are thanked for valuable suggestions; Director of the Institute is thanked for providing necessary facilities. The financial supports received from the Ministry of Environment and Forests, and the Department of Biotechnology, Govt. of India, New Delhi is duly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by B. Borkowska.
Rights and permissions
About this article
Cite this article
Agnihotri, R.K., Mishra, J. & Nandi, S.K. Improved in vitro shoot multiplication and rooting of Dendrocalamus hamiltonii Nees et Arn. Ex Munro: production of genetically uniform plants and field evaluation. Acta Physiol Plant 31, 961–967 (2009). https://doi.org/10.1007/s11738-009-0311-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-009-0311-6