Abstract
Gas exchange rates, chlorophyll fluorescence, pressure–volume relationships, photosynthetic pigments, total soluble sugars, starch, soluble proteins and proline concentrations were investigated in five Olea europaea L. cultivars with different geographical origins (Arbequina, Blanqueta, Cobrançosa, Manzanilla and Negrinha) grown under Mediterranean field conditions. We found considerable genotypic differences among the cultivars. Comparing the diurnal gas exchange rates, we observed that Cobrançosa, Manzanilla and Negrinha had high photosynthetic rate than Arbequina and Blanqueta. The first group reveals to be better acclimated to drought conditions, and appears to employ a prodigal water-use strategy, whereas Blanqueta and Arbequina, with high water-use efficiency, appear to employ a conservative water-use strategy. The degree of midday depression in photosynthesis was genotype dependent, with a maximum in Arbequina and a minimum in Negrinha. The reductions in the photosynthetic rate were dependent from both stomatal and non-stomatal limitations. Elastic adjustment plays an important role as drought tolerance mechanism. The group of cultivars that employ a prodigal water-use strategy revealed high tissue elasticity, whereas Arbequina and Blanqueta revealed high tissue rigidity. We also identified the existence of drought tolerance mechanisms associated with soluble proteins accumulation in the foliage. The high levels of soluble proteins in Arbequina may represent an increased activity of oxidative stress defence enzymes and may also represent a reserve for post stress recovery. In all cultivars, especially in Manzanilla, free proline was accumulated in the foliage. The discussed aspects of drought stress metabolism may have an adaptative meaning, supporting the hypothesis that olive cultivars native to dry regions, such as Cobrançosa, Manzanilla and Negrinha, have more capability to acclimate to drought conditions than cultivars originated in regions with a more temperate climate, like Arbequina and Blanqueta.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Soil and atmospheric water deficits are the most important limiting factors for photosynthesis, growth and survival of plants growing in semiarid climates, such as the Mediterranean. In the field, high irradiance and high temperature also contribute to the reduction in leaf net carbon uptake (Faria et al. 1996). Stomatal control of water losses has been identified as an early event in plant responses to water deficit under field conditions, leading to a limitation of carbon uptake by the leaves (Abd-El-Rahman et al. 1966; Chaves 1991; Cornic and Massacci 1996). When carbon assimilation is limited by the decrease in stomatal conductance during the warmest period of the day, chloroplasts may be subjected to an excess of energy resulting in the down-regulation of photosynthesis or in photoinhibition (Demmig-Adams and Adams 1996).
The olive tree has a reputation of being drought tolerant from very early reports, but few studies have been conducted in the field that quantified its responses to water deficits (Giorio et al. 1999; Bacelar et al. 2007). Among such responses, gas exchange is of particular importance in determining the efficiency of water-use in response to the limited resources. Knowledge on gas exchange of olive leaves is limited and most studies have been conducted on young trees growing in pots (Bongi et al. 1987; Chartzoulakis et al. 1999; Bacelar et al. 2006). In potted olive trees, Angelopoulos et al. (1996) observed that leaf conductance was limiting photosynthesis in trees subjected to mild and moderate water stress, whereas non-stomatal factors influenced photosynthesis only under severe stress conditions. Angelopoulos et al. (1996) also observed that the diurnal course of photosynthesis and leaf conductance in potted trees exposed to the natural environment exhibited a maximum value in the morning, declined towards midday and was more or less constant throughout the afternoon, describing a pattern that is common in Mediterranean woody vegetation (Schulze and Hall 1982; Tenhunen et al. 1990; Fernández and Moreno 1999; Chaves et al. 2002; Ogaya and Peñuelas 2003).
In order to preserve photosynthesis, the olive tree like some plants grown in arid and semi-arid environments, has evolved physiological processes to maintain to some extent tissue turgor and thus stomatal opening (Fernández et al. 1991; Dichio et al. 1997; Chartzoulakis et al. 1999). Lowering of osmotic potential due to net accumulation of compatible solutes in the cytoplasm such as proline, glycine betaine, organic acids and sugars as mannitol and sucrose, is a well established ecophysiological mechanism whereby many plants adjust to low soil water availability (Morgan 1984; Ingram and Bartels 1996; Hare et al. 1998). Nonetheless, the stomatal and the non-stomatal, if existent, limitation of photosynthesis may lead to a drought-induced starvation injury (Levitt 1980) and consequently also metabolic alterations (Souza et al. 2004). Sites at which photosynthetic metabolism may, potentially, be affected by water stress include: (1) Rubisco activity, (2) regeneration of ribulose biphosphate (RuBP) by the photosynthetic carbon reduction (PCR) cycle, (3) supply of ATP and NADPH to the PCR cycle, (4) electron transport and generation of the proton gradient across the thylakoid membrane, (5) light capture and transduction in the photosystems, and (6) use of assimilation products outside the chloroplast (Lawlor 2002; Lawlor and Cornic 2002).
Changes in cell wall elasticity can also contribute to turgor maintenance under drought conditions (Patakas and Noitsakis 1997). Water potential changes more for a given change in tissue water content in leaves with greater bulk modulus of elasticity (ε), leading to larger gradients of water potential between leaves and soil with lower tissue water loss (Niinemets 2001). This improves water uptake from drying soil (Bowman and Roberts 1985) and is a frequently cited mechanism enabling drought-stressed plants to maintain cell volume and avoid deleterious reductions in relative water content (Tyree and Jarvis 1982). In contrast, cells with low ε allow greater cell shrinkage following dehydration. This results in turgor maintenance with lower leaf osmotic potentials, and also higher gradients of water potentials between leaves and the soil (Abrams 1990).
Olive has traditionally been grown in Trás-os-Montes (Northeast Portugal), where it is of considerable economical and social importance. Cultivars most frequently grown in the region are considered to be well adapted to drought. In a previous study (Bacelar et al. 2004), two cultivars originated in Trás-os-Montes reveal to possess different leaf-level mechanisms to cope with summer stress. Nevertheless, there are no studies documenting the physiological responses of these cultivars to drought conditions. The aims of this study were (1) to compare diurnal gas exchange rates (specifically, net CO2 assimilation rate, stomatal and mesophyll conductance, transpiration rate, ratio of intercellular to atmospheric CO2 concentration and intrinsic water-use efficiency) and chlorophyll fluorescence parameters (minimal and maximal fluorescence and maximum quantum yield of PSII) of five olive cultivars, (2) to investigate the quantitative variability of photosynthetic pigments, total soluble sugars, starch, soluble proteins and free proline in the foliage; and (3) to ascertain diurnal changes in stem water potential and leaf water relations determined from the pressure–volume measurements (osmotic potential at full turgor, osmotic potential at turgor loss point, maximum bulk modulus of elasticity and relative water content at turgor loss point).
Materials and methods
Study site
The experiment was conducted in a shallow schistic soil at Mirandela in Northeast Portugal (41°31′N and 7°12′W) at 250 m above sea level. The site has a Mediterranean climate with hot dry summers. Mean annual rainfall is 520.1 mm and minimal rainfall is usually recorded during the summer months, although some periods of drought can occur during winter (Table 1). The warmer months are July/August and the coldest are December/January, with average daily temperatures of 23.6/22.9°C and 6.3/6.1°C, respectively. During the study year (2001), rainfall was rare during the warmer months and plants were subjected to a combination of water deficit, high temperatures and high photosynthetic active photon flux density (PPFD). Measurements were performed under clear sky conditions on a representative day of summer (25 July 2001).
Plant material
We studied five cultivars of field-grown, unirrigated, 10-year-old, own-rooted olive trees. Cobrançosa and Negrinha are native to Trás-os-Montes (Northeast Portugal), Arbequina is the major cultivar in Cataluña (Northeast Spain), Blanqueta is of great importance in Valencia and Alicante (Southeast Spain), and Manzanilla in Extremadura (Centre Spain). Arbequina and Blanqueta are from regions with a Mediterranean climate, tempered by a maritime influence, and Manzanilla comes from the central interior of the Iberian Peninsula, with a climate similar to Trás-os-Montes.
Plant water relations
Stem water potential measurements were used to evaluate tree water status. Predawn (ΨPD) and midday (ΨMD) stem water potentials were measured on six sun-exposed shoots using a pressure chamber (PMS, Corvallis, OR), according to Scholander et al. (1965). Care was taken to minimise water loss during the transfer of the shoot to the chamber by enclosing it in a plastic bag immediately after excision (Turner and Long 1980).
The pressure chamber was also used to obtain pressure–volume curves (P–V) of current year mature leaves. The leaf samples were immediately put in tubes with the petioles sunken in distilled water and kept in darkness until reception at the laboratory. An analysis by water potentials isotherms through a progressive loss of symplasmic water was carried out. At periodic intervals, samples were weighted and water potential was evaluated immediately using a pressure chamber (internally covered with moist paper to reduce transpiration during measurement). Leaves were dried on a laboratory bench at constant temperature of 20°C and the drying period in each curve was about 6–8 h. P–V curves were drawn using a type II transformation (Tyree and Ritcher 1982) and allowed the deduction of the following parameters: osmotic potential at full turgor (ΨΠFT), osmotic potential at turgor loss point (ΨΠTLP), maximum bulk modulus of elasticity (εmax) and relative water content at turgor loss point (RWCTLP).
Gas exchange and chlorophyll fluorescence measurements
Leaf gas exchange measurements were performed using a portable IRGA (ADC-LCA-3, Analytical Development, Hoddesdon, U.K.), operating in the open mode on eight well exposed current year leaves during the morning (0830–0930 hours), midday (1330–1430 hours) and afternoon (1730–1830 hours). Table 2 indicates the mean values of PPFD, air temperature, vapour pressure deficit (VPD), and CO2 concentration during the three periods of gas exchange measurements. Net CO2 assimilation rate (A), stomatal conductance (g s), transpiration rate (E) and the ratio of intercellular to atmospheric CO2 concentration (C i/C a) were estimated from gas exchange measurements using the equations developed by von Caemmerer and Farquhar (1981). Intrinsic water-use efficiency (WUE) was calculated as the ratio of A/g s. Values for liquid phase diffusive conductance to CO2 (g m) were calculated in accordance with Izuta et al. (1996).
In vivo chlorophyll fluorescence was measured with a portable chlorophyll fluorometer (Plant Stress Meter, BioMonitor SCI AB, Umeå, Sweden) at predawn and midday on attached intact leaves similar to those used for gas exchange measurements. Prior to the measurements, a small part of the leaves was kept in the dark for 30 min using cuvettes for dark adaptation. A 5-s light pulse at 400 μmol m−2 s−1 was used. Following convention, we used F 0 to denote minimal fluorescence, which occurs when all PSII reaction centres are open. Maximal fluorescence, which occurs when all PSII reaction centres are closed, was denoted F m. The difference between F 0 and F m is variable fluorescence, F v. Maximum quantum yield of PSII was estimated by the F v /F m ratio (Krause and Weis 1991).
Photosynthetic pigments and metabolites assays
All metabolic compound analyses were made with leaf discs taken at morning (10.00 h) from six fully expanded leaves of comparable physiological age, thereby eliminating developmental effects. Leaf sections of a known area were ground in 80% acetone for chlorophyll and carotenoid determination. Total chlorophyll (Chla+b) and Chl a /Chl b ratio was determined according to Sesták et al. (1971) and total carotenoids (Car) according to Lichtenthaler (1987).
Total Soluble sugars (TSS) were extracted by heating leaf discs in 80% ethanol, according to Irigoyen et al. (1992). TSS were analysed by the reaction of 200 μl of the alcoholic extract with 3 ml of fresh anthrone and placed in a boiling water bath for 10 min. After cooling, the absorbance at 625 nm was determined. After the extraction of the soluble fractions, the solid fraction was used for starch analysis. Starch was extracted with 30% perchloric acid, according to Osaki et al. (1991). The starch concentration was determined by the anthrone method as described above. Glucose was used as a standard for both soluble sugars and starch.
The amount of soluble proteins (SP) was quantified using the method of Bradford (1976). Leaf discs were homogenised in a grinding medium that contained 50 mM phosphate buffer (pH 7.8), 0.1 mM EDTA, 100 μM PMSF and 2% PVP (w/v). Bovine serum albumin was used as a standard.
Proline was determined following the ninhydrin method as described by Bates et al. (1973). Briefly, fresh leaf tissue was extracted in 6 ml of 3% sulfosalicylic acid. After centrifugation at 5,000g for 20 min, 4 ml of the supernatant was added to 2 ml of a mixture of glacial acetic acid and ninhydrin reagent in a 1:1 (v:v) ratio. The reaction mixture was incubated in a water bath at 100°C for 1 h and then portioned against 3 ml of toluene. Absorbance was read in the organic phase at 520 nm. A standard curve was performed with proline. All reagents and chemicals used were of the highest grade of purity commercially available.
Statistics
All data were subjected to an analysis of variance with prior data transformation when required. Proportional data expressed as ratio data were log transformed. Significant different means were separated using the Fisher’s LSD test (P < 0.05).
Results
Plant water relations
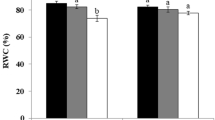
Stem water potential at predawn and midday indicate that olive trees were mildly water-stressed (Table 3). Arbequina and Blanqueta had significantly lower ΨPD than the other cultivars. Stem water potential decreased gradually during the morning reaching a minimum of −2.68 MPa at midday in Arbequina. Differences between ΨPD and ΨMD were higher in Cobrançosa, Manzanilla and Negrinha (above 200%) and lower in Blanqueta (130%). The analysis of P–V curves indicated that both ΨΠFT and ΨΠTLP were higher in Arbequina and Blanqueta (Table 3). In addition, these cultivars had higher RWCTLP and εmax. Cobrançosa had intermediate values of ΨΠFT and ΨΠTLP between that group of cultivars and the group of which included Manzanilla and Negrinha.
Gas exchange rates and chlorophyll fluorescence
Photosynthetic rate was affected by time of day and cultivar. Figure 1 depicts the diurnal changes of A in the five olive cultivars. The values of A followed a pattern characteristic of woody Mediterranean vegetation, with a maximum in the morning that declined towards midday, in a close association with increased evaporative demand and soil water deficits. In the morning, A was lowest in Blanqueta. Later on, at midday, Blanqueta and Arbequina had the lowest A, whereas Negrinha had the highest A. No recovery of A towards the afternoon was observed. On the contrary, Negrinha and Blanqueta had a tendency to drop A. As a consequence, the behaviour between cultivars was the same as in the morning period. The g s values followed a diurnal pattern very similar to those of A (Fig. 1). Nevertheless, g s was relatively more affected during the day than A, which means that the WUE increased from the morning towards the afternoon in all cultivars. In any case, it was evident that Blanqueta had the lowest variation (28%). Among the cultivars, Arbequina and Blanqueta had high WUE, whereas the group of cultivars including Cobrançosa, Manzanilla and Negrinha had low WUE values (Fig. 1). In general, the C i/C a decreased along the day (Fig. 1). However, we observed a much greater reduction in g s compared with C i/C a, from morning to midday. Moreover, Blanqueta had an almost constant C i/C a from midday to afternoon, despite the reduction of g s (Fig. 1).
Diurnal evolution of leaf net CO2 assimilation rate (A), stomatal conductance (g s), mesophyll conductance (g m), transpiration rate (E), ratio of intercellular to atmospheric CO2 concentration (C i/C a) and intrinsic water-use efficiency (WUE) in Arbequina (dark circle), Blanqueta (dark square), Cobrançosa (white circle), Manzanilla (dark triangle) and Negrinha (white square). Each point is the average of eight measurements and the vertical bars represent twice the standard error
The variation of E throughout the day was related to g s and mainly by VPD values. In all the cultivars, except in Arbequina, E increased at midday relatively to the morning period as a result of higher VPD, despite the lowest g s (Fig. 1). Arbequina had an opposite trend, in a more closely association with decreased g s. Meanwhile, E decreased from midday towards the afternoon, due to increased stomatal resistance and/or decreased atmospheric evaporative demand.
Daily recordings of chlorophyll fluorescence parameters (Table 4) showed that F v /F m decreased from predawn to midday in all cultivars, being paralleled by decreases of F m. A tendency to decreased F 0 was also observed, except in Manzanilla (Table 4). Across cultivars, Cobrançosa had the lowest F 0 and F m. The F v /F m values were not significantly different among the cultivars in both periods.
Photosynthetic pigments and metabolites in leaves
Significant differences in Chla+b and Car concentrations and in Chl a /Chl b ratio were observed among the olive cultivars (Table 5). Higher values of Chla+b and Car were observed in the leaves of Blanqueta, Cobrançosa and Manzanilla, but no differences were detected in Chla+b/Car ratio. Arbequina and Cobrançosa had higher Chl a /Chl b ratio and Blanqueta, the lowest.
There were also significant differences in TSS, starch, SP and proline concentrations among cultivars (Table 5). Negrinha had the lowest TSS concentration (25.7% less than Manzanilla), whereas the starch concentration was significantly higher in Cobrançosa. This cultivar had 230% more starch than Arbequina. Nevertheless, Arbequina leaves were richest in SP. Among the cultivars, Manzanilla had the highest proline concentration, whereas Blanqueta and Negrinha had low proline concentrations.
Discussion
The diurnal trends of A in the field-grown olive cultivars (Fig. 1) followed a typical pattern described for woody Mediterranean vegetation (Schulze and Hall 1982; Tenhunen et al. 1990; Fernández and Moreno 1999; Chaves et al. 2002; Ogaya and Peñuelas 2003), with a maximum in the morning and decline at midday. However, we observed that the degree of the midday depression in photosynthesis was genotype dependent, with a maximum in Arbequina and a minimum in Negrinha. The midday depression in photosynthesis is a common phenomenon in higher plants and is the result of a complex effect of many interacting internal and external factors (Xu and Shen 1996). The causes for this depression are still not fully understood and seem to involve mechanisms at both stomatal (Downton et al. 1988) and chloroplastic level (Correia et al. 1990). Our results suggest that the reductions in A in drought-stressed olive plants were dependent on both stomatal and non-stomatal limitations. Stomata closed partly in response to high air temperatures and water vapour pressure deficits, but these factors did not fully explain the closing response, as was observed previously by Faria et al. (1996) in Quercus suber. It is possible that an increased xylem sap ABA concentration (Davies et al. 2000; Liu et al. 2001) and circadian rhythms (Snaith and Mansfield 1986; Correia et al. 1995) may also be involved.
When stomata close in response to drought and CO2 assimilation is reduced, the photosynthetic reduction of O2 via photorespiration increases and serves as a sink for excess excitation energy in the photosynthetic apparatus (Cornic and Briantais 1991; Nogués and Baker 2000). Nevertheless, studies conducted with different species under a variety of conditions provide partly contradictory data on the role of photorespiration during drought stress (Wingler et al. 2000).
The much greater reduction in g s compared with C i/C a, from morning to midday (Fig. 1), indicates that non-stomatal factors may play an important role in limiting photosynthesis when olive cultivars are submitted to prolonged drought under field conditions. This was more evident in Blanqueta that had an almost constant C i/C a, from midday to afternoon, despite the reduction of g s (Fig. 1). Similar results were obtained by Giorio et al. (1999) in a study with field-grown olive trees under water deficit conditions. Possibilities include a higher mesophyll resistance (Fig. 1) and impaired metabolism (Lawlor 2002; Lawlor and Cornic 2002). According to Lawlor (2002), the metabolic limitation of A under drought conditions is primarily caused by decreased RuBP synthesis, probably by impaired ATP synthesis, and not by the inhibition or loss of PCR cycle enzymes, including Rubisco.
Photochemical factors could also be responsible for midday depression of A in olive cultivars. In fact, a decline in photochemical efficiency of PSII, given by F v/F m in dark-adapted leaves (Table 4), was parallel to midday depression of A. However, the F v/F m values were not significantly different among the cultivars in both periods of measurements and differences between values of F v/F m measured in predawn and midday were rather low (Table 4). According to that, the daily decrease of fluorescence parameters rather reflects the typical circadian rhythms than the drought-induced photoinhibition.
Among cultivars, Cobrançosa had the lowest F 0 and F m, namely at midday, indicating a highest absorption efficiency of photons by chlorophyll a in the light harvesting complex and of the reaction centre of PSII (Giorgieva and Yordanov 1993) and a higher non-radiative energy dissipation (Oberhuber and Bauer 1991).
Our study did not show any association between Chla+b concentration and photosynthetic activity/quantum yield in olive cultivars. This was an expected result, since for C3 species, the relationship between quantum yield and chlorophyll is relevant only when Chla+b concentration is below 4 mg dm−2 (Björkman 1981). Apparently, the lower Chla+b concentration of Arbequina and Negrinha (Table 5) was a good way to avoid excessive absorption of light energy, but it occurred in the absence of reduction in chlorophyll fluorescence yield (Table 4). As a consequence, there was a continued efficient use of light captured by chlorophyll in those cultivars. Furthermore, Blanqueta had the lowest Chl a /Chl b ratio (Table 5), which reflects the relative increase in the light harvesting chlorophyll a/b proteins at the expense of the chlorophyll a containing reaction centre complexes (Evans 1993). In addition, the low Chl a /Chl b ratio of Blanqueta is probably associated with a decline in cytochrome f content (Watanabe et al. 1994), which causes the reduction in electron transport capacity, and may also help to explain the low A of Blanqueta.
In general, g s was relatively more affected than A (Fig. 1), which means that WUE increased from the morning towards the afternoon in all cultivars. Nevertheless, olive cultivars have different water-use behaviours. Passioura (1982) pointed out that two types of water-use behaviour may be employed in woody plants. The prodigal water-use behaviour is beneficial in conditions, where water supply is interrupted for short periods only. In this situation, there is little danger of serious desiccation despite rapid water-use, and it enables a plant to grow quickly. The conservative water-use behaviour is beneficial in conditions, where a long, dry period prevails, enabling the plant to use the available water efficiently. According to this theory of plant water-use behaviour, the group of cultivars including Cobrançosa, Manzanilla and Negrinha, with high g s, high C i/C a and low WUE that is positively correlated with A, appears to employ a prodigal or non-conservative strategy, whereas Blanqueta and Arbequina, with high WUE, appear to employ a conservative strategy in the use of water. Nevertheless, our data showed that the reductions in g s for Arbequina could not prevent lower ΨMD values (Table 3). This result, combined with the fact that leaves are anatomically less protected against water loss (Bacelar et al. 2004), suggest that Arbequina have developed some mechanisms linked to drought tolerance. In fact, Arbequina leaves have high εmax (Table 3) and high SP concentration (Table 3). Moreover, we found evidence for the feedforward hypothesis for stomatal closure in Arbequina in response to air drought as proposed by Farquhar (1978), because there was evidence of decreasing E at high VPD (i.e. at midday), whereas the relationship between E and VPD of the other cultivars well abided by feedback effect (Monteith 1995).
We observed that Arbequina and Blanqueta had high εmax (Table 3). The high values of εmax (i.e. high tissue rigidity) of those cultivars were indicative of cell wall adjustment, reduced turgor loss volumes and tightening of the cell walls around the protoplasts, suggesting a cell size reduction (Lemcoff et al. 2002). Inelastic cell walls, although precluding turgor maintenance at low water content, do have several advantages over elastic cell walls (Patakas et al. 2002). In plants, in which there is osmotic adjustment, a rigid cell may be more effective at maintaining cell/tissue integrity on rehydration after a period of stress (Patakas et al. 2002). Rigid cells may also help maintain lower water potential at any given volume than do elastic ones (Patakas et al. 2002). This can result in an increase in the gradient in water potential between the soil and the plant, thereby promoting a more effective water uptake from drying soils and/or accelerating recovery after re-watering (Bowman and Roberts 1985). Conversely, the group of cultivars that employ a prodigal water-use strategy (Cobrançosa, Manzanilla and Negrinha), revealed low εmax (i.e. high tissue elasticity), what may reflect changes on cell wall composition (Munoz et al. 1993). More elastic cell walls can shrink more easily when subjected to stress, which helps maintain higher turgor pressure and protects cell walls from rupturing (Joly and Zaerr 1987). In fact, those cultivars tend to maintain turgor pressure at the expense of more water being lost at zero turgor (lower RWCTLP; Table 3). Thus, the low εmax of Cobrançosa, Manzanilla and Negrinha would probably reduce the fluctuation of both cell turgor and xylem pressure potential, and may have ecological significance by buffering the olive plants against short-term changes in water content (Fann et al. 1994). The parallelism between εmax and RWCTLP confirmed that the volumetric modulus of elasticity controlled RWCTLP values, and that elastic tissues require a more pronounced water deficit in order to lose turgor (Torrecillas et al. 1995). This drought tolerance mechanism observed in Cobrançosa, Manzanilla and Negrinha plants, permits a greater utilization of nutrients and assimilates for growth (Munns 1988), while turgor-mediated processes, such as elongative growth or photosynthesis, can be maintained (Bradford and Hsiao 1982).
The results revealed some differences in leaf osmotic potential among the cultivars (Table 3). Manzanilla and Negrinha had lower ΨΠFT and ΨΠTLP, suggesting a greater capability for osmotic adjustment. However, since those cultivars had low εmax, we believe that it was mostly a consequence from simple passive solute concentration resulting from dehydration (Morgan 1984). Some studies have already dealt with ΨΠ decrease in olive cultivars as a result of water deficit in the leaf tissue (Xiloyiannis et al. 1988; Dichio et al. 1997; Chartzoulakis et al. 1999; Bacelar et al. 2006). In our experiment, we indeed observed the accumulation of TSS and proline in the foliage (Table 5). However, osmotic adjustment was probably accomplished mainly by accumulation of a wide range of other metabolites and inorganic ions. In fact, the compounds involved in osmotic adjustment differ widely among plant species (Patakas et al. 2002). Gucci et al. (1997) reported that osmotic adjustment in olive leaves under salt stress was accomplished primarily by accumulation of inorganic ions, despite the osmotic contribution of soluble carbohydrates.
Although it is highest A, Negrinha had the lowest TSS concentration (25.7% less than Manzanilla). As highlighted by Chaves (1991), it is difficult to establish a clear relationship between the sugar content of the leaves and the photosynthetic activity, which may be partly explained by the complex compartmentation of sugars in the leaf.
On the other hand, the starch concentration was significantly lower in Arbequina (Table 5), probably related with a high export rate of photosynthates to sink organs and/or lower A imposed by drought (Souza et al. 2004).
We observed that in all cultivars, especially in Manzanilla, free proline accumulates in the foliage for further osmotic adjustment (Table 5). Proline within the cell can act as an osmolyte with compatibility for enzymes and other cell macromolecules, therefore protecting them from drought stress induced damage (Hare et al. 1998). Osmotic adjustment produced by proline accumulation causes a drop of the osmotic potential in plant tissues (Hare and Cress 1997). Lower osmotic potentials allow leaves to withstand a greater evaporative demand without loss of turgor. Moreover, proline has a protective action which prevents membrane damage and protein denaturation during severe drought stress (Hare et al. 1998; Ain-Lhout et al. 2001). It has also been proposed that proline can act as an electron acceptor, avoiding damage of photosystems due to their photoinibition by activated oxygen species (Hare et al. 1998). Accumulation of proline, which is a common metabolic response to water deficit, salinity and cold stress in many higher plants (Delauney and Verma 1993), may also facilitate the continued synthesis of nitrogenous compatible solutes using excess photochemical energy available when stomata are closed (Smirnoff et al. 1985). This process seems to be species related. In fact, proline accumulation in two Mediterranean shrubs (Halimium halimifolium L. and Pistacia lentiscus L.) during increasing water deficit was twice the amount found in olive tree (Ain-Lhout et al. 2001). Despite its known role in osmotic adjustment, proline has been considered, in some studies, a symptom of injury (Irigoyen et al. 1992), probably resulting from an excessive protein breakdown during water deficits (Levitt 1980). Sofo et al. (2004) reported that in olive trees, the proline content increases in relation to the severity of stress, particularly in leaves and medium roots. In this study, we observed low levels of proline, so it may rather be a consequence of moderate water stress conditions and not an induced beneficial response. In fact, as we observed, olive cultivars have other mechanisms of drought resistance such as stomata closure and elastic adjustment.
Arbequina leaves exhibited higher levels of SP (Table 5). Changes of soluble protein contents are important to understand the impact of stress on cell proteolysis and protein synthesis (Santos and Caldeira 1999). During drought periods, plants undergo many physiological changes and induce a large number of genes for adaptation (Ingram and Bartels 1996). Under water deficit conditions, a typical change in gene expression is the induction of genes involved in the synthesis of various osmolytes and low-molecular-weight proteins, e.g. dehydrins and late embryogenic-abundant proteins (Ingram and Bartels 1996). Moreover, the increase of SP in Arbequina may represent increased activity of oxidative stress defense enzymes. In fact, under mild water deficit, an increase in activities of superoxide dismutase, glutathione reductase and catalase has been reported (Baisak et al. 1994). The accumulation of leaf proteins under water deficit may also represent a reserve for post stress recovery and with probable implications in stress tolerance (Millard 1988).
Cobrançosa, Manzanilla and Negrinha seem to be well acclimated to the region, with high A along the day, and appear to use a prodigal water-use strategy, whereas Blanqueta and Arbequina appear to employ a conservative water-use strategy. Elastic adjustment plays an important role as drought tolerance mechanism. The group of cultivars that employ a prodigal water-use strategy revealed high tissue elasticity, whereas Arbequina and Blanqueta revealed high tissue rigidity. The high tissue elasticity may help Cobrançosa, Manzanilla and Negrinha plants to maintain elongative growth and photosynthesis under moderate water stress conditions. We also identified the existence of drought tolerance mechanisms of O. europaea plants, associated with SP accumulation in the foliage. The high levels of SP in Arbequina may represent an increased activity of oxidative stress defence enzymes and may also represent a reserve for post stress recovery.
In conclusion, the results of this study reveal that olive cultivars, native to dry regions, such as Cobrançosa, Manzanilla and Negrinha, have more capability to acclimate to drought conditions than cultivars originated in regions with a more temperate climate, like Arbequina and Blanqueta.
References
Abd-El-Rahman AA, Shalaby AF, Balegh M (1966) Water economy of olive under desert conditions. Flora 156:202–219
Abrams MD (1990) Adaptations and responses to drought in Quercus species of North America. Tree Physiol 7:227–238
Ain-Lhout F, Zunzunegui M, Diaz Barradas MC, Tirado R, Clavijo A, Gracia Novo F (2001) Comparison of proline accumulation in two Mediterranean shrubs subjected to natural and experimental water deficit. Plant Soil 230:175–180
Angelopoulos K, Dichio B, Xiloyannis C (1996) Inhibition of photosynthesis in olive trees (Olea europaea L.) during water stress and rewatering. J Exp Bot 47:1093–1100
Bacelar EA, Correia CM, Moutinho-Pereira JM, Gonçalves BC, Lopes JI, Torres-Pereira JM (2004) Sclerophylly and leaf anatomical traits of five field-grown olive cultivars growing under drought conditions. Tree Physiol 24:233–239
Bacelar EA, Santos DL, Moutinho-Pereira JM, Gonçalves BC, Ferreira HF, Correia CM (2006) Immediate responses and adaptative strategies of three olive cultivars under contrasting water availability regimes: changes on structure and chemical composition of foliage and oxidative damage. Plant Sci 170:596–605
Bacelar EA, Santos DL, Moutinho-Pereira JM, Lopes JI, Gonçalves BC, Ferreita TC, Correia CM (2007) Physiological behaviour, oxidative damage and antioxidative protection of olive trees grown under different irrigation regimes. Plant Soil 292:1–12
Baisak R, Rana D, Acharya PBB, Kar M (1994) Alterations in the activities of active oxygen scavenging enzymes of wheat leaves subjected to water stress. Plant Cell Physiol 35:489–495
Bates LS, Waldren RP, Tear ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 156:205–207
Björkman O (1981) Responses to different quantum flux densities. In: Lange OL, Nobel PS, Osmond CB, Ziegler H (eds) Physiological plant ecology II. Encyclopedia of plant physiology, vol 12A. Springer, Berlin, pp 57–108
Bongi G, Mencuccini M, Fontanazza G (1987) Photosynthesis of olive leaves: effect of light, flux density, leaf age, temperature, peltates, and H2O vapour pressure deficit on gas exchange. J Am Soc Hortic Sci 112:143–148
Bowman WD, Roberts SW (1985) Seasonal changes in tissue elasticity in chaparral shrubs. Physiol Plant 65:233–236
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilising the principle of protein-dye binding. Anal Biochem 72:248–254
Bradford KJ, Hsiao TC (1982) Physiological responses to moderate water stress. In: Lange OL, Nobel PS, Osmond CB, Ziegler H (eds) Physiological plant ecology II. Encyclopedia of plant physiology, vol 12B. Springer, Berlin, pp 263–324
Chartzoulakis K, Patakas A, Bosabalidis AM (1999) Changes in water relations, photosynthesis and leaf anatomy induced by intermittent drought in two olive cultivars. Environ Exp Bot 42:113–120
Chaves MM (1991) Effects of water deficits on carbon assimilation. J Exp Bot 42:1–16
Chaves MM, Pereira JS, Maroco J, Rodrigues ML, Ricardo CPP, Osório ML, Carvalho I, Faria T, Pinheiro C (2002) How plants cope with water stress in the field? Photosynthesis and growth. Ann Bot 89:907–916
Cornic G, Briantais JM (1991) Partitioning of photosynthetic electron flow between CO2 and O2 reduction in a C3 leaf (Phaseolus vulgaris L.) at different CO2 concentrations and during drought stress. Planta 183:178–184
Cornic G, Massacci A (1996) Leaf photosynthesis under drought stress. In: Baker NR (ed) Photosynthesis and the environment. Kluwer, Dordrecht, pp 347–366
Correia MJ, Chaves MM, Pereira JS (1990) Afternoon depression in photosynthesis in grapevine leaves—evidence for a high light stress effect. J Exp Bot 41:417–426
Correia MJ, Pereira JS, Chaves MM, Rodrigues ML, Pacheco CA (1995) ABA xylem concentrations determine maximum daily leaf conductance of field-grown Vitis vinifera L. plants. Plant Cell Environ 18:511–521
Davies WJ, Bacon MA, Thompson DS, Sobeih W, Rodríguez LG (2000) Regulation of leaf and fruit growth in plants growing in drying soil: exploitation of the plants chemical signalling system and hydraulic architecture to increase the efficiency of water use in agriculture. J Exp Bot 51:1617–1626
Delauney AJ, Verma DPS (1993) Proline biosynthesis and osmoregulation in plants. Plant J 4:215–223
Demmig-Adams B, Adams WW (1996) The role of xanthophylls cycle carotenoids in the protection of photosynthesis. Trends Plant Sci 1:21–26
Dichio B, Nuzzo V, Xiloyannis C, Celano G, Angelopoulos K (1997) Drought stress-induced variation of pressure–volume relationships in Olea europaea L. cv “Coratina”. Acta Hortic 449:401–409
Downton WJS, Loveys BR, Grant WJR (1988) Non-uniform stomatal closure induced by water stress causes purative non-stomatal inhibition of photosynthesis. New Phytol 110:503–509
Evans JR (1993) Photosynthetic acclimation and nitrogen partitioning within a lucerne canopy. I. Canopy characteristics. Aust J Plant Physiol 20:55–67
Fann S, Blake TJ, Blumwald E (1994) The relative contribution of elastic and osmotic adjustments to turgor maintenance in woody species. Physiol Plant 90:408–413
Faria T, García-Plazaola JI, Abadía A, Cerasoli S, Pereira JS, Chaves MM (1996) Diurnal changes in photoprotective mechanisms in leaves of cork oak (Quercus suber L.) during summer. Tree Physiol 16:115–123
Farquhar GD (1978) Feedforward responses of stomata to humidity. Aust J Plant Physiol 5:787–800
Fernández JE, Moreno F (1999) Water use by the olive tree. In: Kirkham MB (ed) Water use in crop production. The Haworth Press, Binghamton, pp 101–162
Fernández JE, Moreno F, Cabrera F, Arrue JL, Martín-Aranda J (1991) Drip irrigation, soil characteristics and the root distribution and root activity of olive trees. Plant Soil 133:239–251
Giorgieva K, Yordanov I (1993) Temperature dependence of chlorophyll fluorescence parameters of pea seedlings. J Plant Physiol 142:151–155
Giorio P, Sorrentino G, d’Andria R (1999) Stomatal behaviour, leaf water status and photosynthetic response in field-grown olive trees under water deficit. Environ Exp Bot 42:95–104
Gucci R, Lombardini L, Tattini M (1997) Analysis of leaf water relations in leaves of two olive (Olea europaea) cultivars differing in tolerance to salinity. Tree Physiol 17:13–21
Hare D, Cress WA (1997) Metabolic implications of stress-induced proline accumulation in plants. Plant Growth Regul 21:79–102
Hare PD, Cress WA, Van Staden J (1998) Dissecting the roles of osmolyte accumulation during stress. Plant Cell Environ 21:535–553
Ingram J, Bartels D (1996) The molecular basis of dehydration tolerance in plants. Annu Rev Plant Physiol Plant Mol Biol 47:377–403
Irigoyen JJ, Emerich DW, Sánchez-Díaz M (1992) Water stress induced changes in concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago sativa) plants. Physiol Plant 84:55–60
Izuta T, Umemoto M, Horie K, Aoki M, Totsuka T (1996) Effects of ambient levels of ozone on growth, gas exchange rates and chlorophyll contents of Fagus crenata seedlings. J Jpn Soc Atmos Environ 31:95–105
Joly RJ, Zaerr JB (1987) Alteration of cell-wall water content and elasticity in Douglas-fir during periods of water deficit. Plant Physiol 83:418–422
Krause GH, Weis E (1991) Chlorophyll fluorescence and photosynthesis: the basics. Plant Physiol 42:313–349
Lawlor DW (2002) Limitation to photosynthesis in water-stressed leaves: stomata vs. metabolism and the role of ATP. Ann Bot 89:871–885
Lawlor DW, Cornic G (2002) Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant Cell Environ 25:275–294
Lemcoff JH, Guarnaschelli AB, Garau AM, Prystupa P (2002) Elastic and osmotic adjustments in rooted cuttings of several clones of Eucalyptus camaldulensis Dehnh. from southeastern Australia after a drought. Flora 197:134–142
Levitt J (1980) Responses of plants to environmental stresses. II. Water, radiation, salt and other stresses. Academic Press, New York, p 657
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148:350–382
Liu L, McDonald AJS, Stadenberg I, Davies WJ (2001) Stomatal and leaf growth response to partial drying of root tips in willow. Tree Physiol 21:765–770
Millard P (1988) The accumulation and storage of nitrogen by herbaceous plants. Plant Cell Environ 11:1–8
Monteith JL (1995) A reinterpretation of stomatal responses to humidity. Plant Cell Environ 18:357–363
Morgan JM (1984) Osmoregulation and water stress in higher plants. Annu Rev Plant Physiol 35:299–319
Munns R (1988) Why measure osmotic adjustment? Aust J Plant Physiol 15:717–726
Munoz FJ, Dopico B, Labrador E (1993) Effect of osmotic stress on the growth of epicotyls of Cicer arietinum in relation to changes in cell wall composition. Physiol Plant 87:552–560
Niinemets Ü (2001) Global-scale climatic controls of leaf dry mass per area, density, and thickness in trees and shrubs. Ecology 82:453–469
Nogués S, Baker NR (2000) Effects of drought on photosynthesis in Mediterranean plants grown under enhanced UV-B radiation. J Exp Bot 51:1309–1317
Oberhuber W, Bauer H (1991) Photoinhibition of photosynthesis under natural conditions in ivy (Hedera helix L.) growing in an understory of deciduous trees. Planta 185:545–553
Ogaya R, Peñuelas J (2003) Comparative seasonal gas exchange and chlorophyll fluorescence of two dominant woody species in a Holm Oak forest. Flora 198:132–141
Osaki M, Shinano T, Tadano T (1991) Redistribution of carbon and nitrogen compounds from the shoot to the harvesting organs during maturation in field crops. Soil Sci Plant Nutr 37:117–128
Passioura JB (1982) Water in the soil-plant-atmosphere continuum. In: Lange OL, Nobel PS, Osmond CB, Ziegler H (eds) Physiological plant ecology II. Encyclopedia of plant physiology, vol 12B. Springer, Berlin, pp 5–33
Patakas A, Noitsakis B (1997) Cell wall elasticity as a mechanism to maintain favorable water relations during leaf ontogeny in grapevines. Am J Enol Vitic 48:352–358
Patakas A, Nikolaou N, Zioziou E, Radoglou K, Noitsakis B (2002) The role of organic solute and ion accumulation in osmotic adjustment in drought-stressed grapevines. Plant Sci 163:361–367
Santos CV, Caldeira G (1999) Comparative responses of Helianthus annuus plants and calli exposed to NaCl. I. Growth rate and osmotic adjustment in intact plants and calli. J Plant Physiol 155:769–777
Scholander PF, Hammel HT, Bradstreet ED, Hemmingsen EA (1965) Sap pressure in vascular plants. Science 148:339–346
Schulze E-D, Hall AE (1982) Stomatal responses, water loss and CO2 assimilation rates of plants in contrasting environments. In: Lange OL, Nobel PS, Osmond CB, Ziegler H (eds) Physiological plant ecology II. Encyclopedia of plant physiology, vol 12B. Springer, Berlin, pp 181–230
Sesták Z, Castky J, Jarvis PG (1971) Plant photosynthetic production. Manual of methods. Dr. W. Junk Publishers, The Hagge, p 818
Smirnoff N, Winslow MD, Stewart GR (1985) Nitrate reductase activity in leaves of barley (Hourdeum vulgare) and durum wheat (Triticum durum) during field and rapidly applied water deficits. J Exp Bot 36:1200–1208
Snaith PJ, Mansfield TA (1986) The circadian rhythm of stomatal opening: Evidence for the involvement of potassium and chloride fluxes. J Exp Bot 37:188–199
Sofo A, Dichio B, Xiloyannis C, Masia A (2004) Effects of different irradiance levels on some antioxidant enzymes and on malondialdehyde content during rewatering in olive tree. Plant Sci 166:293–302
Souza RP, Machado EC, Silva JAB, Lagôa AMMA, Silveira JAG (2004) Photosynthetic gas exchange, chlorophyll fluorescence and some associated metabolic changes in cowpea (Vigna unguiculata) during water stress and recovery. Environ Exp Bot 51:45–56
Tenhunen JD, Sala Serra A, Harley PC, Dougherty RL, Reynolds JF (1990) Factors influencing carbon fixation and water use by Mediterranean sclerophyll shrubs during summer drought. Oecologia 82:381–393
Torrecillas A, Guillaume C, Alarcón JJ, Ruiz-Sánchez MC (1995) Water relations of two tomato species under water stress and recovery. Plant Sci 105:169–176
Turner NC, Long MJ (1980) Errors arising from rapid water loss in the measurement of leaf water potential by the pressure chamber technique. Aust J Plant Physiol 7:527–537
Tyree MT, Jarvis PJ (1982) Water in tissues and cells. In: Lange OL, Nobel PS, Osmond CB, Ziegler H (eds) Physiological plant ecology II. Encyclopedia of plant physiology, vol 12B. Springer, Berlin, pp 35–77
Tyree MT, Ritcher H (1982) Alternative methods of analysing water potential isotherms: some cautions and clarifications. II. Curvilineality in water potential isotherms. Can J Bot 60:911–916
von Caemmerer S, Farquhar GD (1981) Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 153:376–387
Watanabe N, Evans JR, Chow WS (1994) Changes in the photosynthetic properties of Australian wheat cultivars over the last century. Aust J Plant Physiol 21:169–183
Wingler A, Lea PJ, Quick WP, Leegood RC (2000) Photorespiration: metabolic pathways and their role in stress protection. Philos Trans R Soc Lond B Biol Sci 355:1517–1529
Xiloyiannis C, Pezzarosa B, Jorba J, Angelini P (1988) Effects of soil water content on gas exchange in olive trees. Adv Hortic Sci 2:58–63
Xu D-Q, Shen Y-K (1996) Midday depression of photosynthesis. In: Pessarakli M (ed) Handbook of photosynthsis. Marcel Dekker, New York, pp 451–459
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by W. Filek.
Rights and permissions
About this article
Cite this article
Bacelar, E.A., Moutinho-Pereira, J.M., Gonçalves, B.C. et al. Physiological responses of different olive genotypes to drought conditions. Acta Physiol Plant 31, 611–621 (2009). https://doi.org/10.1007/s11738-009-0272-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-009-0272-9