Abstract
A variety of ecophysiological parameters were monitored in leaves of Hevea brasiliensis (rubber tree) during seasonal leaf senescence. Higher levels of hydrogen peroxide and malondialdehyde, and lower content of total protein and efficiency of photochemistry of photosystem II (PSII) were observed in the senescent leaves (SL) compared to the mature leaves (ML). A significant decrease in the contents of chlorophyll (Chl) and carotenoids (Car) in SL was also observed, but with increase in ratio of Car/Chl. Moreover, activities of superoxide dismutases, catalase, and glutathione reductase in SL were strongly suppressed. In contrast, the activities of guaiacol peroxidase (POD) and ascorbate peroxidase (APX), and the contents of reduced ascorbate, total ascorbate, reduced glutathione and total glutathione were considerably increased in SL compared to ML. In addition, α-pinene, β-pinene, sabinene and total monoterpene pool in SL were drastically decreased. Taken together, these results indicate that the enhanced activities of POD and APX, and further activation of ascorbate-glutathione cycle conferred an important photoprotection against oxidative stress in senescent leaves of rubber trees. The increased Car/Chl could give the protection against photoxidation as well.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The senescing process of leaves is characterized with dramatic exacerbation in lipid peroxidation, degradation of chlorophyll (Chl), proteins and other macromolecules, conversion of peroxisomes into gloxysomes, and a marked increase in production of reactive oxygen species (ROS; Gan and Amasino 1997; Pastori and del Río 1997; Corpas et al. 2001; Prochάzkovά et al. 2001; Buchanan-Wollaston 1997). Leaf senescence can be, therefore, regarded as a process of oxidative stress due to the overproduction of ROS, such as hydrogen peroxide (H2O2), superoxide radicals (O2˙¯), hydroxyl radicals (˙OH) and singlet oxygen (1O2; Piquery et al. 2000; Scebba et al. 2004). To counteract the injurious effects of ROS, plants are equipped with antioxidative systems composed of enzymes such as superoxide dismutase (SOD), guaiacol peroxidase (POD) and catalase (CAT) as well as metabolites such as ascorbate, glutathione, tocopherol, and carotenoids (Alscher et al. 1997; Noctor and Foyer 1998). Some previous studies have reported both the decrease (Dhindsa et al. 1981; Hurng and Kao 1994) and increase (Bueno and del Rio 1992; Prochάzkovά et al. 2001) in the activities of various antioxidative enzymes during leaf senescence. On the other hand, a substantial decrease in all the components of the mitochondrial ascorbate-glutathione cycle has also been observed in senescing leaves of pea (Pastori and del Río 1997).
Although the physiological functions of volatile isoprenoids have not yet clearly been established, it has been shown that leaves producing isoprene and specific monoterpene (e.g. α- and β-pinene) withstand higher oxidative stress than those in which isoprene or monoterpene production is inhibited (Sharkey and Singsaas 1995; Peňuelas and Munné-Bosch 2005; Sharkey and Yeh 2001; Loreto et al. 2004). Therefore, volatile isoprenoids have been suggested to be involved in scavenging ROS and potentially protecting plants against photo-oxidative stress (Zeidler et al. 1997; Loreto et al. 2001; Loreto and Velikova 2001). Correspondingly, non-volatile isoprenoids, mostly carotenoids, are believed to confer photoprotection during dismounting of photosynthetic machinery in senescing leaves (Merzlyak and Solovchenko 2002). The volatile isoprenoids productions are exclusively dependent on photosynthesis (Sharkey and Yeh 2001) and share a common biochemical production pathway and localization with nonvolatile isoprenoids (carotenoids; Logan et al. 2002; Affeck and Yakir 2002). However, little information is available on the actual roles of volatile isoprenoids in scavenging ROS and protecting photosynthesis in relation to progression of leaf senescence.
It has been recently found that the leaves of Hevea brasiliensis (rubber tree) produce and emit monoterpene (Klinger et al. 2002). The rubber tree is widely cultivated in tropical regions for economical purpose, including the marginal tropical areas of China. However, it is an evergreen tree in its native habitats, but deciduous in the marginal tropical areas of China, such as in Xishuangbanna of southern Yunnan where the daily temperature in winter is about 6–8°C lower than in the summer. The change in leaf-habit of the rubber tree in these marginal tropical areas might be caused by chilly temperature. During this chilly period, although no visible injury to leaves of rubber trees has been observed, the low temperature could have adversely affected their physiology and hence ultimately result in senescence and defoliation.
The aim of the present study was to clarify the relationship between oxidative stress and leaf senescence, and antioxidative activities during leaf senescence in rubber trees in a marginal tropical area of China. For this purpose, the components of antioxidative metabolites of ascorbate, glutathione, nonvolatile isoprenoids (carotenoids) and volatile isoprenoids (monoterpenes), and the activities of antioxidative enzymes: SOD, POD, and CAT, as well as stress makers such as the contents of pigments, protein and malondialdehyde (MDA), and the photochemical efficiency of PSII were measured in leaves of rubber trees in relation to the progress of leaf senescence in response to chilly season.
Materials and methods
Study site and leaf sampling
This study was conducted in a rubber tree plantation stand at the Xishuangbanna Tropical Botanical Garden (21°41′N, 101°25′E, and 570 masl), Chinese Academy of Sciences, SW China. Mean annual air temperature is about 21.7°C and annual precipitation is about 1,560 mm. There is a rainy season from May to October, and a well-defined dry season from November to April.
Rubber tree is an evergreen tree in its native habitats but deciduous in the present region. Its old leaves defoliate in early March and the new leaves emerge about 2 weeks later. Uppermost canopy leaves of rubber trees by an iron tower within the rubber-tree plantation were sampled on clear midday on 10–11 November and 15–16 December 2005, 11–12 January, 7–8 February and 19–20 February 2006, respectively. The leaf samples were immediately submerged in liquid nitrogen and then taken to laboratory for assays.
Chl fluorescence
The midday maximum (F v/F m) and actual photochemical efficiency [ΔF/F m′ = (F m′–F t)/F m′] of photosystem II (PSII) were detected from the canopy leaves of the rubber trees under natural light with a portable fluorescence system (FMS-2.02, Hansatech, King’s Lynn, U.K.). F v/F m was measured from the leaves after 20 min dark adaptation. There are not significant differences in maximum photosynthetic photo flux density values during study period (data not shown), thus, it is possible to compare changes in ΔF/F m′ during leaf senescence.
Determination of contents of photosynthetic pigments, protein and H2O2
Contents of chlorophyll (Chl) and carotenoids (Car) of the leaves were measured according to Lichtenthaler and Wellburn (1983). Total content of foliar protein was measured according to Lowry et al. (1951) using bovine serum albumin as a standard. The level of H2O2 was determined according to Velikova et al. (2000).
Determination of MDA content
The content of MDA was analyzed by the method described by Hodges et al. (1999) with slight modification, for taking into account the possible influence of interfering compounds in the assay for thiobarbituric acid (TBA)-reactive substances. Leaf tissues were repeatedly extracted with 4 ml 5% (w/v) trichloroacetic acid (TCA). The homogenate was centrifuged at 15,000×g for 15 min and an aliquot of appropriately diluted sample was added to a test tube with an equal volume of either: (1) −TBA solution containing 20% (w/v) TCA and 0.01% butylated hydroxytoluene (BHT); or (2) +TBA solution containing the above solution plus 0.65% (w/v) TBA. Samples were heated at 95°C for 25 min, then after cooling, the absorbance was read at 440, 532 and 600 nm. MDA equivalents were calculated as 106 × ((A – B)/157,000), where A = [(Abs 532+TBA) – (Abs 600+TBA) – (Abs 532−TBA – Abs 600−TBA)], and B = [(Abs 440+TBA – Abs 600+TBA) × 0.0571].
Analysis of enzyme activity
Leaf fresh material was homogenized with a mortar and pestle in 4 ml ice-cold 0.2 M phosphate buffer (pH 7.8) containing 0.1 mM EDTA, 0.3% (w/v) Triton X-100, 1% (w/v) PVP, and 10 mM dithiothreitol. The homogenate was centrifuged at 15,000×g for 15 min and the supernatant was used for assays of enzyme activity and protein content. All the assay steps were carried out at 0 ± 4°C. CAT (EC 1.11.1.6) activity was measured in the presence of 10 mM H2O2 by monitoring the decrease in absorbance at 240 nm in phosphate buffer (pH 7.0), and expressed as ΔA240 min−1 mg−1 protein. APX (EC 1.11.1.11) activity was measured in the presence of 0.5 mM ascorbic acid and 1.0 mM H2O2 by monitoring the decrease in absorbance at 290 nm in phosphate buffer (pH 7.0), and expressed as ΔA290 min−1 mg−1 protein. GR (EC 1.6.4.2) activity was measured in the presence of 0.5 mM GSSG and 0.15 mM NADPH by monitoring the decrease in absorbance at 340 nm in phosphate buffer (pH 7.0), and expressed as ΔA340 min−1 mg−1 protein. POD (EC 1.11.1.7) activity was measured in the presence of 16 mM guaiacol and 10 mM H2O2 by monitoring the increase in absorbance at 470 nm in phosphate buffer (pH 7.0), and expressed as ΔA470 min−1 mg−1 protein. SOD (EC 1.15.1.1) activity was measured by the photochemical method as described by Giannopolitis and Ries (1977), and one unit of SOD activity was defined as the amount of enzyme which produced a 50% inhibition of nitroblue tetrazolium reduction at 560 nm.
Determination of antioxidative metabolites
Fresh leaf material was homogenized in an ice bath with 4 ml 5% (w/v) TCA. The homogenate was centrifuged at 15,000×g for 15 min and the supernatant was used for assays of contents of ascorbate and glutathione. The content of reduced ascorbate (AsA) was analyzed according to the methods described by Arakawa et al. (1981), which were based on the reduction of ferric ion to ferrous ion with AsA in acid solution, followed by formation of the red chelate between ferrous ion and bathophenanthroline, which absorb at 534 nm. After the reduction of oxidized ascorbate (DHA) to AsA by dithiothreitol, the total AsA content was measured as described above, DHA content was determined by subtraction of AsA from the total AsA content. The contents of reduced and oxidized forms of glutathione were measured by the method described by Doulis et al. (1997) via the increase in absorbance at 412 nm following addition of GR for determination of reduced glutathione (GSH) or GR and NADPH for determination of oxidized glutathione (GSSG) to a solution containing extract and 5,5′-Dithiobis (2-nitrobenzoic acid).
Analysis of monoterpene content
Leaf fresh material was submerged in liquid nitrogen and then the sample was homogenized in ice-cold pentane under liquid nitrogen. A non-terpenoid volatile internal standard, dodecane was used to avoid interference of terpenes. It was added to the pentane extraction procedure before grinding in order to quantify the recovery. Detailed assays of monoterpene concentration were conducted as described by Llusià and Peňuelas (2000).
Statistical analysis
Statistical analysis was performed with software SPSS (Chicago, IL, USA) using one-way ANOVAs (LSD method) to evaluate differences in assayed parameters among different months.
Results
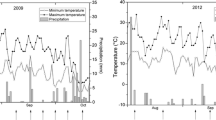
During the study period from August 2005 to February 2006, the mean maximum monthly temperature (MAT) remained relatively stable from August to October, and then reached lowest value of 23.9°C in December (Fig. 1). Mean monthly temperature (MMT) was continuously decreased from August to December, and reached lowest value of 16.7°C in December. Thereafter, a moderate increase in MAT and a slight increase in MMT were observed. However, mean minimum monthly temperature attained lowest value of 13.1°C in January, then with a slight increase in February. The monthly rainfall remained relatively stable from September to December, and then reached lowest value of 4 mm. However, the monthly rainfall was increased to 16.6 mm in February.
The monthly rainfall (bars), mean maximum monthly temperature (open circle), mean monthly temperature (filled circle) and mean minimum monthly temperature (filled triangle) during the experimental period from August 2005 to February 2006, recorded by the Menglun Meteorological Station of Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences
The contents of photosynthetic pigments varied slightly in the leaves of the rubber trees from November 2005 to January 2006, thereafter, about 80% decrease in Chl (P < 0.001), 35–50% decrease in Car (P < 0.05), and 18% decrease in Chla/Chlb (P < 0.05) as well as threefold increase in Car/Chl (P < 0.001) were observed in the leaves sampled on 19–20 February 2006 relative to in leaves from November 2005 to January 2006, respectively (Fig. 2). Similarly, the content of total protein increased from November 2005 to January 2006, afterwards sharply decreased, reaching the values approximately 60–70% lower in February 2006 than those from November 2005 to January 2006 (P < 0.001; Fig. 3a). Based on loss of photosynthetic pigments and total proteins, the following stages of leaf development of the present rubber trees were defined: mature leaves (ML; November 2005 until January 2006), and senescent leaves (SL; 19–20 February 2006).
The contents of H2O2 and MDA in the leaves of rubber trees were increased as leaf senescence progressed. H2O2 content of SL was about 20% higher than that of ML (P < 0.05; Fig. 3b), and approximately 2–5-folds increase in MDA content was observed in SL (P < 0.001; Fig. 3c). On the other hand, maximum photochemical efficiency of PSII (F v/F m) and actual photochemical efficiency of PSII (ΔF/F m′) at midday were decreased by about 20 and 30% in SL versus ML (P < 0.001), respectively (Fig. 4). In addition, the content of MDA was correlated negatively with the content of total protein (r 2 = 0.48, P < 0.001, Fig. 5a) and F v/F m (r 2 = 0.47, P < 0.001, Fig. 5b). Conversely, F v/F m was related positively with total protein (r 2 = 0.62, P < 0.001; Fig. 5c).
Activities of SOD, CAT, and GR were considerably lower in SL than in ML, decreasing by about 71, 54, 58% (P < 0.001), respectively (Fig. 6a–c). In contrast, activities of POD and APX were increased by 60–96% and 1.41–1.96-fold in SL (P < 0.001), respectively (Fig. 6d, e). The content of reduced ascorbate was increased by 43–69% (P < 0.01), and the content of total ascorbate was increased by 32–71% (P < 0.05) in SL when compared to ML (Fig. 7a). DHA content and ratio of AsA to total ascorbate (DHA + AsA) were increased slightly during the study periods (data not shown). On the other hand, the contents of reduced glutathione and total glutathione were increased by 7.2–35.3% and 10–22% in SL, respectively (Fig. 7b). However, there were no considerable differences in content of GSSG and ratio of GSH to total glutathione (GSH + GSSG) between SL and ML (data not shown). In addition, there were decreases of about 35% in ratio of H2O2 to AsA in SL versus ML (P < 0.05; Fig. 7c).
The changes in activities of antioxidative enzymes of superoxide dismutases (SOD, a), catalase (CAT, b), glutathione reductase (GR, c), guaiacol peroxidase (POD, d), and ascorbate peroxidase (APX, e) in leaves of Hevea brasiliensis trees from November 2005 to February 2006. Data are the mean ± SE (n = 6)
The contents of reduced ascorbate (AsA, filled circle), total ascorbate (filled triangle) (a), reduced glutathione (GSH, open circle) and total glutathione (open triangle) (b) as well as hydrogen peroxide/reduced ascorbate (H2O2/AsA, c) in leaves of Hevea brasiliensis trees from November 2005 to February 2006. Data are the mean ± SE (n = 6)
The contents of volatile isoprenoids were drastically decreased during leaf senescence, approximately 98% decrease of α-pinene (P < 0.001), 94% decrease of β-pinene (P < 0.001), and 80% decrease of sabinene (P < 0.001) as well as 94% decrease of total monoterpene pool (P < 0.001) were observed in SL versus ML (Fig. 8). These results suggested that leaf senescence strongly affected the biosynthesis and accumulation of volatile isoprenoids.
Discussion
In the present experimental period, although the monthly rainfall was decreased to 4 mm and minimum monthly temperature reached the lowest values of 13.1°C recorded in January (Fig. 1), rubber tree would not suffer from drought because its deep taproot could absorb deep underground water. However, tropical tree species such as rubber trees, coffee, and mango are most vulnerable to low temperature. Therefore, it could be speculated that low temperature could be the main factor triggering rubber tree leaves senescence. Indeed, an easily observed event during leaf senescence is the loss of chlorophyll. In the present study, the contents of Chl, Car, and total protein were significantly lower in SL than in ML (Figs. 2, 3a). This further supports the idea that leaf senescence involves the degradation of protein (Lutts et al. 1996), nucleic acids (Buchanan-Wollaston 1997), and membranes (Trippi and Thimann 1983), as well as the loss of chlorophyll (Smart 1994). In contrast, the contents of H2O2 and MDA (indicative of oxidative lipid metabolism) in SL were significantly higher than those in ML (Fig. 3b, c). This was consistent with results of the increase in contents of H2O2 and MDA during leaf senescence reported by other researchers (Dhindsa et al. 1981; Hurng and Kao 1994; Ye et al. 2000; Marie 1995). The simultaneous increase in contents of MDA and H2O2 reveals that elevated oxidative stress during leaf senescence results in increased lipid degradation or lipid peroxidation.
Increased oxidative stress during leaf senescence was accompanied with reduction of photochemical efficiency of PSII (Fig. 4). Midday F v/F m and ΔF/F m′ strongly decreased in SL versus ML (Fig. 4), indicating the down-regulation or even irreversible damage of PSII reaction centers (Someralo and Krause 1989). Furthermore, the content of MDA was negatively correlated with F v/F m (Fig. 5b), it was consistent with the report of Mishra and Singhal (1992) on the MDA accumulation with the decline in F v/F m and ΔF/F m′. The lipids are utilized in maintenance of protein conformations, which are required for optimal electron transport. However, the lipid peroxidation might result in dysfunction of the proteins, and thus could slow down PSII electron transport and decline in the photochemical efficiency. The positive correlation of total protein with F v/F m (Fig. 5c) and the negative correlation of MDA with total protein (Fig. 5a) support this idea as stated above.
Low levels of ROS especially H2O2 are known to act as signal molecules initiating several protective mechanisms against oxidative stress (Desikin et al. 2001; Knight and Knight 2001). However, excessive ROS load as the case in the present senescent leaves (Fig. 3b) can cause unrecoverable membrane damage (Rao et al. 1997). Therefore, under this circumstance, plants must activate mechanism of antioxidative protection to withstand oxidative stress. In the present study, a significant decrease in SOD activity in SL was observed, with increased levels of H2O2, this was possibly caused by a significant decrease in CAT activity (Figs. 3b, 6a, b). It further supports the notion that ROS accumulations during leaf senescence were generally attributed to a decrease in antioxidative activities (Droillard et al. 1989; Pastori and Trippi 1993). Besides CAT, POD and APX also play important roles in scavenging of H2O2. In the present study, their activities were higher in SL than in ML (Fig. 6d, e). This supports the hypothesis that activation of POD and APX occurs under higher H2O2 levels to prevent H2O2 reaching too high level (Kang et al. 2003a, b).
Under oxidative stress condition, it is believed that H2O2 generated in plant cells is mainly scavenged by the ascorbate–glutathione cycle (Noctor and Foyer 1998). In this cycle, APX reduces H2O2 to water using AsA as the electron donor; the resulting DHA recycled to AsA using GSH as the electron donor, and the GSSG is converted back to GSH by NAD(P)H-dependent GR (Foyer and Halliwell 1976). The contents of reduced ascorbate and glutathione were significant increased, together with a significant increase in APX activity in SL (Figs. 6e, 7a, b). This suggests that the capacity of antioxidative metabolites to scavenge H2O2 by the ascorbate–glutathione cycle is robust in SL. If so, however, it appears unreasonable that higher level of H2O2 accumulation was observed in SL relative to ML (Fig. 3b). It has been proposed that the ratio of H2O2 to AsA rather than the absolute H2O2 content is a better indicator of the redox balance in plant cells (Kingston-Smith et al. 1997). The lower H2O2/AsA ratio was observed in SL versus ML (Fig. 7c), indicating that, although SL undergo elevated oxidative stress as indicated by H2O2 levels, they may potentially be able to accommodate higher level of H2O2 because some components of antioxidative systems such as POD, APX, and ascorbate–glutathione cycle as stated above are further activated at this stage.
Isoprenoids can also scavenge ROS and dissipate excess excitation energy, thus alleviating lipid peroxidation. In the present study, non-volatile isoprenoids, mostly carotenoids, was decreased with leaf senescence, but Car/Chl was significantly higher along with decreased level of Chl in SL (Fig. 2). This will lead the senescent leaves to reduce the amount of light energy harvested by the antenna complex, and thereby relatively enhance the capacities of Car to dissipate excess energy as heat, to scavenge ROS and to inhibit lipid peroxidation. On the other hand, it has been reported that volatile isoprenoids may serve as an antioxidative metabolites in leaves (Loreto et al. 1998, 2004; Peňuelas and Llusià 1999). In the present study, monoterpene concentrations (Fig. 8) as well as monoterpene/Chl (data not shown) was much lower in SL than in ML. The volatile isoprenoids biosynthesis is exclusively dependent on photosynthesis (Sharkey and Yeh 2001) and share a common biochemical synthesis pathway and localization with nonvolatile isoprenoids (carotenoids) (Logan et al. 2002; Affeck and Yakir 2002). Therefore, when photosynthetic pigments in SL were significantly degraded, volatile isoprenoids (α-pinene, β-pinene, and sabinene) biosynthesis and accumulation also significantly decreased.
In conclusion, the results obtained here have demonstrated that (1) the senescent leaves of rubber trees confronted to elevated oxidative stress, were able to further activate some components of antioxidative system such as POD, APX and ascorbate–glutathione cycle to withstand oxidative stress and thus possibly postpone the senescing process; (2) the biosynthesis of volatile isoprenoids (monoterpene) was suppressed in senescent leaves, but non-volatile isoprenoids (carotenoids) may in turn confer further photoprotection at this stage.
Abbreviations
- H2O2 :
-
Hydrogen peroxide
- MDA:
-
Malondialdehyde
- Chl:
-
Chlorophyll
- Car:
-
Carotenoids
- PSII:
-
Photosystem II
- SOD:
-
Superoxide dismutase (EC 1.15.1.1)
- CAT:
-
Catalase (EC 1.11.1.6)
- GR:
-
Glutathione reductase (EC 1.6.4.2)
- POD:
-
Guaiacol peroxidase (EC 1.11.1.7)
- APX:
-
Ascorbate peroxidase (EC 1.11.1.11)
- AsA:
-
Reduced ascorbate
- DHA:
-
Oxidized ascorbate
- GSH:
-
Reduced glutathione
- GSSG:
-
Oxidized glutathione
- F v/F m :
-
Maximum photochemical efficiency of PSII
- ΔF/F m′:
-
Actual photochemical efficiency of PSII
- ROS:
-
Reactive oxygen species
- TCA:
-
Trichloroacetic acid
- TBA:
-
Thiobarbituric acid
References
Affeck HP, Yakir D (2002) Protection by isoprene against singlet oxygen in leaves. Plant Physiol 129:269–277
Alscher R, Donahue J, Cramer CL (1997) Reactive oxygen species and antioxidants: relationship in green cells. Physiol Plant 100:224–233
Arakawa N, Tsutsumi K, Sanceda NG, Kurata T, Inagaki C (1981) A rapid and sensitive method for the determination of ascorbic acid using 4, 7-diphenyl-1, 10-phenanthroline. Agric Biol Chem 45:1289–1290
Buchanan-Wollaston V (1997) The molecular biology of leaf senescence. J Exp Bot 48:181–199
Bueno P, del Rio LA (1992) Purification and properties of glyoxysomal cuperozinc superoxide dismutases from watermelon (Citrullus vulgaris Scrad). Plant Physiol 98:331–336
Corpas FJ, Barroso JB, del Río LA (2001) Peroxisomes as a source of reactive oxygen species and nitric oxide signal molecules in plant cells. Trends Plant Sci 6:145–150
Desikan R, Mackerness SAH, Hancock JT, Neill SJ (2001) Regulation of the Arabidopsis transcriptome by oxidative stress. Plant Physiol 127:159–172
Dhindsa RA, Plumb-Dhindsa P, Thorpe TA (1981) Leaf senescence: correlated with increased permeability and lipid peroxidation, and decreased levels of superoxidase and catalase. J Exp Bot 126:93–101
Doulis A, Debian N, Kingston-Smith AH, Foyer CH (1997) Characterization of chilling sensitivity in maize. I. Differential localization of antioxidants in maize leaves. Plant Physiol 114:1031–1037
Droillard MJ, Bureau D, Paulin A (1989) Changes in activities of superoxide dismutases during aging of petals of cut carnations (Dianthus caryophyllus). Physiol Plant 76:149–154
Foyer CH, Halliwell B (1976) The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta 133:21–25
Gan S, Amasino RM (1997) Making sense of senescence. Plant Physiol 113:313–319
Giannopolitis N, Ries SK (1977) Superoxide dismutase. I. Occurrence in higher plants. Plant Physiol 59:309–314
Hodges DM, Delong JM, Forney CF, Prange RK (1999) Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and interfering compounds. Planta 207:604–611
Hurng WP, Kao CH (1994) Lipid peroxisomes and antioxidative enzymes in senescing tobacco leaves during post flooding. Plant Sci 96:41–44
Kang GZ, Wang CH, Sun GC, Wang ZX (2003a) Salicylic acid changes activities of H2O2-metabolizing enzymes and increases the chilling tolerance of banana seedlings. Environ Exp Bot 50:9–15
Kang GZ, Wang ZX, Sun GC (2003b) Participation of H2O2 in enhancement of cold chilling hardening by salicylic acid in banana seedlings. Acta Bot Sin 45(5):567–573
Kingston-Smith AH, Thomas H, Foyer CH (1997) Chlorophyll a fluorescence, enzyme and antioxidant analyses provide evidence for the operation of alternative electron sinks during leaf senescence in a stay-green mutant of Festuca pratensis. Plant Cell Environ 20:1323–1337
Klinger LF, Li QJ, Guenther AB, Greenberg JP, Baker B, Bai JH (2002) Assessment of volatile organic compound emissions from ecosystems of China. J Geophys Res 107:4603–4624
Knight H, Knight MR (2001) Abiotic stress signaling pathways: specificity and cross-talk. Trends Plant Sci 6:262–267
Lichtenthaler HK, Wellburn AR (1983) Determinations of total carotenoids and chlorophyll and chlorophyll a and b of leaf extracts in different solvents. Biochem Soc Trans 603:591–592
Llusià J, Peňuelas J (2000) Seasonal patterns of terpene content and emission from seven Mediterranean woody species in field conditions. Am J Bot 87:133–140
Logan BA, Monson RK, Potosnak MJ (2002) Biochemistry and physiology of foliar isoprene production. Trends Plant Sci 5:477–481
Loreto F, Főrster A, Dϋrr M, Csiky O, Seufert G (1998) On the monoterpene emission under heat stress and on the increased thermotolerance of leaves of Quercus ilex L. fumigated with selected monoterpenes. Plant Cell Environ 21:101–107
Loreto F, Mannozzi M, Maris C, Nascetti P, Ferranti F, Pasqualini S (2001) Ozone quenching properties of isoprene and its antioxidant role in leaves. Plant Physiol 126:993–1000
Loreto F, Pinelli P, Manes F, Kollist H (2004) Impact of ozone on monoterpene emissions and evidence for an isoprene-like antioxidant action of monoterpenes emitted by Quercus ilex leaves. Tree Physiol 24:361–367
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Lutts S, Kinet JM, Bouharmount J (1996) NaCl-induced senescence in leaves of rice (Oryza sativa L.) cultivars differing in salinity resistance. Ann Bot 78:389–398
Marie O (1995) Alteration in lipid composition and antioxidative protection during senescence in drought stressed plants and non-drought stressed plants of Pisum sativum. Plant Physiol Biochem 33:547–553
Merzlyak MN, Solovchenko AE (2002) Photostability of pigments in ripening apple fruit: a possible photoprotective role of carotenoids during plant senescence. Plant Sci 163:881–888
Mishra RK, Singhal GS (1992) Function of photosynthetic apparatus of intact wheat leaves under high light and heat stress and its relationship with peroxidation of thylakoids. Plant Physiol 98:1–6
Noctor G, Foyer C (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol 49:249–279
Pastori GM, del Río RA (1997) Natural senescence of pea leaves: an activated oxygen-mediated function for peroxisomes. Plant Physiol 113:411–418
Pastori GM, Trippi VS (1993) Antioxidative protection in a drought-resistant strain during leaf senescence. Physiol Plant 87:227–231
Peňuelas J, Llusià J (1999) Seasonal emission of monoterpenes by the Mediterranean tree Quercus ilex in field conditions: relations with photosynthetic rates, temperature and volatility. Physiol Plant 105:641–647
Peňuelas J, Munné-Bosch S (2005) Isoprenoids: an evolutionary pool for photoprotection. Trends Plant Sci 10:166–169
Piquery L, Davoine C, Huault C, Billard JP (2000) Senescence of leaf sheats of ryegrass stuble: changes in enzymes activities related to H2O2 metabolism. Plant Growth Regul 30:71–77
Prochάzkovά D, Sairam RK, Srivastava GC, Singh DV (2001) Oxidative stress and antioxidant activity as the basis of senescence in maize leaves. Plant Sci 161:765–771
Rao MV, Paliyath G, Ormrod DP, Murr DP, Watkins CB (1997) Influence of salicylic acid on H2O2 production, oxidative stress, and H2O2-metabolizing enzymes: salicylic acid-mediated oxidative damage requires H2O2. Plant Physiol 155:137–149
Scebba F, Sebastiani L, Vitaglian C (2004) Activities of antioxidant enzymes during senescence of Prunnus armeniaca leaves. Plant Biol 44:41–46
Sharkey TD, Singsaas EL (1995) Why plants emit isoprene. Nature 374:769
Sharkey TD, Yeh S (2001) Isoprene emission from plants. Annu Rev Plant Physiol Plant Mol Biol 52:407–436
Smart CM (1994) Gene expression during leaf senescence. New Phytol 126:419–448
Somersalo A, Krause GH (1989) Photoinhibition at chilling temperature. Fluorescence characteristics of unhardened and cold acclimated spinach leaves. Planta 177:409–416
Trippi S, Thimann KV (1983) The exudation of solutes during senescence of oat leaves. Physiol Plant 58:21–28
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant system in acid rain-treated bean plants. Protective role of exogenous polymines. Plant Sci 151:59–66
Ye Z, Rodriguez R, Tran A, Hoang H, des los Santos D, Brown S, Vellanoweth RL (2000) The developmental transition to flowering repress ascorbate peroxidase activity and induces enzymatic lipid peroxidation in leaf tissue in Arabidopsis thaliana. Plant Sci 158:115–127
Zeidler JG, Lichtenthaler HK, May HU, Lichtenthaler FW (1997) Is isoprene emitted by plants synthesized via the novel isopentenyl pyrophosphate pathway? Z Naturforsch [C] 52:15–23
Acknowledgments
This study was funded by the National Science Foundation of China (project no. 90302013). We are grateful to our colleagues Jiao-lin Zhang and Jun-jie Zhu for helpful comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Filek.
Rights and permissions
About this article
Cite this article
Chen, JW., Cao, KF. Changes in activities of antioxidative system and monoterpene and photochemical efficiency during seasonal leaf senescence in Hevea brasiliensis trees. Acta Physiol Plant 30, 1–9 (2008). https://doi.org/10.1007/s11738-007-0070-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-007-0070-1