Abstract
To provide a theoretical basis for revealing the mechanism of winter leaf reddening in evergreen species, the relationships between winter leaf reddening, reactive oxygen species (ROS) and the antioxidant system of Buxus microphylla ‘Wintergreen’ were studied. The pigment changes, ROS production, lipid peroxidation and antioxidants activities of sun leaves during the reddening and regreening processes were investigated, using green shade leaves as controls. The carotenoids in the sun leaves increased linearly with reddening but decreased with the regreening. There was no significant difference in either the superoxide anions (O −·2 ) or malondialdehyde (MDA) changes between the sun and shade leaves, and their O −·2 contents were positively correlated with MDA. In contrast to the shade leaves, the sun leaves showed a trend in which the hydrogen peroxide (H2O2) changes were closely related to the reddening process and positively correlated with carotenoids content but not with the MDA content. A similar trend was observed for catalase (CAT) and ascorbate peroxidase (APX) activity between the sun and shade leaves, but superoxide dismutase (SOD) and peroxidase (POD) activity and the ascorbate (AsA) content differed between these two ecotypes. Furthermore, the sun leaves had higher CAT activity and AsA content than the corresponding shade leaves. These results suggested that H2O2 might play an important role in the winter reddening of sun leaves by promoting the accumulation of carotenoids. In addition, SOD, POD and AsA probably play a photoprotective role in winter-red sun leaves, while the changes in O −·2 , CAT and APX were independent of winter leaf reddening and were more likely responses to stress caused by low temperatures.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The leaves of some evergreen species frequently change color to brown or reddish yellow in winter and are restored to green with springtime warming (Koiwa et al. 1986; Hughes 2011). Previous research on the winter leaf reddening mechanism mainly investigated the relationship between pigment changes in leaves and their photoprotective function (Hughes 2011). It is well documented that leaf reddening in winter evergreen species is mainly caused by the accumulation of anthocyanins (Kaku et al. 1992; Hughes and Smith 2007) or red carotenoids (Ida et al. 1995; Han et al. 2004). According to the photoprotection hypothesis, these red pigments accumulate in leaves to protect photosynthetic tissues from winter high light stress by acting as light attenuators to alleviate excess chlorophyll excitation pressure in underlying cells (Hormaetxe et al. 2005; Carpenter et al. 2014; Hughes et al. 2014). These pigments also act as quenchers of reactive oxygen species (ROS) (Hormaetxe et al. 2004; Velissarios-Phaedon and Yiannis 2006). The former function has been studied directly in detail, but the latter remains a hypothesis because the direct relationship between pigments and ROS in the winter leaf coloration process has not been systematically studied.
Under normal growth conditions, the production of ROS, including hydrogen peroxide (H2O2), superoxide anions (O −·2 ), hydroxyl radical (OH·) and singlet oxygen (1O2), is low in cells (Mittler 2002). However, in winter, green leaves are often subjected to cold temperature stress or chilling-enhanced photo-oxidative stress (Wise 1995; Arora and Rowland 2011). This may disrupt the cellular homeostasis of cells and enhance the production of ROS (Wise 1995; Verhoeven et al. 2005; Ruelland et al. 2009). Excess ROS can cause oxidation of biomolecules and cell death. However, there are a series of antioxidant systems that tightly control ROS production (Mittler 2002). 1O2 is a singular ROS that is produced constitutively in plant leaves under light conditions via chlorophylls that act as photosensitizers, and its production is enhanced under light stress conditions (Triantaphylidès and Havaux 2009). Carotenoids are considered to be the main 1O2 quenchers in chloroplasts (Ramel et al. 2012; Fischer et al. 2013). OH·, produced from O −·2 and H2O2, is one of the most highly reactive ROS (Gill and Tuteja 2010). Superoxide dismutase (SOD) can remove O −·2 , and hence decrease the risk of OH· formation, and H2O2 can be converted into water by other ROS-scavenging mechanisms (Pandhair and Sekhon 2006).
Research on the shrubs of two boxwood species have shown that winter leaf reddening in Buxus sempervirens and B. microphylla is closely related to the accumulation of carotenoids (Ida et al. 1995; Jiang et al. 2015). As an important antioxidant, carotenoids protect plants from ROS damage by scavenging free radicals and by quenching 1O2 and the excited-state light-sensitive molecules (Domonkos et al. 2013). Therefore, in this study, the relationships between carotenoids, ROS and antioxidants in sun and shade leaves of B. microphylla ‘Wintergreen’ were investigated during winter reddening and regreening periods to provide an important theoretical basis for revealing the mechanism underlying winter reddening.
Materials and methods
Plant material
A cultivar of B. microphylla ‘Wintergreen’, introduced from Belgium more than 10 years ago, grown in the Beijing Botanical Garden Seedling Center, China (40°01′N, 116°19′E), was selected for study. This shrub is spherical in nature with an average crown of 1.2 × 1.2 m, and a height of 1.3 m. Shrubs were planted in an east–west array in an open field. The morphology, growth and size were basically similar. According to our preliminary observations and the relevant literature, sun leaves exposed to light turn red in winter and become green again with springtime warming compared with shade leaves that remain green throughout winter (Hughes 2011; Peng et al. 2011).
The color change of B. microphylla sun leaves was divided into three stages during the winter, (1) the reddening process in which color changed from green to red as winter progressed, (2) red stable stage in mid-winter, (3) the regreening process in which color changed from red to green with springtime warming. Three sampling dates were selected in the first (reddening process, October to December 2013) and the third stage (regreening process, February to March 2014) respectively according to the leaf color change process in the 2013–2014 winter. Sun and shade leaves were randomly collected from the middle of the current year branches with south- and north-facing exposure.
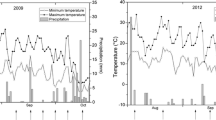
Leaves designated as G-g (green leaves), G-m (intermediate state between green and red leaves) and G-r (red leaves) correspond to three different coloration stages of sun leaves changing from green to red as winter progresses. Similarly, leaves designated as R-r (red leaves), R-m (intermediate state between red and green leaves) and R-g (green leaves) correspond to the different colors of sun leaves changing from red to green with springtime warming. The coloration of all sun leaves sampled is presented in Fig. 1. All samples were immediately frozen in liquid nitrogen and stored at −80 °C until biochemical analysis. The field temperature during the test period was monitored using a HOBO U23-004 data logger (Onset Computer Corp., MA, USA) (Fig. 2).
Winter leaf color changes in B. microphylla ‘Wintergreen’. a, b Sun leaves; c, d shade leaves in the corresponding period. G-g, G-m and G-r represent the different colors of the sun leaves changing from green to red during the reddening process as winter progresses; R-r, R-m and R-g represent the different colors changing from red to green during the regreening process with springtime warming
Biochemical analysis
Chlorophyll and carotenoids contents
An extraction method was used following Zhang (1986), but with 80 % (v/v) acetone as the extraction solvent instead of the acetone–ethanol mixture. Leaves cut into filaments (0.15 g) were immersed in 10 ml 80 % acetone (v/v) and were incubated for 24 h at ambient temperature in the dark until the material was completely white. The absorbance of the extraction solution was measured at 663, 646 and 470 nm using a Biomate 3S UV–Visible Spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) according to Lichtenthaler and Wellburn (1983). A similar method was also used in the total carotenoids determination of red needles in Qilian juniper in Wen and Chen (2014). The contents of chlorophyll a, b and total carotenoids were calculated using the following formulas,
where V = volume of 80 % (v/v) acetone (ml) and W = weight of sample (g). The chlorophyll and carotenoids contents = [mg g−1 fresh weight (FW)].
Determination of ROS (O −·2 and H2O2)
The O −·2 content was measured according to the procedure described by Wang and Luo (1990). Leaves (0.3 g) were ground in a pre-chilled mortar with 5 ml of 50 mM sodium phosphate buffer (PBS, pH 7.8) in an ice bath. The homogenates were centrifuged at 4000×g for 15 min at 4 °C. One milliliter of the reaction mixture, which consisted of 0.5 ml supernatant, 50 mM PBS (pH 7.8) and 10 mM hydroxylamine hydrochloride, was incubated at 25 °C for 1 h. Then, 1 ml of 17 mM p-amino-benzene sulfonic acid and 1 ml 7 mM α-naphthylamine were added, and mixed well. After 20 min of incubation at 25 °C, the absorbance was recorded at 530 nm. Standard curves were obtained using different concentrations of NaNO2 for each independent experiment. The O −·2 content (nmol g−1 FW) was calculated according to a chemical equation as follows: NH2OH + 2O −·2 + H+→ NO2 − + H2O2 + H2O.
The H2O2 level was determined according to the method of Sergiev et al. (1997). Frozen leaf tissue (0.3 g) was ground in a pre-chilled mortar with 4 ml 0.1 % (w/v) TCA in an ice bath. The extraction solution was centrifuged at 4000×g for 15 min at 4 °C. The absorbance of the reaction mixture consisted of 0.5 ml supernatant, 0.5 ml 10 mM potassium phosphate buffer (pH 7.0) and 1 ml 1 M KI, was measured at 390 nm. The H2O2 content was determined using a standard curve.
Malondialdehyde (MDA) assay
The MDA content was determined according to Li et al. (2000). Leaves (0.3 g) were homogenized in 4 ml 5 % (w/v) trichloroacetic acid (TCA) solution in an ice bath. The homogenate was centrifuged at 10,000×g for 20 min. The supernatant was collected and make up to a final volume of 4 ml for the next set of measurements. The reaction mixture, which consisted of 1 ml sample and 1 ml 0.67 % (w/v) thiobarbituric acid (TBA, dissolved in 20 % TCA), was incubated in boiling water for 30 min, and the reaction was stopped by placing the reaction tube in an ice bath. Then, the mixture was centrifuged again at 10,000×g for 5 min, and the absorbance of the supernatant was read at 450, 532 and 600 nm.
Extraction and assay of antioxidant enzymes
Antioxidant enzymes were extracted according to Prochazkova et al. (2001) and Wang et al. (2009). Using a pre-cooled mortar and pestle in an ice bath, a frozen leaf sample (0.3 g) was ground in 5 ml of 100 mM PBS (pH 7.5), containing 0.5 mM ethylene diamine tetraacetic acid (EDTA) and 2 % polyvinylpyrrolidone (PVP-40), with the addition of 2 mM ascorbate (AsA) in the case of the ascorbate peroxidase (APX) assay. The extract was centrifuged at 12,000×g for 20 min at 4 °C and the supernatant was collected as enzyme extracts for the following assays.
SOD activity was assayed at 560 nm using the nitroblue tetrazolium (NBT) method according to Dhindsa et al. (1981). The reaction mixture was composed of 1.7 ml 50 mM PBS (pH 7.8), 0.3 ml 130 mM l-methionine, 0.3 ml 750 μM nitroblue tetrazolium, 0.3 ml 100 μM EDTA-Na2 and 0.1 ml enzyme extract (same volume of PBS as the control). One unit of SOD activity was defined as the amount of enzyme required to cause 50 % inhibition in the reduction of NBT.
Catalase (CAT) activity was determined by monitoring the decrease in absorbance at 240 nm according to Prochazkova et al. (2001). The 2.7 ml mixture contained 100 mM PBS (pH 7.0) and 0.2 ml enzyme extract. Then, 0.3 ml 10 mM H2O2 was added to initiate the reaction. A decrease in absorbance of 0.01 per minute at 240 nm was defined as one unit of enzyme activity.
APX activity was determined by monitoring the rate of drop in absorbance (per min) at 290 nm as a result of ascorbate oxidation according to Nakano and Asada (1981) and Wang et al. (2009). The 2.7 ml reaction mixture contained 50 mM PBS (pH 7.4), 0.5 mM AsA, 0.1 mM EDTA, and 50 μl enzyme extract. Then, 0.3 ml of 10 mM H2O2 was added to initiate the reaction.
Peroxidase (POD) activity was determined according to guaiacol oxidation caused by H2O2 at 470 nm (Prochazkova et al. 2001). The 3 ml reaction mixture contained 100 mM PBS (pH 7.0), 20 mM guaiacol and 12 mM H2O2. Then, 0.1 ml enzyme extract (same volume of PBS as control) was added and scanned at 470 nm immediately. An increase in absorbance of 0.1 per minute at 470 nm was defined as one unit of enzyme activity.
AsA content
The AsA content was determined according to the method of Kampfenkel et al. (1995). Frozen leaf material (0.3 g) was ground to a fine powder in a mortar (placed on ice) that was pre-chilled using liquid N2. Then, 4 ml of 6 % (w/v) TCA was added. The mixture was continually homogenized in an ice bath until it was completely thawed, after which it was centrifuged at 4000×g for 15 min at 4 °C. The supernatant was transferred to a reaction vessel (on ice) and adjusted to a volume of 4 ml using 6 % (w/v) TCA for AsA analysis. The assay mixture contained 0.2 ml of sample extract, 0.6 ml of 0.2 M PBS (pH 7.4), 0.2 ml of double-distilled H2O, 1.0 ml of 10 % TCA, 0.8 ml of 4.2 % H3PO4, 0.8 ml of 0.4 % of 2,2′-dipyridyl dissolved in 70 % (v/v) ethanol and 0.4 ml of 3 % FeCl3. The mixture was immediately and vigorously mixed after the addition of FeCl3. Then, incubate at 42 °C in a water bath for 40 min, and the absorbance was read at 525 nm. The AsA content (μmol g−1 FW) was determined using a standard curve.
Statistical analysis
All statistical analyses were performed using Microsoft Excel 2007 software (Microsoft Corp, Richmond, CA, USA) and SPSS software (version 18.0 SPSS Inc., Chicago, IL). Multiple comparisons were carried out using one-way ANOVA followed by Duncan’s multiple range test when significant differences were detected. Mean comparisons between sun and shade leaves in each period were subjected to an independent sample t test. Correlations between ROS, carotenoids and MDA were evaluated using Pearson’s correlation coefficient. Three replications were performed for all experiments.
Results
Changes in pigment contents
The chlorophyll content decreased (Fig. 3a) and the carotenoids content increased (Fig. 3b) linearly in the sun leaves as the color changed from green to red during the reddening process. In contrast, these pigments exhibited the opposite pattern during the regreening process. The chlorophyll content increased but there was no significant difference between R-r and R-m (Fig. 3c) while carotenoids declined linearly (Fig. 3d) as the color changed from red to green. In addition, the carotenoids content in the winter-red sun leaves was higher than that in the green shade leaves during the corresponding period, but the chlorophyll content was lower. Unlike the sun leaves, the chlorophyll and carotenoids contents in shade leaves did not show a linear change over the winter.
Changes in the pigment contents of B. microphylla ‘Wintergreen’ leaves. a, b The reddening process; c, d the regreening process. G-g, G-m and G-r represent the different colors of sun leaves changing from green to red during the reddening process as winter progresses; R-r, R-m and R-g represent the different colors of sun leaves changing from red to green during the regreening process with springtime warming. Each value is the mean ± SE. Different letters denote significant differences at P < 0.05. * and ** indicate significant differences at P < 0.05 and P < 0.01, respectively, between the sun and shade leaves
Changes in ROS and MDA
MDA accumulation has been used as an important bio-marker of oxidative stress-induced damage caused by ROS. The O −·2 , H2O2 and MDA contents (Fig. 4a–c) in the sun leaves increased during reddening process as the winter progressed and reached the highest values at the G-r stage, which were significantly higher than that at the G-g stage. Correlation analysis showed that the O −·2 content in the sun leaves was significantly positively correlated with the carotenoids and MDA, and H2O2 was positively correlated with the carotenoids but not with MDA during the reddening process (Table 1). In addition, no significant difference was found in either the O −·2 or MDA content between the sun and shade leaves (Fig. 4a, c). This result indicated that the changes in the O −·2 content did not occur specifically as a result of the reddening of the sun leaves and that the two types of ecological leaves were subjected to the same oxidative damage. However, the changes in the H2O2 content were different; the sun leaves had consistently higher H2O2 levels than the corresponding shade leaves (Fig. 4b).
Changes in the O2−·, H2O2 and MDA contents of B. microphylla ‘Wintergreen’ leaves. a-c The reddening process; d–f the regreening process. G-g, G-m and G-r represent the different colors of sun leaves changing from green to red during the reddening process as winter progresses; R-r, R-m and R-g represent the different colors of sun leaves changing from red to green during the regreening process with springtime warming. Each value is the mean ± SE. Different letters denote significant differences at P < 0.05. * and ** indicate significant difference at P < 0.05 and P < 0.01, respectively, between the sun and shade leaves
The O −·2 and MDA contents in the sun leaves showed a downward trend with the lowest values measured in the R-g leaves (Fig. 4d, f), while the H2O2 remained relatively constant as the color changed from red to green. Correlation analysis performed on the sun leaf data indicated that O −·2 was positively related to the carotenoids and MDA, in contrast to the H2O2 content, during the regreening process (Table 1). In addition, the O −·2 and MDA contents in the sun and shade leaves were similar at the R-m and R-r stages and decreased to the lowest values at the R-g stage (Fig. 4d, f). In contrast to the sun leaves, the H2O2 content in the shade leaves remained unchanged initially but then increased at the R-g stage (Fig. 4e), but this value was much lower than that of the corresponding sun leaves (Fig. 4e).
Changes in the antioxidant system
Antioxidant system changes in the leaves of B. microphylla ‘Wintergreen’ during the reddening process are shown in Fig. 5a–e. Changes in the SOD, CAT and POD activities in the sun leaves exhibited similar trends, i.e., an increase at the G-m stage and a decrease at the G-r stage. Compared with the G-g stage, leaves at the G-r stage had lower SOD activity (Fig. 5a, b) but higher POD activity (Fig. 5d). Both the APX activity and the AsA content increased as winter progressed but the former reached a maximum value at the G-r stage (Fig. 5c) while the latter had the highest value at the G-r and G-m stages (Fig. 5e).The above results demonstrate that the antioxidant systems were mobilized in cooperation and responded synchronously in sun leaves, with the same changes in the trends of their activity. In addition, the change in CAT activity was similar between these two ecotypes, but it was higher in sun leaves compared with the corresponding shade leaves (Fig. 5b), and no difference was found in APX activity between the sun and shade leaves (Fig. 5c). However, the SOD and POD activities and AsA content of the sun leaves were different from those of the shade leaves, in which the SOD activity continued to increase (Fig. 5a), the POD activity decreased before increasing (Fig. 5d) and the AsA content remained constant before increasing (Fig. 5e). Furthermore, the sun leaves had a higher AsA content than the shade leaves during each step of the color changing process (Fig. 5e).
Changes in the antioxidant system of B. microphylla ‘Wintergreen’ leaves. a–e The reddening process; f–j the regreening process. G-g, G-m and G-r represent the different colors of sun leaves changing from green to red during the reddening process as winter progresses; R-r, R-m and R-g represent the different colors of sun leaves changing from red to green during the regreening process with springtime warming. Each value is the mean ± SE. Different letters denote significant differences at P < 0.05. * and ** indicate significant difference at P < 0.05 and P < 0.01, respectively, between the sun and shade leaves
The antioxidant system changes in the leaves of B. microphylla during the regreening process are presented in Fig. 5f–j. In the sun leaves, both the SOD and APX activities decreased at the R-m stage and then increased at the R-g stage (Fig. 5a, h). In contrast, the CAT activity increased at the R-m stage and decreased at the R-g stage (Fig. 5g). The POD activity increased linearly (Fig. 5i), but the AsA content remained steady (Fig. 5j) with spring time warming. Compared with R-g, the winter-red sun leaves (R-r) had higher CAT and APX activities (Fig. 5g, h) and lower POD activity (Fig. 5i) but the same SOD activity and AsA content (Fig. 5f, j). In addition, there was no difference in the change in the CAT and APX activities (Fig. 5g, h) between the sun and shade leaves, although the CAT activity was higher than that of the corresponding sun leaves. This result was similar to what occurred during the reddening process. However, the SOD and POD activities and the AsA content differed between the sun and shade leaves. In the shade leaves, the SOD and POD activities decreased after the first increase (Fig. 5f, i) and the AsA content decreased (Fig. 5j) with springtime warming. Furthermore, the sun leaves had a higher AsA content than the shade leaves at each step during the color changing process (Fig. 5j), similar to the trend in the reddening process.
Discussion
According to Koiwa et al. (1986), the winter reddening process of B. sempervirens leaves was accompanied by simultaneous chloroplast disintegration into chromoplasts which were restored to functional chloroplasts in regreening leaves. Consistent with their results, our study also showed similar pigment changes in B. microphylla ‘Wintergreen’ leaves during the reversible color transformation process (Fig. 3). However, during the regreening process, no difference was found in the chlorophyll content of the sun leaves at the R-r and R-m stages, though their colors were significantly different. This might be attributed to the carotenoids having already degraded without the immediate de novo synthesis of chlorophyll at the R-m stage (Ruelland et al. 2009). Therefore, the total carotenoids content can better reflect the degree of discoloration of sun leaves compared with chlorophyll. We should point out that the Lichtenthaler and Wellburn (1983) equation for total carotenoids was usually used for the “usual” yellow carotenoids present in green leaves and it could be less precise for the quantification of red carotenoids.
Excess ROS generated under low temperatures can cause oxidative damage to plants, and this damage would be exacerbated in the presence of light (Wise 1995). In this study, there was no difference in the O −·2 content between the sun and shade leaves in the same period, and their change trends were also similar during overwintering (Fig. 4a, d). This indicated that O −·2 may not be involved in sun leaf winter reddening, although the O −·2 content was positively related to carotenoids (Table 1). MDA is the ultimate product of membrane lipid peroxidation and its content is often related to the extent of oxidative damage caused by ROS. Our present findings clearly revealed that the sun and shade leaves showed similar changes in the MDA (Fig. 4c, f) content in winter, and their O −·2 contents were positively correlated with MDA (Table 1). These findings led us to believe that sun leaves undergo winter reddening, and that were exposed to high light did not suffer from additional damage compared with the green shade leaves, and O −·2 may have caused cell membrane lipid peroxidation.
The H2O2 content in the sun leaves was consistently higher than that of the corresponding shade leaves (Fig. 4b, e), suggesting that the winter photo-oxidative stress promoted the accumulation of H2O2 in the sun leaves. It is known that as antioxidants, carotenoids can protect photosynthetic organisms from oxidative damage by quenching 1O2 (Ramel et al. 2012; Fischer et al. 2013). According to Bouvier et al. (1998), down-regulation of CAT induced the synthesis of carotenoids in excised green pericarp discs, indicating that H2O2 might play an important role in mediating carotenoids synthesis by acting as a novel class of secondary messengers. Under photo-oxidative stress, H2O2 is sufficient for 1O2-mediated oxidative damage protection though a reduction in the degree of carotenoid breakdown by the activation of de novo carotenoid synthesis (Chang et al. 2013; Iigusa et al. 2005). In our study, the reddening process was accompanied by an increase in H2O2 (Fig. 4b), and the level of H2O2 was correlated with carotenoids but not with the MDA content (Table 1). Therefore, it is conceivable that H2O2 may play an important role in sun leaf winter reddening by promoting carotenoids accumulation for photoprotection.
Although plants actively produce H2O2 as a signaling molecule, its presence above threshold levels is also toxic. Thus, plants also have scavenging mechanisms, including enzymatic and nonenzymatic antioxidants, to ensure that H2O2 level are controlled in cells (Shabnam and Pardha-Saradhi 2016). Located in the peroxisome, CAT directly removes H2O2 and is indispensable for H2O2 detoxification under stressed conditions (Garg and Manchanda 2009). However, APX has a higher affinity for H2O2 than CAT (Mittler 2002). APX is involved in scavenging H2O2 in water–water and AsA-GSH cycles and utilizes AsA as the specific electron donor (Gill and Tuteja 2010; Sofo et al. 2015). In our research, the APX activities of the sun and shade leaves of B. microphylla increased synchronously during the cold period in winter (Fig. 5c, h), whereas the CAT activities declined (Fig. 5b, g). The reduction in CAT was specific to low temperature treatments and might trigger the induction of APX by increasing the H2O2 level (Distelbarth et al. 2013). This indicated that APX may be more effective in maintaining the cold hardiness of both sun and shade leaves compared with CAT, which was consistent with some studies (e.g., Cansev et al. 2011). Therefore, CAT and APX were probably generated in response to low temperature stress and did not play a role in sun leaf winter reddening. The observation of higher CAT activity in sun leaves compared with shade leaves was consistent with their high accumulation of H2O2.
The significant differences between the sun and shade leaves with respect to the changes in SOD (Fig. 5a, f) and POD (Fig. 5d, i) activities and the AsA content (Fig. 5e, j) throughout the overwintering period suggested that these three antioxidants were not only affected by low temperature but also by high light. It is known that SOD can convert O −·2 into H2O2 (Breusegem et al. 2001). In the sun leaves, the SOD activity decreased at the G-r stage (Fig. 5a), though the O −·2 (Fig. 4a) and H2O2 (Fig. 4b) contents were much higher. This may have occurred because excess H2O2 inhibited the SOD activity during that period (Casano et al. 1997). However, POD and AsA may enable the reactivation of SOD by reducing H2O2 production and through the continuous scavenging of O −·2 (Zipor and Oren-Shamir 2013), and together these antioxidants played a role in photoprotection. POD is widely involved in plant growth and development (Passardi et al. 2005). Our research revealed that the increase in POD activity corresponded to the regreening process of the sun leaves, but the role this enzyme plays in the regreening process requires further investigation. High light can enhance the AsA content (Yang et al. 2008). Studies have also shown that AsA can maintain excess energy dissipation as a cofactor of violaxanthin de-epoxidase (Eskling et al. 1997). Therefore, a higher AsA content (Fig. 5e, j) in the sun compared with the shade leaves was not only due to the higher level of H2O2 (Fig. 4b, e), but was also related to the higher light energy absorption of the sun leaves.
In conclusion, H2O2 which was positively correlated with carotenoids but not with the MDA content during the entire overwintering period may play an important role in sun leaf winter reddening by promoting the accumulation of carotenoids. By contrast, the level of O −·2 in the sun leaves was similar to that in the shade leaves, which suggests that it is independent of sun leaf winter reddening, but mainly causes plant cell membrane lipid peroxidation under low temperature stress. SOD, POD and AsA have a high sensitivity to light oxidative stress. They participate in regulating H2O2 production and play a photoprotective role in the winter-red sun leaves of B. microphylla ‘Wintergreen’ by cooperating with carotenoids. However, CAT and APX are independent of sun leaf winter reddening and their changes are probably common responses to low temperature.
Author contribution statement
Xue-ru Jiang and Jin-gen Peng designed experiments; Xue-ru Jiang carried out experiments; Xue-ru Jiang, Jin-gen Peng and Yan Liu analyzed experimental results and wrote the manuscript. Meng-xue Jia critically revised the manuscript. Rong-fu Gao provided technical assistance. Ling Guo provided experimental materials. All authors read and approved the manuscript.
References
Arora R, Rowland LJ (2011) Physiological research on winter-hardiness: deacclimation resistance, reacclimation ability, photoprotection strategies, and a cold acclimation protocol design. HortScience 46:1070–1078
Bouvier F, Backhaus RA, Camara B (1998) Induction and control of chromoplast-specific carotenoid genes by oxidative stress. J Biol Chem 273:30651–30659
Breusegem FV, Vranová E, Dat JF, Inzé D (2001) The role of active oxygen species in plant signal transduction. Plant Sci 161:405–414
Cansev A, Gulen H, Eris A (2011) The activities of catalase and ascorbate peroxidase in olive (Olea europaea L. cv. Gemlik) under low temperature stress. Hortic Environ Biotechnol 52:113–120
Carpenter KL, Keidel TS, Pihl MC, Hughes NM (2014) Support for a photoprotective function of winter leaf reddening in nitrogen-deficient individuals of Lonicera japonica. Molecules 19:17810–17828
Casano LM, Gómez LD, Lascano HR, González CA, Trippi VS (1997) Inactivation and degradation of CuZn-SOD by active oxygen species in wheat chloroplasts exposed to photooxidative stress. Plant Cell Physiol 38:433–440
Chang HL, Kang CY, Lee TM (2013) Hydrogen peroxide production protects Chlamydomonas reinhardtii against light-induced cell death by preventing singlet oxygen accumulation through enhanced carotenoid synthesis. J Plant Physiol 170:976–986
Dhindsa RA, Plumb-Dhindsa P, Thorpe TA (1981) Leaf senescence: correlated with increased permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J Exp Bot 126:93–101
Distelbarth H, Nägele T, Heyer AG (2013) Responses of antioxidant enzymes to cold and high light are not correlated to freezing tolerance in natural accessions of Arabidopsis thaliana. Plant Biol 15:982–990
Domonkos I, Kis M, Gombos Z, Ughy B (2013) Carotenoids, versatile components of oxygenic photosynthesis. Prog Lipid Res 52:539–561
Eskling M, Arvidsson PO, Åkerlund HE (1997) The xanthophyll cycle, its regulation and components. Physiol Plantarum 100:806–816
Fischer BB, Hideg É, Krieger-Liszkay A (2013) Production, detection, and signaling of singlet oxygen in photosynthetic organisms. Antioxid Redox Sign 18:2145–2162
Garg N, Manchanda G (2009) ROS generation in plants: boon or bane? Plant Biosyst 143:81–96
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Han Q, Katahata S, Kakubari Y, Mukai Y (2004) Seasonal changes in the xanthophyll cycle and antioxidants in sun-exposed and shaded parts of the crown of Cryptomeria japonica in relation to rhodoxanthin accumulation during cold acclimation. Tree Physiol 24:609–616
Hormaetxe K, Hernández A, Becerril JM, García-plazaola (2004) Role of red carotenoids in photoprotection during winter acclimation in Buxus sempervirens leaves. Plant Biol 6:325–332
Hormaetxe K, Becerril JM, Fleck I, Pintó M, García-Plazaola JI (2005) Functional role of red (retro)-carotenoids as passive light filters in the leaves of Buxus sempervirens L.: increased protection of photosynthetic tissues? J Exp Bot 56:2629–2636
Hughes NM (2011) Winter leaf reddening in ‘evergreen’ species. New Phytol 190:573–581
Hughes NM, Smith WK (2007) Seasonal photosynthesis and anthocyanin production in ten broadleaf evergreen species. Funct Plant Biol 34:1072–1079
Hughes NM, Carpenter KL, Keidel TS, Miller CN, Waters MN, Smith WK (2014) Photosynthetic costs and benefits of abaxial versus adaxial anthocyanins in Colocasia esculenta ‘Mojito’. Planta 240:971–981
Ida K, Masamoto K, Maoka T, Fujiwara Y, Takeda S, Hasegawa E (1995) The leaves of the common box, Buxus sempervirens (Buxaceae), become red as the level of a red carotenoid, anhydroeschscholtzxanthin, increases. J Plant Res 108:369–376
Iigusa H, Yoshida Y, Hasunuma K (2005) Oxygen and hydrogen peroxide enhance light-induced carotenoid synthesis in Neurospora crassa. FEBS Lett 579:4012–4016
Jiang XR, Peng JG, Guo L, Gao RF, Liu Y (2015) Relationship between winter leaf reddening and carotenoids, reactive oxygen species in Buxus microphylla L. J Beijing For Univ 37:93–99 (in Chinese)
Kaku S, Iwaya-Inoue M, Toki K (1992) Anthocyanin influence on water proton NMR relaxation times and water contents in leaves of evergreen woody plants during the winter. Plant Cell Physiol 33:131–137
Kampfenkel K, Montagu MV, Inzé D (1995) Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Anal Biochem 225:165–167
Koiwa H, Ikeda T, Yoshida Y (1986) Reversal of chromoplasts to chloroplasts in Buxus leaves. Bot Mag Tokyo 99:233–240
Li HS, Sun Q, Zhao SJ, Zhang WH (2000) Assay of malondialdehyde in plants. Experiment principle and technology of plant physiology and biochemistry. Higher Education Press, Beijing, pp 260–261 (in Chinese)
Lichtenthaler HK, Wellburn AR (1983) Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem Soc Trans 11:591–592
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate specific peroxidases in spinach chloroplasts. Plant Cell Physiol 22:867–880
Pandhair V, Sekhon BS (2006) Reactive oxygen species and antioxidants in plants: an overview. J Plant Biochem Biot 15:71–78
Passardi F, Cosio C, Penel C, Dunand C (2005) Peroxidases have more functions than a Swiss army knife. Plant Cell Rep 24:255–265
Peng JG, Liu Y, Guo L, Gao RF (2011) Color change and chlorophyll fluorescence kinetics characteristics of Buxus microphylla leaves during the period of overwintering and regreening. J Beijing For Univ 33:27–34 (in Chinese)
Prochazkova D, Sairam RK, Srivastava GC, Singh DV (2001) Oxidative stress and antioxidant activity as the basis of senescence in maize leaves. Plant Sci 161:765–771
Ramel F, Birtic S, Cuiné S, Triantaphylidès S, Ravanat JL, Havaux M (2012) Chemical quenching of singlet oxygen by carotenoids in plants. Plant Physiol 158:1267–1278
Ruelland E, Vaultier MN, Zachowski A, Hurry V (2009) Cold signaling and cold acclimation in plants. Adv Bot Res 49:35–150
Sergiev I, Alexieva V, Karanov E (1997) Effect of spermine, atrazine and combination between them on some endogenous protective systems and stress markers in plants. Compt Rend Acad Bulg Sci 51:121–124
Shabnam N, Pardha-Saradhi P (2016) Floating and submerged leaves of Potamogeton nodosus exhibit distinct variation in the antioxidant system as an ecophysiological adaptive strategy. Funct Plant Biol 43:346–355
Sofo A, Scopa A, Nuzzaci M, Vitti A (2015) Ascorbate peroxidase and catalase activities and their genetic regulation in plants subjected to drought and salinity stresses. Int J Mol Sci 16:13561–13578
Triantaphylidès C, Havaux M (2009) Singlet oxygen in plants: production, detoxification and signaling. Trends Plant Sci 14:219–228
Velissarios-Phaedon K, Yiannis M (2006) Mesophyll versus epidermal anthocyanins as potential in vivo antioxidants: evidence linking the putative antioxidant role to the proximity of oxy-radical source. J Exp Bot 57:2203–2210
Verhoeven AS, Swanberg A, Thao M, Whiteman J (2005) Seasonal changes in leaf antioxidant systems and xanthophyll cycle characteristics in Taxus x media growing in sun and shade environments. Physiol Plant 123:428–434
Wang AG, Luo GH (1990) Quantitative relation between the reaction of hydroxylamine and superoxide anion radicals in plants. Plant Physiol Commun 6:55–57 (in Chinese)
Wang X, Peng Y, Singer JW, Fessehaie A, Krebs SL, Arora R (2009) Seasonal changes in photosynthesis, antioxidant systems and ELIP expression in a thermonastic and non-thermonastic Rhododendron species: a comparison of photoprotective strategies in overwintering plants. Plant Sci 177:607–617
Wen L, Chen T (2014) Responses of pigmentation and δ13C in Qilian juniper to cold and drought stresses under natural conditions in the Qilian Mountains, China. Acta Physiol Plant 36:2211–2218
Wise RR (1995) Chilling-enhanced photooxidation: the production, action and study of reactive oxygen species produced during chilling in the light. Photosynth Res 45:79–97
Yang Y, Han C, Liu Q, Lin B, Wang J (2008) Effect of drought and low light on growth and enzymatic antioxidant system of Picea asperata seedlings. Acta Physiol Plant 30:433–440
Zhang XZ (1986) Determination of chlorophyll content in plants by acetone-ethanol mixture method. Liaoning Agric Sci 3:26–28 (in Chinese)
Zipor G, Oren-Shamir M (2013) Do vacuolar peroxidases act as plant caretakers? Plant Sci 199–200:41–47
Acknowledgments
This work was supported by the Beijing Municipal Science and Technology Commission (Z08050602970801). The authors are grateful to Beijing Botanical Garden Seedling Center for conservation of the testing plants.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by J Gao.
Xue-ru Jiang and Jin-gen Peng contributed equally to this work.
Rights and permissions
About this article
Cite this article
Jiang, Xr., Peng, Jg., Jia, Mx. et al. Relationship between leaf reddening, ROS and antioxidants in Buxus microphylla during overwintering. Acta Physiol Plant 38, 199 (2016). https://doi.org/10.1007/s11738-016-2206-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-016-2206-7