Abstract
In this work, the effect of sucrose on photosynthetic activity during in vitro culture was studied. Experiments were carried out using uniform somatic embryo-derived germlings of Gentiana kurroo (Royle) confirmed by chromosome counting and flow cytometry technique. Photosynthetic activity was measured by chlorophyll a fluorescence and gas exchange method. The efficiency of photosynthetic apparatus as measured by the ratio F v/F m, Yield and qP (light phase of photosynthesis) was the highest when the medium was supplemented with 0.3% sucrose which well corresponded with plant gas exchange. Taking all data into consideration for the best development of photosynthetic apparatus and the most efficient of net photosynthesis of studied germlings would be medium supplemented with 0.2–0.4% of sucrose.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Genus Gentiana consists of a number of species with some of them (Gentiana lutea and G. kurroo) being confirmed as the main producers of secondary metabolites used for wide spectrum of medicinal purposes.
Gentiana kurroo (Royle), cross-pollinated species, endangered, legally protected by Indian law, important medicinal plant of temperate/subtemperate regions is heavily extracted from its natural habitat (Raina et al. 2003). Among several species and forms of in vitro propagated gentians (Morgan et al. 1997; Momčilović et al. 1997; Hosokawa et al. 1996, 1998; Bach and Pawłowska 2003; Mikuła and Rybczyński 2001; Mikuła et al. 2005), the Gentiana kurroo in vitro cultures distinguished very high morphogenic potential of protoplasts, cells, tissues and explants, originating from its various ontogenic stages (Fiuk et al. 2003). The high morphogenic potential is expressed by very rich somatic embryo production with the application of a combination of two systems of plant growth regulators during long-term cultures (Mikuła and Rybczyński 2001). Micropropagation through somatic embryogenesis leads to the production of clonal germlings and it is used for a number of plant genera including Gentiana ssp (Bach and Pawłowska 2003; Mikuła and Rybczyński 2001; Mikuła et al. 2005). Vegetative propagation requires not only an effective system for germling production but also the system should be helpful and stimulative for the induction of full autotrophy of developing germlings. Despite widely accepted in vitro propagation technology, the plantlet physiology including photosynthesis is not fully understood yet and data available in literature are limited and often controversial (Langford and Wainwright 1987; Van Huylenbroeck and Debergh 1996; Talavera et al. 2005; Fuentes et al. 2005a, b; Lucchesini et al. 2006). Exogenous sugars added routinely to the medium, play a substantial role in culture development and also in acclimatisation of derived plantlets. In vitro leaves act as nutrient storage structures in acclimatisation and sustain the growth of new leaves, ex vitro formed (Borkowska 2000). However, the high exogenous sucrose content in the medium diminishes the photosynthetic ability of cultured shoots/plantlets (Fuentes et al. 2005a, b).
The low photosynthetic capability of cultured plantlets is attributed to poor development of photosynthetic apparatus, low activity of enzymes of CO2 fixation, inadequate gas exchange and stomata deformation (Capellades et al. 1990; Rival et al. 1997; Zenkteler and Borkowska 2002). In the case of constant temperature and constant quality and intensity of light in growth chamber, the chemical composition of the medium plays a key role in the development and activity of photosynthetic apparatus.
During the past years, interest has been growing in the practical application of Chl a fluorescence as a highly sensitive method for the determination of various aspects of photosynthetic apparatus. This approach has been also applied to the study of photosynthesis of in vitro growing germlings (Cappelades et al. 1990; Hdider and Desjardin 1994; Borkowska 2000; Fuentes et al. 2005a). Unfortunately, the literature on analyses of fluorescence induction transient and the process that quench fluorescence is often confusing because of the diverse and duplicated nomenclature. There are many examples in recent publications of different terms being used to define the same fluorescence level or parameter (Rosenqvist and van Kooten 2003; Baker and Rosenqvist 2004). Because of the subject of the paper it is worth saying that Chl a fluorescence method is very informative on the status and activity of photosynthetic apparatus and is widely accepted (Krause and Weis 1991; Lazar 1999, White and Critchley 1999; Maxwell and Johnson 2000; Baker and Rosenqvist 2004; Lichtenthaler et al. 2005), it should be assisted by other methods of measurements of photosynthesis—especially gas exchange. (Grout 1988; Capellades et al. 1991; Rival et al. 1997; Zhu et al. 2004; Fuentes et al. 2005a, b).
In this work, an attempt was made to assess the role of sucrose added to the medium at different concentrations in: (1) growth and development of Gentiana kurroo Royle germlings derived via somatic embryogenesis in vitro culture, and (2) status and activity of photosynthetic apparatus evaluated by Chl a fluorescence technique, and gas exchange.
Material and methods
Plant material and culture conditions
Experiments were carried out on the 5/6-month-old germlings of Gentiana kurroo (Royle) derived from somatic embryos (Fiuk et al. 2003). Somatic embryogenesis was induced in culture of the leaf blade explants. Excised embryos were implanted on conversion medium (MS 1962) supplemented with 0.5 mg dcm-3 GA3 + 1.0 mg dcm-3 Kin + 0.5 mg dcm-3 NAA + 30.0 g dcm-3 sucrose. After 2 months, germlings were subcultured on MS (1962) medium supplemented with sucrose at different concentrations. The experiment was carried out twice. In the first, preliminary experiment, four concentrations of sucrose were used: 0.0, 0, 0.3, 0.6, 1.0%. The second experiment was set up on the base of the result of the first one and several more concentrations (%) of sucrose were applied: 0.0, 0.1, 0.2, 0.3, 0.4, 0.5, 1.0 and 3.0 (standard). Media were solidified by adding 8.0 g dcm-3 of Difco-Bacto agar and pH 5.6 was adjusted before autoclaving. Media were sterilized at 121°C with the pressure of 1 atm for 20 min. The cultures were maintained in the jars, filled with 80 ml of culture medium and kept in growth chamber under white cold fluorescence light. The intensity of PPFD reaching the cultures was, on average, 25 μmol m-2 s-1, with a 16/8 h photoperiod. Germlings were individually cultured one plant per jar. For each sucrose concentration, out of 20 germlings, six or seven most morphologically uniformed were selected and analysed.
Measurements of germlings used
Data on fresh and dry matter, and leaf area were collected. Additionally, plant growth ratio (i.e. final to initial fresh matter) was calculated (Table 1).
Confirmation of uniformity of germlings used
Uniformity of experimental material was assessed by nuclear DNA measurement of green leaf mesophyll cells with the help flow cytometry. Single leaf of each of the randomly selected 22 germlings was excised from shoots and chopped with sharp razor blade in a l plastic Petri dish with 1 ml nucleus-isolation buffer. The buffer was composed of 0.1 M Tris, 2.5 mM MgCl2.6H20, 0.1% Triton X-100, pH = 7.0 supplemented with DAPI (2μg ml-3) for ploidy or propidium iodide (50μg ml-3) and ribonuclease A (50μg ml-3) and was also used for nuclear DNA content estimation. After chopping, the suspension of nucleuses was passed through a 50 μm mesh nylon filter. For each sample 5,000–10,000 nuclei were analyzed directly after preparation using a Partec CCA (Müster, Germany) flow cytometer, equipped with a mercury UV lamp and an argon laser. Analysis was replicated ten times for each germling for DNA content estimation. Histograms were analysed using a DPAC V2.2 computer program. Percentage nuclei containing 2 and 4C were calculated. Total nuclear DNA content was calculated using linear relationship between 2C pick position gentian/gentian ex vitro, on the histogram of fluorescence intensities (Thiem and Śliwińska 2003).
Chromosome number assessment
Mitotic chromosomes were analysed in root tips originating from germlings. Chromosome number was estimated with the application of Feulgen’s methods consisted on the use of Schiff regent. Initially, root tips were pre-treated in the presence of 8-hydroxyquinoline (0.0002M) in room temperature (2 h) and later transferred to refrigerator at 4°C for the next 2 h and fixed in mixture of glacial acetic acid and 96% ethanol (1:3) during 2–4 h. Fixed material was washed in sterile distilled water to remove fixative. For root tip hydrolysis, 5N HCL at 20°C for 50 min. was employed. Schiff’s reagent treatment took about 2 h in darkness and after that plant material was washed with sulphuric water during 30 min. After water washing, plant material was transferred on to basal microscopic glass and squeezed in drop of 45% acetic acid (Fras and Małuszyńska 2003)
Chlorophyll a fluorescence
Chlorophyll a (Chl a) fluorescence was measured using the Photosynthesis Yield Analyzer (MINI-PAM, Waltz, Germany). The analyses of fluorescence induction transient, the process of quenching fluorescence and name of parameters were used according to those elaborated for MINI-PAM and described in Handbook of Operation MINI-PAM 1999. After dark adaptation for minimum 20 min, the fluorescence parameters F v/F m and F o were obtained. Values of F v/F m reflect the potential (maximal) quantum efficiency of PSII and are referred to as an indicator of plant photosynthetic performance, with optimum value 0.830. An alternative expression to F v/F m is F v/F o—more sensitive to changes in photochemical efficiency (Maxwell and Johnson 2000; Skórska 2000). This parameter was calculated on the base of measured by MINI-PAM F o and F m. Minimal fluorescence F o determines energy emission from antenna in competition with energy transferred to RC (reaction centre) of PSII (Lazar 1999; Skórska 2000). The most useful parameter, which measures effective yield of photochemical energy conversion of light-adapted leaves is Yield (Y, Genty-parameter, marked also as ΦPSII). This parameter determines the proportion of the light absorbed by chlorophyll associated with PSII that is used in photochemistry (Maxwell and Johnson 2000; Skórska 2000; Borkowska 2001). Light energy absorbed undergoes one of the three fates: it can be used for photosynthesis (photochemical energy conversion at PSII centres); excess energy can be dissipated as heat (at antenna); or can be re-emitted as light (at RC level). These three processes occur in competition (White and Critchley 1999; Maxwell and Johnson 2000; Zhu at al. 2004). On the basis of these considerations, the so-called ‘quenching coefficients’ qP, NPQ and qN were defined. MINI-PAM system was developed to separate these three types of fluorescence quenching.
The ability of photosynthetic apparatus to acclimate to rapid changes of light intensity was determined with Light Curves (LCs) for chosen sucrose concentrations. The recording of LCs involves nine consecutive measurements, which MINI-PAM performs automatically. Before starting LC recording, a sample was well adapted to a moderate light intensity, which is close to the light intensity experienced by the plant in its natural environment. LC as measured with the MINI-PAM contains different information than the conventional light response curves. LC allows an insight into the physiological flexibility, with which the photosynthetic apparatus adapts to rapid changes of light intensity. Measurements of LCs were started after full darkness and ended at 1,200 (mol m-2 s-1.
The measurements were conducted on the central and apex areas of ‘upper’ leaf blade and were performed on 15–22 leaves (with the exception for LC = 8–10) taken off 6–7 culture vessels and three sucrose concentrations (0.0, 0.2 and 3.0%). Thus, for each sucrose concentration, 30–42 measurements were carried out.
Gas exchange measurements
Photosynthesis net (based on CO2 absorption), stomata resistance and internal CO2 concentration were measured by infrared gas analysis (IRGA) method using Photosynthesis System LICOR 6200, Lincoln, Nebraska. During measurements the PPFD was 90 (mol m-2 s-1 (at measuring chamber level) and the temperature was constant at 22°C. Three uniform germlings (replications) were selected and for each, three measurements were carried out. Thus, presented data are mean ± SE, n = 9.
Statistical analysis
Obtained results, whenever it was possible, were elaborated statistically by analysis of variance and the differences between combinations were estimated by HSD (Honestly Significant Difference) at P = 0.05 of Tukey’ test using Statgraphics Plus 4.1 (Statistical Graphics Corp., USA). Yield was evaluated by function of standard deviation, and estimation of LCs was based on their shape. The estimation of F v/F m was based on its absolute value where 0.800 was a threshold. Presented data are mean ± SE (where indicated), n = 4–7, 9 or 30–42 depending on parameter.
Results
Culture growth and development
Experimental material originated from cultures with regard to the morphological potential studies of leaf blade explants of G. kurroo. Germlings derived from somatic embryos during 5/6 months lasting culture, in majority reached multi-leaf stage of development (Plate 1 A,B). Their root system was not uniform. Number of roots and their elongation growth varied. The ratio of plant growth was different for each of studied sucrose concentration and varied from 2.69 to 4.75 being more effective in the range of 0.2–0.4% (Table 1). The stage of germlings development predisposed each of them for transferring to hardening culture and subsequently into ex vitro conditions (Plate 1 C,D).
All randomly selected 22 studied germlings presented the same DNA content for 2C level (Plate 1E) with stable chromosome number 2n = 26 (Plate1F) as confirmed by flow cytometry measurements of the nuclei DNA content and chromosome number counting.
Photosynthetic status
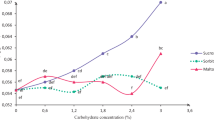
In preliminary experiment, the maximal photochemical activity F v/F m was below the optimal value of 0.800 but evidently higher for the cultures growing on the medium with 0.3% of sucrose (Fig. 1 a). The Yield (Y), effective photochemical activity, was twofold higher for germlings growing on medium containing 0.3% than remaining treatments (Fig. 1b). Also quenching analysis showed that the sucrose concentration of 0.3% was the most favourable to convert light energy into chemical form, represented by coefficient qP (Fig. 2).
Potential (a) and effective photochemical activity (b) of Gentiana kurroo (Royle) germlings cultured on Murashige and Skoog medium (1962) in relation to different sucrose concentration in medium
Relationship among three forms of quenching in relation to different sucrose concentration in Murashige and Skoog medium (1962)
The importance of sucrose in regulation of photosynthetic activity was investigated more detailed in the second experiment, in which sucrose was used in lower and higher doses than 0.3%. The potential efficiency of photosynthetic apparatus, as measured by the ratio F v/F m was similar in wide range of sucrose concentration (0.2–1.0%) with the highest value (nearly 0.800) at 0.3%. The lowest value of F v/F m was recorded for cultures growing without sucrose or on standard sucrose concentration (3.0%) (Fig. 3a). At this sucrose treatment, Y (Fig. 3b) and photochemical quenching (qP) (Table 2) were significantly higher than in all other concentrations. These were associated with the highest values of non-photochemical quenching (qN and NPQ) (Table 2). Furthermore, germlings gas exchange was most intensive for the 0.3% of sucrose in the medium (Fig. 4). Photosynthesis as based on CO2 absorption, in general, well corresponded with stomata resistance (Fig. 5). On the other hand however, no relation was found between rate of photosynthesis and internal CO2 concentrations. The internal CO2 in germling tissues, cultured on both sucrose-free and highest sucrose medium was significantly higher than in all other concentrations (Fig. 6). Besides the described above key parameters for light and dark phases of photosynthesis, additional measurements (F o and F v/F o) showed that the 0.3% sucrose was the most effective for functionality of photosynthetic apparatus (with F o having the lowest and F v/F o the highest values) (Table 3).
Maximal (a) and effective photochemical activity (b) in relation to sucrose concentration in Murashige and Skoog medium (1962)
The shape of LCs for Yield showed that exposure to photoinhibitory irradiance reduced photochemical efficiency. However, germlings cultured on medium containing 0.2% of sucrose had ability for the best acclimation to increasing PAR (Fig. 7a). Distinct differences among treatments were found in quenching parameters. LC representing photochemical quenching was in the highest position in wide range of light irradiance for cultures growing on the medium containing 0.2% sucrose. The lowest adaptive capacity to light stress was found in cultures growing on medium with standard sucrose concentration (Fig. 7b). No distinct effect of sucrose concentration was observed for qN, (Fig. 7c). On the contrary, substantial differences among treatments were recorded for NPQ LCs; the greatest energy dissipation with increasing PPFD was found in germlings grown without sucrose (Fig. 7d), Fig. 8.
The developmental stages of germlings of Gentiana kurroo (Royle) used for the experiments. a Globular stages of somatic embryo regeneration on the upper surface of leaf blade on MS medium supplemented with 2.0 mg dcm3 Dicamba + 0.5 mg dcm3 Thidiazuron, b isolated somatic embryo in the stage for conversion culture, c Germling with stage of various level of leaf blade formation, d Germling with well developed leaf blade used for experiments, f Flow cytometry estimation of 2C DNA of green leaf mesophyll cell nucleus of germling, e Diploid chromosome number (2n = 26) of regenerant
Discussion
Results of this study showed that sucrose level in medium plays an important role in growth and development of flow-cytrometricly uniformed (Kim et al. 2003) Gentiana kurroo germlings derived from somatic embryos (Fiuk et al. 2003).
Under the standard in vitro conditions, chlorophyllous cultures develop low (if any) photosynthetic activity. This is due to suboptimal conditions in culture vessels: very low light intensity, limited gas exchange and high level of exogenous sugar. In several species, photoautotrophic growth could be promoted when the sugar in the medium is reduced or omitted. Results presented in this paper demonstrate that degree of autotrophy of gentian cultures was affected by the external sucrose in the medium. Moderate level of sucrose in medium (0.2–0.4%) was beneficial for development of phototosynthetic capability of gentian germlings as assessed by F v/F m and Y. The 0.800 value for F v/F m (close to optimum) recorded at 0.3% sucrose tells that the photosynthetic apparatus of germlings was well developed. This coincides with the best conversion of light energy to chemical form (qP) and also with intensity of germlings gas exchange. Hdider and Desjardis (1994) also reported significantly higher F v/F m ratios and higher quantum yield when strawberry were cultured on lower sucrose concentration or even sucrose-free medium comparably to 3 and 5%. Similarly to us, they also found that data on F v/F m ratios and quantum yield well corresponded with plantlets gas exchange.
Decrease in photosynthetic activity, due to higher sucrose level in medium, could be, at least partially explained by the feedback inhibition or down regulation of Rubisco, as reported by Hdider and Desjardis (1994) and Rival et al. (1997). Also Fuentes et al. (2005b) concluded that exogenous sucrose level decreases photosynthetic activity of coconut plantlets. At 0.3% sucrose in medium, also the ratios of F v/F m and F v/F o were close to their optimal values, while in all other concentrations they were evidently lower. It must be taken into consideration that F v/F m is relatively inert and changes are detectable when stress is either prolonged or severe, therefore, concluding based only on this ratio can be misleading (for example that the photosynthetic apparatus was undisturbed). Although, F v/F o contains the same basic information, it is more sensitive and reflects quite dynamically (Duran-Serantes et al. 2002; Lichtenthaler et al. 2005).
Although, standard measurements performed under given light intensity (momentary) are widely used, in some cases they should be assisted with more informative LC measurements. Such measurements provide insight into physiological flexibility, with which stressed plant adapts its photosynthetic apparatus to rapid changes of PPFD. Contrary to LC measured using IRGA method the measurement of LCs with Chl a fluorescence technique gives data for each parameter describing photophysical and photochemical fate of absorbed energy (White and Critchley 1999; Rascher et al. 2000; Baker and Rosenqvist 2004). Obtained LCs showed distinct differences in germlings reaction to amount of available carbon in medium.
Throughout the whole range of PAR (from 0 up to 1,220 μmol m-2 s-1), the effective photochemical activity (Y) and photochemical quenching (qP) were highest for germlings cultured on 0.2% sucrose. The patterns of the changes of Y and qP LCs for standard sucrose concentration (3.0%) and sucrose-free medium showed very low “plasticity” of photosynthetic apparatus under our experimental conditions. However, the highest and the lowest position NPQ LCs for sucrose-free and sucrose-3.0% medium, respectively, shows that enhanced tolerance to stress is proportional to less amount of sugar in the medium.
Taking all the above into account, it can be said that for germlings of G. kurroo, most suitable would be medium that contains sucrose in the range of 0.2–0.4%, as it resulted in relatively good development and functioning of photosynthetic apparatus. Both, no sucrose addition and standard sucrose concentration (i.e. 3.0%) are harmful for photosynthetic apparatus and its activity of G. kurroo.
The efficiency of photosynthetic processes, for plants growing under different environmental conditions, not always directly relates to molecular and biomass production levels. The in vitro culture of plantlets can be considered as shade-plants, meaning that their photosynthetic apparatus is adapted to low irradiance (Borkowska 2005). In our experiments, adaptation of photosynthetic apparatus to the environment of in vitro culture was the best at the presence of sucrose concentration 0.3% but it did not correspond with leaf area and biomass accumulation. This might be due to changes in the level of light compensation point resulting with lower germlings mass and area of leaves recorded at the end of subculture and with lack of relation with amount of sucrose added to medium. Despite the fact that the higher photosynthesis of germlings cultured in vitro did not lead to their higher biomass, it can be assumed that it would positively affect germlings during acclimation to and appearance in ex vitro conditions.
References
Bach A, Pawłowska B (2003) Somatic embryogenesis in Gentiana pneumonanthe L. Acta Biologia Cracoviensia, Series Botanica 45:79–86
Baker NR, Rosenqvist E (2004) Application of chlorophyll fluorescence can improve crop production strategies: an examination of future possibilities. J Exp Bot 55(403):1607–1621
Borkowska B (2000) Development and physiological status of micropropagated strawberry plants rooted ex vitro and planted to different substrates. Acta Hort 530:333–338
Borkowska B (2001) Morphological and physiological characteristics of micropropagated strawberry plants rooted in vitro and ex vitro. Scientia Hort 89:195–206
Borkowska B (2005) The photosynthetic activity of plants growing under different environmental conditions. Int J Fruit Sci 5(2):3–16
Capellades M, Fontarnau R, Carulla C, Debergh P (1990) Environment influences anatomy of stomata and epidermal cells in tissue-cultured Rosa multiflora. J Am Soc Hort Sci 115:141–145
Capellades M, Lemeur R, Debergh P (1991) Effects of sucrose on starch accumulation and rate of photosynthesis in rosa cultured in vitro. Plant Cell Tiss Org Cult 25:21–26
Deng R, Donnelly DJ (1993) In vitro hardening of red raspberry by CO2 enrichment and reduced medium sucrose concentration. HortScience 28:1048–1051
Duran-Serantes B, Gonzales L, Reigosa MJ (2002) Comparative physiological effects of three allelochemicals and two herbicides on Dactylis glomerata. Acta Physiol Plantarum 24(4):385–392
Fiuk A, Rajkiewicz M, Rybczyński JJ (2003) Gentiana kurroo (Royle) in tissue culture (summary in English). Biotechnologia 3(62):267–274
Fras A, Małuszyńska M (2003) Regeneration of diploid and tetraploid plants of Arabidopsis thaliana via callus. Acta Biologica Cracoviensia, Ser Botanica 45/2:145–152
Fuentes G, Talavera C, Desjardins Y, Santamaria J (2005a) High irradiance can minimize the negative effect of exogenous sucrose on the photosynthetic capacity of in vitro grown coconut plantlets. Biol Plantarum 49:7–15
Fuentes G, Talavera C, Opereza C, Desjardins Y, Santamaria J (2005b) Exogenous sucrose can decrease in vitro photosynthesis but improve field survival and growth of coconut (Cocos nucifera L.) in vitro plantlets. In Vitro Cell Dev Biol Plant 41:69–76
Grout BWW (1988) Photosynthesis of regenerated germlings in vitro and the stress of transplanting. Acta Hort 230:129–135
Handbook of Operation MINI-PAM (1999) Heinz Walz GmbH, Germany
Hdider C, Desjardins Y (1994) Effects of sucrose on photosynthesis and phosphoenol pyruvate carboxylase activity in in vitro cultured strawberry plantlets. Plant Cell Tiss Org Cult 36:27–33
Hosokava K, Nakano M, Oikawa Y, Yamamura S (1996) Adventitious shoot regeneration from leaf, stem and root explants of commercial cultivars of Gentiana. Plant Cell Repts 15:578–581
Hosokawa K, Oikawa Y, Yamamura S (1998) Mass propagation of ornamental gentian in liquid medium. Plant Cell Repts 17:747–751
Hdider C, Desjardins Y (1994) Effects of sucrose on photosynthesis and phosphoenolpyruvate carboxylase activity on in vitro cultured strawberry plantlets . Plant Cell Tiss Org Cult 36:27–33
Kim K–M, Baenziger PS, Rybczyński JJ, Arumuganathan K (2003) Characterisation of ploidy levels of wheat microspore-derived plants using laser flow cytometry. In Vitro Cell Dev Biol Plant 39:663–668
Krause GH, Weis E (1991) Chlorophyll fluorescence and photosynthesis: the basics. Ann Rev Plant Mol Biol 42:313–349
Langford PJ, Wainwright H (1987) Effects of sucrose concentration on the photosynthetic ability of rose shoots in vitro. Ann Bot 60:633–640
Lazar D (1999) Chlorophyll a fluorescence induction. Bioch Bioph Acta 1412:1–28
Lichtenthaler HK, Buschmann C, Knapp M (2005) How to correctly determine the different fluorescence parameters and the chlorophyll fluorescence decrease ration Rfd of leaves with the PAM fluorymetr. Photosynthetica 43:379–393
Lucchesini M, Monteforti G, Mensuali –Sodi A, Serra G (2006) Leaf ultrastructure, photosynthetic rate and growth of myrtle plantlets under different in vitro culture conditions. Biol Plantarum 50:161–168
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence—a practical guide 51(345):659–668
Mikuła A, Rybczyński JJ (2001) The effect of the preculture treatment and primary explant origin on somatic embryogenesis of Gentiana cruciata (L.), G. pannonica (Scop.) and G. tibetica (King). Acta Physiol Plant 23:15–25
Mikuła A, Fiuk A, Rybczyński JJ (2005) Induction, maintenance and preservation of embryogenic competence of Gentiana cruciata L. cultures. Acta Biologica Cracoviensia Seria Botanica 47:227–236
Momčilović I, Grubišić D, Nešković M (1997) Micropropagation of four Gentiana species (G. lutea, G. cruciata, G. purpurea, G. acaulis). Plant Cell Tiss Org Cult 49:141–144
Morgan ER, Butler RM, Bicknell RA (1997) In vitro propagation of Gentiana cerina and G. corymbifera. NZL J Crop Hortic Sci 25:1–8
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15:473–497
Raina R, Behera MC, Chand R, SharmaY (2003) Reproductive biology of Gentiana kurroo Royle. Curr Sci 85:667–610
Rascher U, Liebig M, Luttge U (2000) Evaluation of instant light-response curves of chlorophyll fluorescence parameters obtained with a portable chlorophyll fluorometer on site in the field. Plant Cell Environ 23:1397–1405
Rival A, Beule T, Lavergne D, Nato A, Havaux M, Puard M (1997) Development of photosynthetic characteristics in oil palm during in vitro micropropagation. J Plant Physiol 150:520–527
Skórska E (2000) UV-B light reaction of selected crops. Habiltation Thesis (in Polish summary in English). Agricultural University of Szczecin.
Talavera C, Contreras F, Espadas F, Fuentes G (2005) Cultivating in vitro coconut palms (Cocos nucifera) under glasshouse conditions with natural light, improves in vitro photosynthesis nursery survival and growth. Plant Cell Tiss Org Cult 83:287–292
Thiem B, Śliwińska E (2003) Flow cytometry of nuclear DNA content in cloudberry (Rubus chamaemorus) in vitro culture. Plant Sci 164:129–134
Van Huylenbroeck JM, Debergh PC (1996) Impact of sugar concentration in vitro on photosynthesis and carbon metabolism during ex vitro acclimatization of Spatthiphyllum germlings. Physiol Plant 96:298–304
White AJ, Critchley C (1999) Rapid light curves: a new fluorescence method to assess the state of the photosynthetic apparatus. Photosyn Res 59:63–72
Zenkteler E, Borkowska B (2002) The photosynthetic status of in vitro strawberry shoots. Acta Hort 567:309–312
Zhu Xin-Guang, Ort DR, Whitmarsh J, Long SP (2004) The slow reversibility of photosystem II thermal energy dissipation on transfer from high to low light may cause large losses in carbon gain by crop canopies: a theoretical analysis. J Exp Bot 55(400):1167–1175
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Z. Krupa.
Rights and permissions
About this article
Cite this article
Rybczyński, J.J., Borkowska, B., Fiuk, A. et al. Effect of sucrose concentration on photosynthetic activity of in vitro cultures Gentiana kurroo (Royle) germlings. Acta Physiol Plant 29, 445–453 (2007). https://doi.org/10.1007/s11738-007-0054-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-007-0054-1