Abstract
Carbon source plays an important role in the induction of embryogenic callus (EC). Therefore it is necessary to determine the effective carbohydrate for improving this process. In this study, different carbohydrates as sucrose, maltose, and sorbitol with different levels (0–30 g/L) were tested in the callus induction of Pyrus communis. At first, in solid culture, calli were divided in relation to their morphological appearance in EC and non-EC. EC was white or yellow with the granular aspect. The morphological study revealed the highest frequency of EC (28.5%) obtained at 30 g/L sucrose. Biochemical analyses showed EC exhibited both soluble sugar and protein high contents, while the starch was at the lowest level. As well as flow cytometry showed EC were genetically similar to donor explants. With transferring the EC into the liquid culture, then, subculturing calli along with 2 ml cell suspension on solid medium led to the globular embryo.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In plant cell and tissue culture, non-zygotic or somatic cells are induced to form embryos by a complex process of cell divisions, eventually developing into complete plants and thus demonstrating the phenomenon of totipotency (Mujib et al. 2005). According to the definition of this phenomenon, all normal living cells possess the potential to regenerate entire organisms (Thorpe and Stasolla 2001). Somatic embryogenesis (SE) is an asexual form of plant propagation in nature that mimics many of the events of sexual reproduction. The control of somatic embryo development involves the temporal expression of the different sets of genes that allow the dividing cell to progress through the different stages of SE (Tang and Newton 2006). Common features for embryogenic cells include small size, dense cytoplasmic contents, large nuclei, prominent enlarged nucleoli, small vacuoles (Pierik 1997). SE is particularly suited for breeders of woody plants who are looking to use biotechnology to shorten the length of conventional breeding programs. Somaclonal variation and genetic transformation preferably require embryogenic cultures that can be maintained for long periods (Druart 1999). Overall, SE is an alternative method for the clonal propagation of intact plants in breeding (Wallin et al. 1995). SE was reported for several woody angiosperms including Prunus persica (Meng and Zhou 1981), Prunus amygdalus (Antonelli 1992), Prunus avium (David et al. 1992), Pyrus communis (Qingrong et al. 2003), Vitis vinifera (Acanda et al. 2013) and Salix viminalis (Gronroos et al. 1989) and also gymnosperm such as Larix × marschlinsii (Von Aderkas et al. 2015). Pear is one of the most important temperate fruit crops. Its species belong to the genus Pyrus, Maloideae subfamily (Pomoideae) in the Rosaceae family (Morgan et al. 1994). Propagation difficulties in Pyrus especially rooting of this genus caused SE to be considered as a suitable method for overcoming these problems. Qingrong et al. (2003) have studied SE through young leaves in Pyrus communis. In their study, somatic embryo regeneration rate was 55.5 and 47.3% in cultivars ‘D’Anjou’ and ‘Red D’Anjou’, respectively. In SE process, the primary response is the production of embryogenic callus. The typical feature of embryogenic callus has been described with respect to histological and biochemical changes. Among histological and cellular ultra-structural studies on the morphological features of embryogenic callus, the study of Shang et al. (2009) reported that the embryogenic callus develops nodule-like structures, which are formed by small, tightly packed, hemispherical cells. The surface of some embryogenic calli was covered with a fibrillar-like structure named extracellular matrix. In contrast, the non-embryogenic calli were covered by oval or sphere cells or small clusters of cells. Swedlund and Locy (1993) studied the effect of carbon source on the production of embryogenic callus in maize; they reported that the presence of sorbitol in medium resulted in the growth of only embryogenic callus whereas medium containing sucrose led to the subsequent growth of both embryogenic and non-embryogenic callus. Furthermore, they detected a significant amount of sorbitol dehydrogenase in embryogenic callus while non-embryogenic callus contained a low level of the aforementioned enzyme. Abbasi et al. (2016) reported that there are differences in biochemical traits of embryogenic and non-embryogenic calli. Their results revealed that somatic embryos exhibited the highest level of total carbohydrate, starch, ascorbic acid, and total free amino acids. In Pyrus communis, Chevreau and Bell (2005) reported that somatic embryos were morphologically abnormal and failed to convert into plantlets. James et al. (1984) in their report stated that Malus adventitious embryos did not respond; hence, there is little information in the literature about the germination of Pyrus somatic embryo and its conversion to plant. The majority of studies have not been successful, so it is necessary to conduct basic studies in this case. Following the studies have been conducted so far on embryogenic callus in different fruit trees, the present study is aimed at investigating the producing embryogenic callus in pear. First, it should be evaluated the induction phase of somatic embryo production. This phase is related to embryogenic callus. Carbohydrates are one of the effective factors on the induction of embryogenic callus. In this investigation, some carbohydrates, with regard to the studied species so far, have been chosen. The chosen carbohydrates include sucrose, sorbitol, and maltose. The sucrose is prevalent carbohydrate in media. Since the final product of photosynthesis is sorbitol in the majority of temperate fruit trees, we chose sorbitol as one of the candidates for carbohydrate in Pyrus communis SE medium. There are many reports which confirm maltose role in SE (Li et al. 1998; Reidiboym-Talleux et al. 1998; Ganesan and Jayabalan 2005). Most studies have focused on the morphological and morphogenic aspects of SE. Although, there are few studies on the morphological aspects of Pyrus communis SE. On the other hand, few studies have considered the biochemical process in SE. Knowledge of the biochemical process can assist in the improvement of SE induction. However, it is not any research has been done on the biochemical studies related to the induction of somatic embryogenesis in Pyrus communis have not been reported so far. In this research, we examined the production of embryogenic calli by testing different carbohydrates and levels added into culture media on cotyledons of Pyrus communis cv. ‘Dar Gazi’ and evaluated biochemical traits under these treatments. Finally, genetic stability of embryogenetic callus was evaluated by flow cytometry.
Materials and Methods
Plant Material
In June, almost 50 days after full bloom, young fruits of Pyrus communis cv. ‘Dar Gazi’ was collected to obtain immature cotyledonary explants from Emam Reza orchard, (latitude 36° 20′ N, longitude 59° 34′ E) Mashhad, Iran. Fruits were washed in running tap water for 45 min, followed by submerging in 1% commercial bleach (containing 5% sodium hypochlorite) with 0.01% Tween 80 for 10 min and finally rinsed in 70% ethanol for 10–15 s. Cleaned fruits were surface sterilized in 0.1% mercuric chloride for 8 min. After washing three times with sterile distilled water, the fruits were surface dried by placing on filter paper and the seeds were dissected out. The excised cotyledon was used for embryogenic callus induction.
Callus Induction
The basal medium containing Murashige and Skoog (1962) salts and its vitamins supplemented with 0.1% casein hydrolysate, 1 mg/L 1‑Naphthalene acetic acid (NAA) and 2 mg/L N6-Benzyladenine (BA) were used and solidified with 0.8% agar. Three carbohydrates, including sucrose, sorbitol, and maltose with different concentrations (0, 6, 12, 18, 24, and 30 g/L) were used. The pH of the media was adjusted to 5.8 prior to autoclaving. Treatments were carried out with three replications (five explants in each replication). All cultures were maintained at 23 ± 1 °C in continuous darkness and sub-cultured onto fresh medium every 30 d.
Biochemical Analyses
For biochemical assays, calli grown 90 days after cultures under different treatments were evaluated for total protein, total sugar, and starch contents.
Total Protein
300 mg fresh weight of callus were macerated at 4 °C with 1 ml extraction buffer (pH 7.0) containing 50 mM sodium phosphate dibasic, 0.2 Mβ-mercaptoethanol, 17.3 mM sodium dodecyl sulphate (SDS), and 1 mM phenyl methyl sulfonyl fluoride (PMSF), and were centrifuged at 4 °C for 20 min at 8300 × g. The supernatant containing total soluble proteins was removed and the pellet stored at −20 °C. Soluble proteins were sedimented at 0 °C by adding two volumes of 100% ethanol into the supernatant and then centrifuged at 4 °C for 20 min at 12,850 × g. (Cangahuala-Inocente et al. 2014). Protein content was determined by the Bradford (1976) method, using bovine serum albumin as standard. The absorbance was read using a CE2502 spectrophotometer at 595 nm.
Total Sugar
The pellet from the protein extraction was macerated using 2 ml methanol: chloroform: water (MCW) (12:5:3) and centrifuged for 10 min at 2000 rpm. The supernatant was recovered, and the pellet was re-extracted using 2 ml MCW. One part chloroform and 1.5 part water were added for every four parts of the supernatant, followed by centrifuging for 10 min at 2000 rpm, from which two phases were obtained. The upper aqueous phase was removed for dosage using 0.2% anthrone. The sugar concentrations were calculated using glucose as standard. The absorbance was read in a CE2502 spectrophotometer at 620 nm (Cangahuala-Inocente et al. 2014).
Total Starch
The pellet from the sugar extraction was ground with 1 ml of 30% perchloric acid and centrifuged for 15 min at 10,000 rpm. The supernatant containing starch was maintained, and the pellets were re-extracted two-folds. The supernatants were combined and the pellets eliminated. The starch concentrations were calculated using glucose as standard. The absorbance was read in a CE2502 spectrophotometer at 620 nm (Cangahuala-Inocente et al. 2009a).
Flow Cytometric Analysis
The nuclei were isolated by chopping approximately 100 mg tissue with a sharp razor blade in 500 μl of a buffer containing 200 mm Tris-HCl, pH 7.5, 4 mm MgCl2, 0.1% Triton X-100 (Cangahuala-Inocente et al. 2014). The homogenate was then filtered through a 50-μM nylon mesh and 50 μg/ml of propidium iodide and 50 μg/ml of RNase (Sigma) were added to the samples (Acanda et al. 2013). After a 10-min incubation period, the samples were analyzed using Flow Cytometer (BD FACSCalibur). Data analysis and figure preparation were performed using ModFit LT 4.1.
Liquid Culture
After doing a biochemical test, the best carbohydrate and concentration was selected, and then 400 mg fresh weight of 3‑month-old embryogenic callus was entered to liquid culture. Liquid culture medium containing MS supplemented with 0.1% casein hydrolysate and 3% sucrose, 500 mg/L glutamine, 1% Polyethylene glycol. The pH of each medium was adjusted to 5.8 with NaOH or HCl prior to autoclaving at 98 kPa and 121 °C. These liquid media were incubated on an orbital rotary shaker (150 rpm) at 24 °C in continuous darkness and maintained by weekly replacement of 75% of the medium with fresh same medium (Acanda et al. 2013).
The Two-phase Culture
After 4 weeks, calli were transferred from liquid culture to solid culture, but along with the 2 ml cell suspension. The solid media containing MS supplemented with 3% sucrose, 0.25% charcoal activated, 0.1 mg/L indole butyric acid, 0.03 mg/L benzyl adenine, and 0.8% agar. The pH of each medium was adjusted to 5.8 with NaOH or HCl prior to autoclaving at 98 kPa and 121 °C. After 4 weeks, globular embryo masses were observed in two-phase culture (Acanda et al. 2013).
Ultrastructural Studies
Scanning electron microscopy (SEM) is carried out based on the protocol described by Saeed and Shahzad (2015). The samples were prefixed in 5% glutaraldehyde buffer (0.1 M phosphate buffer, pH 7.2) for 2 h at room temperature. After dehydration through a graded ethanol series, the samples are dried with a CO2 critical-point drying system and sputtered with gold and observed with a scanning electron microscope.
Statistical Analysis
The experiment was conducted in a completely randomized factorial design with three replications consisted of five explants in each plate. Data were analyzed using Minitab Version 16 software and means were compared with LSD test at the 5% level of confidence.
Results
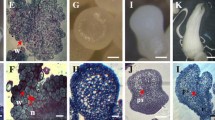
In order to diagnosis embryogenic callus and the selection of the best medium for producing embryogenic callus, the effects of type and concentrations of carbohydrates were evaluated on the frequency of embryogenic callus, protein, sugar and starch contents. The higher levels of sucrose (40–90 g/L) and 16/8 photoperiodic conditions have no any response, therefore, were not presented here. On immature cotyledon explants of Pyrus communis cv. ‘Dar Gazi’ collected was observed the production of embryogenic and non-embryogenic calli. The kind of carbohydrate and concentration significantly (P ≤ 0.01) influenced the embryogenic callus production observed only in sucrose at the levels 24 and 30 g/L (Table 1). Embryogenic callus was white or yellow and with the granular aspect. Non-embryogenic callus was translucent and watery or friable and non-granular observed in other treatments. The results are presented in Table 1. Then callus subjected to biochemical analyses. These analyses showed the carbohydrate type and concentration interaction was a significant effect on protein (Fig. 1) soluble sugar (Fig. 2) and starch (Fig. 3) contents in callus (P < 0.05). These interaction effects represented that the highest protein content (0.070 mg g−1 FW) in callus was related to 3% sucrose and the lowest observed in control (free of carbohydrate), 6, 12 and 30 g/L sorbitol (Fig. 1). 0.397 mg g−1 FW of callus grown under 3% sucrose was soluble sugar, and this content was the highest sugar among of other treatments versus 0.328 mg g−1 FW of callus grown in control, was the lowest sugar (Fig. 2). The starch status in the calli also varied depending on the type of carbohydrate and concentration supplied in the medium. When the medium contained sucrose, the callus starch content reduced. So that, 3% sucrose treatment had the lowest amounts of starch (0.2310 mg g−1 FW) (Fig. 3). Based on Fig. 4, there is the positive relationship between EC frequency and Protein (R2 = 0.8932) (Fig. 4a) as well as, soluble sugar (R2 = 0.6518) (Fig. 4b) while it was observed the negative relationship between EC frequency and starch content in callus (R2 = 0.4695) (Fig. 4c). The flow cytometry analysis showed embryogenic calli were predominantly diploid (2n = 2X = 34) (98.11%). Fig. 5 shows all of the embryogenic calli were diploid similar to donor explants. Therefore, it can be said that medium with formulation presented in this investigation had the lowest variety because of somaclonal variation is not often favorable. The growth of embryogenic callus increased in liquid culture and in the two-phase culture, granular structures gradually developed to the globular stage (Figs. 6 and 7).
The Histograms of relative fluorescence intensity of PI (Propidium Iodide) obtained after the analysis of nuclei isolated from diploid embryogenic callus Pyrus communis L. cv. ‘Dar Gazi’ (a) and diploid Pyrus communis field-grown plant (b). In each panel, peak 1 corresponds to nuclei at the G0/G1 phase
Discussion
The formation of the somatic embryo as zygotic embryo associates with the accumulation of storage materials and the consumption of other materials. It is clear that the accumulation of storage materials such as protein, RNA, polyamines, and some other components as well as the consumption of the cellular ingredients occurs during the embryogenic callus induction process. These metabolisms take place as a result of the cells tend to embryogenesis pathway. Hence, some materials increase and some others decrease. Cangahuala-Inocente et al. (2009b) suggested variations in the levels of protein, starch, amino acids, and polyamines occurred during the induction phase of SE. Blanc et al. (2002) reported that embryogenic induction was associated with high starch content in Hevea brasiliensis callus, but in this experiment contrary to the observation of Blanc et al. (2002), embryogenic callus was successfully achieved from callus with the lowest starch. It is interesting to point out that not only callus with the lowest starch led to embryogenic callus, but the best differentiation of callus to globular embryo was obtained from the callus containing low starch. Martin et al. (2000) stated that the embryogenic callus of Medicago arborea had the highest levels of reducing sugars and the lowest starch levels, these are in agreement with the results of this survey. Nieves et al. (2003) in their study on the biochemical characteristics of embryogenic callus of Saccharum sp. Var CP-5243 reported that this kind of callus had total protein higher than non-embryogenic callus. Mukherjee et al. (2001) reported that the biochemical characteristics of embryogenic callus have more proteins compared with non-embryogenic callus, also in study of Warchoł et al. (2015) was revealed that the embryogenic callus of Cordyline australis with various stages of embryo development had higher level of protein than the non-regenerable one, whereas level of sugars increased only in callus with embryos in cotyledonary stage which are similar to the results of this survey.
Santos Filho et al. (2014) ascribed variation in protein content to the amino acids metabolism. Ng et al. (2016) showed high levels of primary metabolites in general, especially glutamine, arginine, and lysine in embryogenic callus of Boesenbergia rotunda. Blanc et al. (1999) called the type of carbohydrate in the embryogenesis expression medium proposed as an environmental change that had a profound effect on somatic embryo production. Visarada et al. (2002) stated the expression of embryogenesis depends on the interaction between the genotypes and culture conditions. In this study, 3% sucrose had the highest amount of protein, sugar and the lowest starch, on the other hand; these biochemical analyses were consistent with results that obtained from morphological evaluation. In the morphological evaluation, the highest embryogenic callus was related to 3% sucrose. The higher amounts of soluble sugar and protein in embryogenic callus which obtained with the application of 30 g/L sucrose, show 30 g/L sucrose could with sugar accumulation in embryogenic cells caused osmotic stress. On the other hand, 30 g/L sucrose acting as a primary metabolite and is used in the formation of protein carbon skeleton. Therefore, the reason for the positive effect of 30 g/L sucrose refers to providing osmotic stress and its nutritive effect. Based on the results of flow cytometry, it is characterized environmental factors, media formulation, genetic factor, type of explant, and culture condition, the interval of subcultures, were favorite; consequently, embryogenic calli genetic stability was preserved. Therefore, preservation of genetic stability assists in normal embryo formation. With the overall view of the results presented in Figs. 1, 2 and 3, it is revealed the highest amounts of accumulated materials in embryogenic callus were related to sugar. The acceptable reason for reducing of starch content and increasing sugar was related to converting the starch to sugar in embryogenic callus for providing required energy in embryogenesis pathway, for this reason, the starch content reduced and reverse, the amount of sugar increased. Borji et al. (2018) considered the starch primary source of energy for cellular proliferation and growth in Avena sativa. Therefore, the consumption of these starch grain provides energy for somatic embryos development confirming the results of this study.
Conclusion
In the present study, a protocol for embryogenic callus was optimized. Based on the results of this research, embryogenic callus had high soluble sugar and protein contents and the lowest starch. The uses of solid culture as a first step, then followed by liquid culture and two-phase culture, respectively, led to the globular embryo.
References
Abbasi BH, Ali H, Yücesan B, Saeed S, Rehman K, Khan MA (2016) Evaluation of biochemical markers during somatic embryogenesis in Silybum marianum L. 3 Biotech 6(1):1–8
Acanda Y, Prado MJ, Gonzalez MV, Rey M (2013) Somatic embryogenesis from stamen filaments in grapevine (Vitis vinifera L. cv. Mencía): changes in ploidy level and nuclear DNA content. In Vitro Cell Dev Biol Plant 49:276–284
Antonelli M (1992) Regeneration from almond cotyledon: induction of proembryonal masses. Acta Hortic 300:255–259
Blanc G, Lardet L, Martin A, Jacob JL, Carron MP (2002) Differential carbohydrate metabolism conducts morphogenesis in embryogenic callus of Hevea brasiliensis. J Exp Bot 53:1453–1462
Blanc G, Michaux-ferriere N, Teisson C, Lardet L, Carron MP (1999) Effects of carbohydrate addition on the induction of somatic embryogenesis in Hevea brasiliensis. Plant Cell Tissue Organ Cult 59:103–112
Borji M, Bouamama-Gzara B, Chibani F, Teyssier C, Ammar AB, Mliki A, Zekri S, Ghorbel A (2018) Micromorphology, structural and ultrastructural changes during somatic embryogenesis of a Tunisian oat variety (Avena sativa L. var ‘Meliane’). Plant Cell Tissue Organ Cult 132(2):329–342
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cangahuala-Inocente GC, Silveira V, Caprestano CA, Ducroque JPHJ, Floh EIS, Guerra MP (2009b) Biochemical and histological changes during zygotic embryogenesis in Acca sellowiana. Plant Growth Regul 59:103–115
Cangahuala-Inocente GC, Silveira V, Caprestano CA, Floh EIS, Guerra MP (2014) Dynamics of physiological and biochemical changes during somatic embryogenesis of Acca sellowiana. In Vitro Cell Dev Biol Plant 50:166–175
Cangahuala-Inocente GC, Steiner N, Maldonado SB, Guerra MP (2009a) Patterns of protein and carbohydrate accumulation during somatic embryogenesis of Acca sellowiana. Pesqui Agropecu Bras 44:217–224
Chevreau E, Bell R (2005) Pyrus spp. Pear and Cydonia spp Quince. In: Litz RE (ed) Biotechnology of fruit and nut crops. Biotechnology in agriculture. CABI, Wallingford, p 543
David H, Domon JM, Miannay N, Sulmont G, Dargent R, David A (1992) Evidence for early stages of somatic embryo development in a protoplast-derived cell culture of Prunus avium. Physiol Plant 85:301–307
Druart PH (1999) Somatic embryogenesis in Prunus species. In: Jain SM, Gupta PK, Newton RJ (eds) Somatic embryogenesis in woody plants. Kluwer, Dordrecht, pp 215–235
Ganesan M, Jayabalan N (2005) Carbon source dependent somatic embryogenesis and plant regeneration in cotton, Gossypium hirsutum L. cv. SVPR2 through suspension cultures. Indian J Exp Biol 43(10):921–925
Gronroos L, Von Arnold S, Eriksson T (1989) Callus production and somatic embryogenesis from floral explants of basket willow (Salix viminalis L.). J Plant Physiol 134:558–566
James DJ, Passey AJ, Deeming DC (1984) Adventitious embryogenesis and the in vitro culture of apple seed parts. J Plant Physiol 115:217–229
Li XY, Huang FH, Murphy JB, Gbur EE (1998) Polyethylene glycol and maltose enhance somatic embryo maturation in loblolly pine (Pinus taeda L.). In Vitro Cell Dev Biol Plant 34(1):22–26
Martin AB, Cuadrado Y, Guerra H, Gallego P, Hita O, Martin L, Dorado A, Villalobos N (2000) Differences in the contents of total sugars, reducing sugars, starch and sucrose in embryogenic and non-embryogenic calli from Medicago arborea L. Plant Sci 154:955–960
Meng X, Zhou W (1981) Induction of embryoid and production of plantlets in vitro from endosperm of peach. Acta Agric Univ Peking 4:95–98
Morgan DR, Soltis DE, Robertson KR (1994) Systematic and evolutionary implications of rbcL sequence variation in Rosaceae. Am J Bot 81:890–903
Mujib A, Banerjee S, Ghosh PD (2005) Origin, development and structure of somatic embryos in selected bulbous ornamentals: BAP as inducer. In: Mujib A, Samaj J (eds) Somatic embryogenesis. Springer, Berlin, pp 15–25
Mukherjee A, Debata BK, Mukherjee PS, Malik SK (2001) Morphohistobiochemical characteristics of embryogenic and non-embryogenic callus cultures of Sweet potato (Ipomoea batatas L.). Cytobios 106:113–124
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Ng TL, Karim R, Tan YS, Teh HF, Danial AD, Ho LS, Khalid N, Appleton DR, Harikrishna JA (2016) Amino acid and secondary metabolite production in embryogenic and non-embryogenic callus of fingerroot ginger (Boesenbergia rotunda). PLoS ONE 11(6):e156714
Nieves N, Segura-Nieto M, Blanco MA, Sanches A, Gonzalez M, Gonzalez JL, Castillo B (2003) Biochemical characterization of embryogenic and non-embryogenic calluses of sugarcane. In Vitro Cell Dev Biol Plant 39:343–345
Pierik RLM (1997) In vitro culture of higher plants. Kluwer, Dordrecht
Qingrong S, Qingzhong L, Ruihua Z (2003) Somatic embryo genesis from in vitro leaves of pear. Acta Hortic Sin 30:85–86
Reidiboym-Talleux L, Diemer F, Sourdioux M, Chapelain K, Grenier-De march G (1998) Improvement of somatic embryogenesis in wild cherry (Prunus avium). Effect of maltose and ABA supplements. Plant Cell Tiss Organ Cult 55(3):199–209
Saeed T, Shahzad A (2015) High frequency plant regeneration in Indian Siris via cyclic somatic embryogenesis with biochemical, histological and SEM investigations. Ind Crops Prod 76:623–637
Santos Filho PR, Santos BR, Barbosa S, Vieira LR, Freitas NC, Dias DF, Santos MH (2014) Growth curve, biochemical profile and phytochemical analyses in calli obtained from the procambium segments of Bacupari. Braz Arch Biol Technol 57(3):326–333
Shang HH, Liu CL, Zhang CJ, Li FL, Hong WD, Li FG (2009) Histological and ultrastructural observation reveals significant cellular differences between agrobacterium transformed embryogenic and non-embryogenic calli of cotton. J Integr Plant Biol 51:456–465
Swedlund B, Locy RD (1993) Sorbitol as the primary carbon source for the growth of embryogenic callus of maize. Plant Physiol 103:1339–1346
Tang W, Newton RJ (2006) Genome-wide expression analysis of genes involved in somatic embryogenesis. In: Mujib A, Samaj J (eds) Somatic embryogenesis. Springer, Berlin, pp 69–83
Thorpe TA, Stasolla C (2001) Somatic embryogenesis. In: Bhojwani SS, Soh WY (eds) Current trends in the embryology of lingiosperms. Kluwer, Dordrecht, pp 279–336
Visarada KBRS, Sailaja M, Sarma NP (2002) Effect of callus induction media on morphology of embryogenic in rice genotypes. Biol Plant 45:495–502
Von Aderkas P, Teyssier C, Charpentier JP, Gutmann M, Pâques L, Le Metté C, Ader K, Label P, Kong L, Lelu-Walter MA (2015) Effect of light conditions on anatomical and biochemical aspects of somatic and zygotic embryos of hybrid larch (Larix× marschlinsii). Ann Bot 115(4):605–615
Wallin A, Nyman M, Svensson M (1995) Somatic embryogenesis in apple (Malus). In: Jain S, Gupta P, Newton R (eds) Somatic embryogenesis in woody plants. Kluwer, Dordrecht, pp 445–460
Warchoł M, Skrzypek E, Kusibab T, Dubert F (2015) Induction of somatic embryogenesis and biochemical characterization of Cordyline australis (G. Forst.) Endl. ‘Red Star’ callus. Sci Hortic 192:338–345
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
A. Ameri, G.H. Davarynejad, N. Moshtaghi and A. Tehranifar declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Ameri, A., Davarynejad, G.H., Moshtaghi, N. et al. The Role of Carbohydrates on The Induction of Somatic Embryogenesis and The Biochemical State of The Embryogenic Callus in Pyrus communis L. Cv. ‘Dar Gazi’. Erwerbs-Obstbau 62, 411–419 (2020). https://doi.org/10.1007/s10341-020-00518-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10341-020-00518-6