Abstract
Hypericum polyanthemum Klotzsch ex Reichardt, an endemic species of Southern Brazil, was micropropagated on MS medium supplemented with 1.78 μM BAP. Shoot proliferation and rooting was achieved on hormone-free medium and plantlets survived acclimatization. The bioactive compounds: 6-isobutyryl-5,7-dimethoxy-2,2-dimethyl-benzopyran (HP1), 7-hydroxy-6-isobutyryl-5-methoxy-2,2-dimethyl-benzopyran (HP2) and 5-hydroxy-6-isobutyryl-7-methoxy-2,2-dimethyl-benzopyran (HP3) were quantified in the leaves, stems and roots of propagated and acclimatized plantlets and compared with the field-grown plants. The HPLC analysis revealed that the three benzopyrans are accumulated in the aerial parts and the concentration varied with the age of the plant whereas the roots were capable of accumulating only HP3. Greatest yield of HP1 (7.12 mg/g DW) was quantified in the leaves of the acclimatized plantlets, whereas the flowers of the plants from natural habitat displayed higher amounts of HP2 (11.04 mg/g DW) and HP3 (13.99 mg/g DW).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The flora of Southern Brazil is rich in medicinal plants and the unrestricted collection is resulting in an overexploitation of the natural resources without proper replenishment. From the genus Hypericum, widely distributed in temperate regions, approximately 20 species are found and some of them are used in the traditional medicine as anti-inflammatory and wound healing agents (Jiménes 1980; Mentz et al. 1997). In recent years, the pharmaceutical potential increase of Hypericum perforatum with antiviral (Diwu 1995), anticancer (Agostinis et al. 2002) and antidepressive (Chatterjee et al. 1998; Singer et al. 1998) activities were demonstrated and other Hypericum species have also been found to exert pronounced effects on the central nervous system (Öztürk 1997). Some of the Southern Brazilian species demonstrated antidepressive (Daudt et al. 2000), antimicrobial (Dall’Agnol et al. 2003) and antiproliferative activities (Ferraz et al. 2005a). Among them, Hypericum polyanthemum showed a remarkable analgesic activity (Viana et al. 2003) and three main compounds, 6-isobutyryl-5,7-dimethoxy-2,2-dimethyl-benzopyran (HP1), 7-hydroxy-6-isobutyryl-5-methoxy-2,2-dimethyl-benzopyran (HP2) and 5-hydroxy-6-isobutyryl-7-methoxy-2,2-dimethyl-benzopyran (HP3) were isolated from the aerial parts of the plant (Fig. 1) (Ferraz et al. 2001) and the monoamine oxidase and antiproliferative activities evaluated (Gnerre et al. 2001; Ferraz et al. 2005b). Due to the relevant biological properties of H. polyanthemum and its highly restricted distribution (Robson 1990), we chose to standardize a protocol for in vitro propagation of the species and to characterize the distribution of the bioactive substances in the leaves, stems and roots of the micropropagated and acclimatized plantlets and compare with the field-grown plants by reversed-phase HPLC.
Materials and methods
Plant material
Plants of H. polyanthemum Klotzsch ex Reichardt were collected in the city of Caçapava do Sul, state of Rio Grande do Sul, Brazil, in the spring of 2003. Voucher specimens were deposited in the herbarium of the Universidade Federal do Rio Grande do Sul (ICN) (H. polyanthemum, Bordignon 1429). The aerial parts of the plant were thoroughly washed with tap water, surface sterilized in 70% EtOH for 1 min, rinsed twice with sterile-distilled water, immersed in 1.5% sodium hypochlorite for 10 min, and rinsed four times with sterile-distilled water.
Culture conditions
Shoot tips (0.3–0.5 cm long) were excised and the segments (one per flask) were vertically implanted into 175 ml glass jars with 25 ml of MS medium (Murashige and Skoog 1962) containing 30 g l−1 sucrose supplemented with 0.178, 0.89, 1.78 and 4.45 μM of 6-benzylaminopurine (BAP) alone or in combination with 1.074 μM of α-naphthalene-acetic acid (NAA). The pH of the medium was adjusted to 5.8 prior to the addition of 0.5% agar (extra pure, Merck) and autoclaved for 20 min at 121°C. Medium without plant growth regulators was used as a control. The cultures were maintained at 25 ± 1°C with 16 h light/8 h dark photoperiods (light intensity 45 μmol m−2 s−1). Each treatment consisted of 12 explants and was replicated five times. The number of shoots/explant and the callus formation was determined after 6 weeks in vitro and the microshoots that grew on medium with 1.78 μM BAP were excised and placed on control medium without growth regulators and supplemented with BAP alone (0.178 and 0.89 μM) or in combination with 1.074 μM of NAA. After 4 weeks of growth regenerated plantlets were transferred to hormone-free medium.
Acclimatization
The regenerated plantlets cultured on hormone-free medium with well developed roots were washed with distilled water to remove adhering culture medium, transferred to pots containing a sterile mixture of non-fertilized commercial top soil and vermiculite (2:1, v/v) and covered with plastic film. Acclimatizing plants were kept under controlled environmental conditions at 25 ± 5°C, under a 16 h photoperiod provided by cool white fluorescent lamps (approximately 70 μmol m−2 s−1) for 30 days and transferred to a greenhouse (irradiance of 500 μmol m−2 s−1 at plant level, measured in the middle of sunny days without clouds with a sensor Quantum Li-cor®). The plants were watered as needed.
Benzopyrans determination
Leaves, stems, flowers and roots of field-grown plants, from micropropagated and from acclimatized plantlets were freeze dried and 0.05 g DW of each sample were extracted 15 times × 20 min with 5 ml of n-hexane in an ultrasonic bath. The extracts were combined and evaporated to dryness under reduced pressure. The residue was redissolved in 5 ml of HPLC grade methanol and filtered (0.22 μm pore size, Merck). The injection volume was 20 μl.
Liquid chromatographic separations were performed using a Waters 600 pump and a Waters 2487 dual λ absorbance detector. The separations were carried out with an isocratic solvent system (60% CH3CN, 40% H2O) using a Waters Nova-Pack C18 column (4 μm, 3.9 × 150 mm) adapted to a guard column Waters Nova-Pack C18 60A (3.9 × 20 mm). The flow rate was 1 ml min−1; the detector sensitivity 1.0 Aufs and the detection was performed at 270 nm at room temperature.
Constituents of the extracts were identified by comparison with retention times of the authentic samples (HP1, HP2 and HP3) isolated from the aerial parts of H. polyanthemum in natura as described elsewhere (Ferraz et al. 2001) and the identity and purity of the compounds were confirmed by 1H NMR.
For quantitative analysis, peak areas were used to calculate the amount of the benzopyrans present in different plant material and compared to the standards. The identification was made on the basis of their ultraviolet absorption spectra and retention times. The amount of benzopyrans was calculated by using a calibration curve (absorption at 270 nm vs. mass) measured in μg/ml with: HP1-range of 3.90–250, R 2 = 0.9994; HP2-range of 1.95–500, R 2 = 0.9997; HP3-range of 0.97–250, R 2 = 0.9997. Deviation of repeated estimation of same sample was less than 1%.
The detection limits of the different compounds were 1.69956, 0.01530 and 0.17834 μg/ml and the quantification limits were 5.15046, 0.04637 and 0.54043 μg/ml for HP1, HP2 and HP3, respectively. Benzopyrans contents were expressed as mg/g of freeze dried weight of the plants.
Statistical analysis
In all of the experiments, the layout was totally randomized. One-way or Two-way Analysis of Variance (ANOVA) was applied with a critical value of P ≤ 0.05. Data transformation was done as needed to satisfy ANOVA requirements (Sokal and Rohlf 1981).
Results and discussion
Shoot tips of H. polyanthemum were cultured on MS medium supplemented with 0.178, 0.89, 1.78 and 4.45 μM of BAP alone or in combination with 1.074 μM of NAA. Within 6 weeks of inoculation, multiple shoot (approximately 30) and some callus formation was observed on treatment with 1.78 μM of BAP (Fig. 2a) whereas the medium with lower concentration of BAP (0.178 and 0.89 μM) showed three to four shoots per explant and callus formation (data not shown). Interestingly, the increase of BAP concentration promoted multiplication rate up to 1.78 μM after which the rate was reduced. Explants cultured on control medium without growth regulators did not respond satisfactorily. Similar effect of BAP on shoot development in H. perforatum and H. foliosum has been reported previously (Cellárová et al. 1992; Moura 1998). The absence of rooting in the presence of NAA, as mentioned by the same studies, was also confirmed for H. polyanthemum.
Shootlets were further cultured on MS medium with BAP alone and combined with NAA. With 0.178 μM BAP, the auxine source did not seem to be necessary for rooting (Table 1), whereas with 0.89 μM BAP and 1.074 μM NAA no rooting was observed, suggesting the need of a compensatory effect from a higher exogenous auxine source (Tamas 1987).
For culture multiplication, shoots grown with 0.178 μM BAP were transferred to a hormone-free medium and regenerated plantlets (Fig. 2b) presented 7.22 ± 0.97 shoots and 7.88 ± 0.99 roots after four weeks of culture, not differing significantly from that on medium with 0.178 μM BAP.
The acclimatization stage was successfully performed by transplanting the plantlets to a potting mixture of soil and vermiculite (2:1) under greenhouse conditions (Fig. 2c). The formation of both shoots and roots on the same medium eliminates one step of medium transfer in the propagation of this species, thus reducing the total time and cost required for plant regeneration. Similar results were reported for H. perforatum (Cellárová et al. 1992; McCoy and Camper 2002) and H. brasiliense (Cardoso and Oliveira 1996).
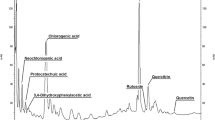
Regenerated plantlets of H. polyanthemum were evaluated in order to study the production of secondary metabolites. The n-hexane extract was analysed by HPLC using a isocratic system composed of acetonitrile and water (60:40% v/v). The assay was shown to be suitable for the characterization of HP1, HP2 and HP3 and a short running time (HP3 20.6 min; HP2 12.4 min; HP1 8.6 min) was achieved. These bioactive substances were quantified in the different parts of the plants as shown in Table 2. The concentration of the benzopyrans decreased in the in vitro micropropagated plantlets. Nevertheless, in the acclimatized plants the HP1 and HP3 increased approximately threefold in relation to the field-grown plants. HP1 appeared as the main compound in the leaves of the acclimatized plant (Table 2) and surpassed the total amount of the same compound quantified in the field-grown plant at flowering stage, where the main compound quantified was HP3. Similar results were found for the secondary metabolites quantified in regenerated organs of H. perforatum (Pasqua et al. 2003). HP1 and HP2 have not been detected in the roots of all analysed plants. These compounds are present, instead in the leaves, stems and flowers, suggesting a role in the chemical defense of the plant against insects and environmental factors which have been found to alter the concentration of secondary metabolites in Hypericum species (Briskin and Gawienowski 2001; Abreu and Mazzafera 2005) as have harvest date and season (Southwell and Bourke 2001). On the other hand, HP3 was found in all parts of the plants (Table 2). However, its distribution pattern depends on the developmental stage, with higher concentrations being found in the leaves and roots of the acclimatized plantlets. Moreover, it is necessary that plantlets reach an advanced stage of growth to obtain the biosynthesis of all benzopyrans. Furthermore, the difference in benzopyrans concentration may be due to the light intensity with which plantlets were grown (45 and 500 μmol m−2 s−1 for in vitro and acclimatized plantlets, respectively). Thus, the higher contents of the compounds in field-grown plants may be related to the high light intensity (2000 μmol m−2 s−1) to which the plants were exposed. This effect was previously shown for H. perforatum (Briskin and Gawienowski 2001) where a linear relationship was observed between the light intensity and the number of dark glands associated with hypericin contents.
The knowledge of the biosynthesis of HP1, HP2 and HP3 is not available. Nevertheless, an experiment carried out with Ageratum conyzoides (Asteraceae), demonstrated that in the benzopyrans precocene I and II, structurally similar to the above mentioned benzopyrans, the aromatic ring arises from the polyketide route and condenses with dimethyallyl pyrophosphate arising from the mevalonic acid; the subsequent hydroxylation followed by methylation of the aromatic ring yielded the precocenes (Vyas and Mulchandani 1980). Considering that this is an usual route for benzopyrans we could infer that the benzopyrans in H. polyanthemum are formed by a similar pathway, with HP1 being biosynthesized by the methylation of HP2 and/or HP3 by the action of methyl-transferases.
The higher concentration of benzopyrans was found in the flowers of the field-grown plants except for HP1 which was detected in higher concentration in the leaves of the acclimatized plants. HP2 and HP3 were isolated as yellow crystals, contributing to the intense yellow colour of the flowers, while HP1 was colourless.
Flowers of Hypericum have not been extensively investigated, with the presence of flavonoids (quercetin, quercitrin, isoquercitrin, hyperoside, biapigenin), phloroglucinol derivatives (hyperforin) and hypericin being reported for some species (Takel’ova et al. 2000; Maggi et al. 2004). The report that flowers of in vitro grown plants and field-grown plants of H. perforatum have comparable concentrations of medicinal secondary metabolites (Murch et al. 2002) will lead us to investigate the benzopyrans content in different flower ontogenesis phases of in vitro propagated H. polyanthemum plants. Furthermore, based on our results, optimal conditions for large biomass yield and production of secondary metabolites from H. polyanthemum will be further investigated.
Abbreviations
- BAP:
-
6-Benzylaminopurine
- HP1:
-
6-Isobutyryl-5,7-dimethoxy-2,2-dimethyl-benzopyran
- HP2:
-
7-Hydroxy-6-isobutyryl-5-methoxy-2,2-dimethyl-benzopyran
- HP3:
-
5-Hydroxy-6-isobutyryl-7-methoxy-2,2-dimethyl-benzopyran
- HPLC:
-
High performance liquid chromatography
- IAA:
-
Indole-3-acetic acid
- DW:
-
Dry weight
- MS:
-
Murashige and Skoog
- NAA:
-
α-Naphthalene-acetic acid
References
Abreu IN, Mazzafera P (2005) Effect of water and temperature stress on the content of active constituents of Hypericum brasiliense Choisy. Plant Physiol Biochem 43:241–248
Agostinis PA, Vantieghem A, Merlevede W, de Witte P (2002) Hypericin in cancer treatment: more light on the way. Int J Biochem Cell Biol 34:221–241
Briskin DP, Gawienowski MC (2001) Differential effects of light and nitrogen on production of hypericins and leaf glands in Hypericum perforatum. Plant Physiol Biochem 39:1075–1081
Cardoso MA, Oliveira DE (1996) Tissue culture of Hypericum brasiliense Choisy: shoot multiplication and callus induction. Plant Cell Tiss Org Cult 44:91–94
Cellárová E, Kimáková K, Brutovská R (1992) Multiple shoot formation and phenotypic changes of R0 regenerants in Hypericum perforatum L. Acta Biotechnol 12:445–452
Chatterjee SS, Nöldner M, Koch E, Erdelmeier E (1998) Antidepressant activity of Hypericum perforatum and hyperforin: the neglected possibility. Pharmacopsychiatry 31:7–15
Dall’Agnol R, Ferraz A, Bernardi AP, Albring D, Nor C, Sarmento L, Lamb L, Hass M, von Poser GL, Schapoval EES (2003) Antimicrobial activity of some Hypericum species. Phytomedicine 10:141–147
Daudt R, Poser GLV, Staats C, Neves G, Rates SMK (2000) Screening for the antidepressant activity of some species of Hypericum from South Brazil. Phytother Res 14:344–346
Diwu Z (1995) Novel therapeutic and diagonistic applications of hypocrellins and hypericins. Photochem Photobiol 61:529–539
Ferraz ABF, Bordignon S, Staats C, Schripsema J, von Poser GL (2001) Benzopyrans from Hypericum polyanthemun. Phytochemistry 57:1227–1230
Ferraz ABF, Grivicich I, von Poser GL, Faria DH, Kayser GB, Schwartsmann G, Henriques AT, da Rocha AB (2005a) Antitumor activity of three benzopyrans isolated from Hypericum polyanthemun. Fitoterapia 76:210–215
Ferraz ABF, Faria DH, Benneti MN, da Rocha AB, Schwartsmann G, Henriques AT, von Poser GL (2005b) Screening for antiproliferative activity of six southern Brazilian species of Hypericum. Phytomedicine 12:112–115
Gnerre C, von Poser GL, Ferraz AC, Viana A, Testa B, Rates SMK (2001) Monoamine oxidase inhibitory activity of some Hypericum species native to South Brazil. J Pharm Pharmacol 53:1273–1279
Jiménes CR (1980) Hipericaceaes. Flora Ilustrada Catarinense. Editora IOESC, Florianópolis, pp 3–32
Magii F, Ferretti G, Pocceshi N, Menghini L, Ricciutelli M (2004) Morphological, histochemical and phytochemical investigation of the genus Hypericum of the Central Italy. Fitoterapia 75:702–711
McCoy JA, Camper ND (2002) Development of a micropropagation protocol for St. John’s Wort (Hypericum perforatum L.). HortScience 37:978–980
Mentz LA, Lutzemberger LC, Schenkel EP (1997) Da flora medicinal do Rio Grande do Sul: notas sobre a obra literária de D’Ávila. Cad Farm 13:25–47
Moura M (1998) Conservation of Hypericum foliosum Aiton, na endemic Azorean species, by micropropagation. In Vitro Cell Dev Biol Plant 34:244–248
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Murch SJ, Rupasinghe HPV, Saxena PK (2002) An in vitro and hydroponic growing system for hypericin, pseudohypericin, and hyperforin production of St. John’s wort (Hypericum perforatum L. cv New Stem). Planta Med 68:1108–1112
Öztürk Y (1997) Testing the antidepressant effects of Hypericum species on animal models. Pharmacopsychiatry 40:125–128
Pasqua G, Avato P, Monacelli B, Santamaria AR, Argentieri MP (2003) Metabolites in cell suspension cultures, calli, and in vitro regenerated organs of Hypericum perforatum cv. Topas Plant Sci 165:977–982
Robson NKB. (1990) Studies in the genus Hypericum L. (Guttiferae) 8. Sections 29. Brathys (part 2) and 30. Trigynobrathys. Bull Br Mus 20:1–151
Singer AS, Wonnemann M, Muller W (1998) Hyperforin, a major antidepressant constituent of St. John’s Wort, inhibits serotonin uptake by elevating free intracellular Na+1. J Pharmacol Exp Ther 290:1363–1368
Sokal RR, Rohlf FJ (1981) Biometry. Freeman, San Francisco, p 859
Southwell IA, Bourke CA (2001) Seasonal variation in hypericin content of Hypericum perforatum L. (St. John’s wort). Phytochemistry 56:437–441
Takel’ova D, Repcak M, Zemkova E, Toth J (2000) Quantitative changes in dianthrones, hyperforin and flavonoids in the flower ontogenesis of Hypericum perforatum. Planta Med 66:778–780
Tamas LA (1987) Hormonal regulation of apical dominance. In: Davies PJ (ed) Plant hormones and their role in plant growth and development. Martinus Nijhoff, Dordrecht, pp 393–410
Viana AF, Heckler AP, Fenner R, Rates SMK (2003) Analgesic activity of Hypericum caprifoliatum and Hypericum polyanthemum (Guttiferae). Braz J Med Biol Res 36:631–634
Vyas AV, Mulchandani NB (1980) Biosynthesis of precocenes –I and –II, anti-juvenile hormones. Phytochemistry 19:2597–2598
Acknowledgments
This study was supported by CNPq, FAPERGS and Propesq-UFRGS. The authors thank CBIM/UFRGS for the HPLC facilities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Lewak.
Rights and permissions
About this article
Cite this article
Bernardi, A.P.M., Maurmann, N., Rech, S.B. et al. Benzopyrans in Hypericum polyanthemum Klotzsch ex Reichardt cultured in vitro. Acta Physiol Plant 29, 165–170 (2007). https://doi.org/10.1007/s11738-006-0021-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-006-0021-2