Abstract
Although there’s growing information about the long-term oncological effects of robotic surgery for rectal cancer, the procedure is still relatively new. This study aimed to assess the long-term oncological results of total mesorectal excision (TME) performed laparoscopically versus robotically in the setting of rectal cancer. Restrospective analysis of a prospectively maintained database. A total of 489 laparoscopic (L-TME) and 183 robotic total mesorectal excisions (R-TME) were carried out by a single surgeon between 2013 and 2023. The groups were compared in terms of perioperative and long-term oncological outcomes. In the R-TME and L-TME groups, male sex predominated (75.4% and 57.3%, respectively), although the robotic group was significantly greater (p = 0.008). There was no conversion in R-TME group, whereas three (0.6%) converted to open surgery in L-TME group. The R-TME group had a statistically significant higher number of distal rectal tumors (85%) compared to the L-TME group (54.6%). Only three (1.7%) patients in the R-TME group received abdomineperineal resection (APR); in contrast, 25 (5%) patients in the L-TME group received APR (p < 0.001). For R-TME, the mean follow-up was 70.7 months (range 18–138) and for L-TME, it was 60 months (range 14–140). Frequency of completed mesorectum was significantly greater in R-TME group (98.9% vs 94.2%, p < 0.001). The 5 year overall survival rates for R-TME and L-TME groups were 89.6% and 88.7%, respectively. The 5 year disease-free survival for R-TME and L-TME groups were 84.1% and 81.1%, respectively. The local recurrences rates were 7.6% and 6.3%, respectively in R-TME and L-TME groups (p = 0.274). R-TME is characterized by no conversion and improved mesorectal integrity. R-TME had longer operation time. The long-term oncological outcomes were comparable between groups.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since its introduction in the 1980s, Total mesorectal excision (TME) has become a widely adopted technique, significantly enhancing local control post-surgery and remaining a pivotal aspect of rectal cancer surgery [1]. The standard of surgical therapy now includes laparoscopic TME (L-TME), which is universally approved and carried out. L-TME has been shown in many randomized trials to have superior short-term oncological safety, less postoperative pain, improved cosmetic outcomes, faster recovery, and shorter hospital stay than open surgery. Nevertheless, laparoscopic surgery has numerous potential drawbacks, despite its clinical efficacy as a surgical approach for rectal cancer [2, 3]. These include the high conversion rate to open surgery and the limited visibility due to unstable cameras [4, 5]. Notably, due to the limitations of the limited pelvic anatomy, the laparoscopic approch poses technical obstacles in the successful treatment of low rectal cancer, particularly in cases with bulky tumors and in male or overweight patients undergoing neoadjuvant chemoradiotherapy (NCRT).
Theoretically, in an attempt to overcome the drawbacks of laparoscopy, robotic surgery was developed and could provide benefits in limited spaces as for pelvic surgery. Robotic approach has been proving to be safe and to provide good short- and mid-term oncological outcomes for rectal cancer surgery [6]. Besides improved dexterity, the robotic platform provides robust, readily adjustable three-dimensional imaging and a stable platform for surgeons, minimizing fatigue and improving comfort during rectal dissection [7].
This study aims to compare the perioperative and long-term oncological outcomes of L-TME and robotic TME (R-TME).
Methods
Study population
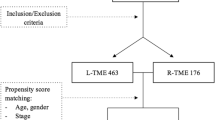
This retrospective study evaluated a consecutive series of patients with rectal cancer undergoing either L-TME or R-TME surgery between January 2013 and June 2023. Data were extracted from a prospectively maintained surgical database. This study was conducted in compliance with the Principles of the Declaration of Helsinki. Informed consent was obtained from each patient.
Primary aim was to report perioperative and long-term oncological outcomes.
Inclusion criteria were:(1) rectal adenocarcinoma; (2) tumor location (15 cm from the anal verge, AV); (3) clinical TNM stage I–III; (4) absence of distant metastases and synchronous tumors; (5) sphincter-saving TME or abdominoperineal resection (APR); 6) elective surgery; (7) curative intent surgery.
Exclusion criteria were: (1) sigmoid resections; (2) Hartmann’s resection; (3) local excision; (4) sistemic metastases; (5) palliative surgery.
The boundary between the anal mucosa and the perineal skin was defined as AV. The distance between the tumor's caudal border and the anal margin was measured through rectal magnetic resonance imaging (MRI), flexible sigmoidoscopy, and digital rectal examination.
Preoperative protocol
Preoperative staging included chest X-ray, carcinoembryonic antigen (CEA) levels, flexible or virtual colonoscopy, torax/abdominopelvic CT and MRI of the pelvic phased array.
NCRT included: (1) Long-term neoadjuvant chemoradiotherapy with concurrent chemotherapy using capecitabine (1600 mg/m2 daily during radiotherapy), with a total dose of 50 Gy given in 25 fractions over 5 weeks. Surgery was undertaken within the timeframe of 4 to 8 weeks following the conclusion of chemoradiotherapy; (2) total neoadjuvant treatment consists of NCRT followed by consolidation chemotherapy. Consolidation chemotherapy involved concurrent administration of oxaliplatin (85 mg/m2) and leucovorin (400 mg/m2) biweekly, followed by a bolus injection of 5-fluorouracil (5-FU) (400 mg/m2), and then infusion with 5-FU (2400 mg/m2). Following June 2018, the consolidation regimen was changed to include capecitabine (1000 mg/m2) twice daily on days 1–14 in addition to oxaliplatin (130 mg/m2) on day one; this was repeated every three weeks for eight cycles; (3) A total of 25 Gy was given in five fractions over 5–7 days to the short-course neoadjuvant radiotherapy group. Within one to four weeks following the end of neoadjuvant radiation therapy, surgery was performed. When there was no chance of positive lateral margins, patients were given short-course radiotherapy.
Without the addition of oral antibiotics, all patients underwent oral mechanical bowel preparation. One hour prior to the start of the surgical incision, a single 400 mg intravenous dosage of ciprofloxacin was given.
Surgical technique
A single surgeon (O.A.), with over 20 years of experience in oncological colorectal surgery, performed all surgical procedures. The surgeries were performed at Maslak Acibadem Hospital in Istanbul, Turkey and Liv Hospital, Department of General Surgery, Istanbul, Turkey. Every patient had a detailed discussion regarding the surgical strategy, which was decided by the surgeon after considering the patient's preferences and the surgeon's assessment. Before the procedure, every patient was given thorough instructions detailing the benefits and drawbacks of both laparoscopic and robotic approach. Both laparoscopic and robotic surgical procedures were previously described in detail [8,9,10].
The Da Vinci Si® or Xi® platform (Intuitive Surgical, Inc., Sunnyvale, CA, USA) was used in the R-TME group.
Any unintended laparotomy that occurred during surgery, regardless of the extent of the incision, was referred to as a conversion.
Pathological report
During the data evaluation process, pathological staging was modified to conform to the 8th edition staging criteria of the American Joint Committee on Cancer (AJCC) [11]. Tumor size, number of harvested lymph nodes, distal resection margin (DRM), circumferential resection margin (CRM), and integrity of the mesorectum were all determined by a thorough analysis of all pathology specimens. The criteria established by Quirke et al. [12] were used to assess the mesorectum’s quality after dissection [12]. Positive CRM was described as direct tumor expansion that occurred within 1 mm of the resected specimen's radial, non-peritonealized surface [13].
Postoperative outcomes and follow-up
The Clavien–Dindo classification was used to assess postoperative complications, which are defined as unfavorable events that occur within 30 days following surgery [14]. Anastomotic leaks were diagnosed and treated in accordance with the protocols set by the International study group of rectal cancer [15].
The follow-up measures included thoraco-abdominopelvic CT assessment every year, colonic examinations at the first, third, fifth, and tenth years after surgery, and monitoring of oncological markers (CEA, carbohydrate antigen 19–9) every 3 months.
Disease-free survival (DFS) was estimated from the date of surgery to the tumor’s recurrence, whereas overall survival (OS) was calculated from the date of surgery to the date of death or the last follow-up. Histological confirmation or radiographic identification of expanding lesions established the diagnosis of recurrence. The STROBE declaration principles for cohort studies were followed by this study [16].
Statistical analysis
Data were analyzed using IBM SPSS Statistics for Windows, version 27 (IBM Corp., Armonk, NY, USA) and 95% confidence level was used. For categorical (qualitative) variables, frequency and percentage (n (%)), for numerical (quantitative) variables mean, standard deviation (mean ± ss), minimum, maximum statistics are given. In the pairwise comparison of the measurements determined in the study according to the groups, LSD/Mann Whitney One-way ANOVA/Kruskal Wallis test was used for intergroup comparisons and Chi-square test was used for the relationships between grouped variables. Chi-square test is a test technique used to determine the relationships between grouped variables. LSD/Mann Whitney is a test technique used to compare two independent groups in terms of a quantitative variable. One-way ANOVA/Kruskal Wallis is a test technique used to compare more than two independent groups (k = group > 2) in terms of a quantitative variable.
Result
Clinical outcomes
A total of 672 patients with rectal cancer were enrolled into two groups: L-TME group (n = 489, 72.7%) and R-TME (n = 183, 37.3%). Table 1 shows patients’ clinical and demographic details. Patients in the R-TME group were younger (54.2 ± 12.8 and 57.2 ± 12.8 years for R-TME and L-TME, respectively) (p = 0.007). Male patients made up to 54% of the L-TME group and 75.4% of the R-TME group, respectively. This was statistically significant (p < 0.001).
Mid-low rectal cancers were significantly more common in the R-TME group (85% and 54.6% for R-TME and L-TME, respectively) (p < 0.001).
Perioperative outcomes
Table 2 lists the perioperative outcomes. No cases of 30 day death was reported. With R-TME, the operation time was substantially longer (158.9 ± 39.1 vs. 116.1 ± 31.7 min, p < 0.001). In terms of estimated blood loss and postoperative hospital stay, there was no significant difference seen. While the majority of patients in both groups were cT3 and node-positive, there was a greater percentage of cT4 patients in the L-TME group (14.8% in R-TME and 24% in L-TME, respectively). Neoadjuvant therapy was administered to most patients however, the R-TME group received more (84.1% and 52.3% for R-TME and L-TME, respectively; p < 0.001).
The R-TME group's patients received more low anterior resections (54.6% vs. 45.6% p = 0.187) and intersphincteric resections (35% vs. 18.2% p < 0.001) because they mostly had distal tumors. Conversely, the L-TME group had a higher frequency of proximal tumors, and there was a marked predilection for anterior resections (8.7% vs. 31.1%, p < 0.001) in this group.
Three patients (1.7%) had APR in the R-TME group, while twenty-five patients (5%) received APR in the L-TME group (p < 0.001). Three patients (0.6%) in the L-TME group had open conversions, whereas there were none in the R-TME group.
Histopathological outcomes
Even though R-TME had a larger mean number of excised lymph nodes, the difference between the two groups was not statistically significant (24.6 ± 9.3 vs 23.5 ± 10.8, p = 0.125). The mean distance of the resection margin (DRM) was longer in L-TME (15.2 ± 14.7 mm and 20.8 ± 15.6 mm for R-TME and L-TME, respectively). Eight (4.3%) patients in R-TME and 16 (3.2%) patients in L-TME had positive CRM involvement (p = 0.236).
A statistical difference was reported between R-TME and L-TME regarding specimen quality (98.9% vs. 94.2%, p < 0.001). There were no notable variations in other histopathology results (Table 3).
Postoperative complications
Table 4 shows that the overall complication rate was 17.5% for L-TME and 16.8% for R-TME (p = 0.132). Symptomatic anastomotic leakage occurred in 16 (8.7%) R-TME patients and 47 (9.6%) L-TME patients. All instances of anastomotic leakage were managed conservatively, involving the maintenance of pelvic drainage until clinical resolution of the infection, with selective use of endo-sponge drainage.
Rectovaginal fistula occurred in five patients in the L-TME group and two patients in the R-TME group.
Two patients, one with R-TME and the other with L-TME, experienced an immediate onset of colonic ischemia. On the third postoperative day, these patients underwent an urgent laparoscopic colon resection and re-anastomosis.
According to Clavien–Dindo’s classification, there was no significant difference in postoperative morbidity.
Oncological outcomes
The mean follow-up were 70.7 months (interval 18–138) for R–TME and 60 months (interval 14–140) for L–TME. R-TME and L-TME had 5 year OS rates of 89.6% and 88.7%, respectively. The 5 year DFS rates for R-TME and L-TME were 81.1% and 84.1%. The Kaplan–Meier curves for OS and DFS are reported in Fig. 1.
Local recurrence occurred in 14 (7.6%) of the R-TME group and in 31 (6.3%) of the L-TME group of patients (p = 0.276). During the follow-up period, 20 patients (10.9%) in the R-TME group and 62 patients (12.6%) in the L-TME group were found to have distant metastases. However, there was no significant statistical difference between the groups.
Discussion
The purpose of this study was to compare the perioperative and long-term oncological results of L-TME and R-TME patients with rectal cancer. R-TME was associated with a statistically significant prolonged operation time compared to L-TME (p < 0.001). There were three (0.6%) conversions to open surgery in L-TME, and none in the R-TME group. The conversion rates in previously published randomized controlled trials showed different rates: COLOR II (16%), CLASICC (16%), ACOSOG Z6051 (11%), ALaCaRT (9%), and COREAN (1.2%) [2, 3, 17,18,19]. The present study showed that the conversion rate (0.6%) is significantly lower than the aforementioned studies. This may be explained by the standardization of techniques, surgeons' experiences, and the learning curve [20, 21]. A study conducted between 2005 and 2012 by the same group showed a 6.5% conversion rate in L-TME [20]. We believe that conversion to open surgery had a negative effect on the survival of rectal cancer patients. In fact, the 10-year results of the aforementioned study showed inferior outcomes in the converted group (DFS 50.0% vs. 78.3% and OS 46.7% vs. 68.5%) [8].
There was no statistically significant difference in the CRM involvement rate between L-TME and R-TME (p = 0.236). Completeness of mesorectum was higher in R-TME with statistical difference (98.9% vs. 94.2%; p < 0.001). The mesorectal completeness and margin-free CRM are key histopathologic factors for achieving improved local recurrences rates [12, 13]. The overall CRM positivity rates were 19% in a Dutch study, 13% in the CLASSİC trial, 10.0% COLOR II, 5.7% ROLARR, and 4.1% COREAN trial [2, 3, 17, 22, 23]. In the current investigation the R-TME and L-TME showed 3.2% and 4.3% CRM involvement, respectively. There was no statistically significant difference in the CRM involvement between the laparoscopic and robotic low-anterior resection groups in the prospective study conducted by Baik et al. [24].
The quality of specimen is considered as a parameter for the evaluation of prognosis. The majority of studies consistently showed that preventing incomplete mesorectum might greatly enhance OS and dramatically lower the chance of recurrence following TME surgery. Classification of a mesorectal specimen was described by Quirke et al. in the CR07 trial, which included 1,156 patients [12]. Surgery was considered complete in 604 (52%), near complete in 398 (34%), and incomplete in 154 (13%). Additionally, they observed that the three-year local recurrence for each of the three groups was 4% for complete, 7% for nearly complete, and 13% for incomplete (p = 0.0039), indicating the greatest possible association between the local recurrence and mesorectal integrity. According to our research, the robotic group had more mesorectal completeness (98.9% vs. 94.2% p < 0.001). This outcome is in line with our earlier results [9, 10, 25,26,27].
Comparable 5-year OS and DFS rates were seen in the L-TME and R-TME groups (p = 0.741 and p = 0.368., respectively). This study revealed similar results in terms of local recurrence rate (p = 0.274). The distant metastasis rates were also comparable in L-TME and R-TME (p = 0.27). In contrast to the present study, our previous research, which compared L-TME and R-TME procedures in male patients with mid-low rectal tumors, revealed substantial differences [26]. Specifically, the R-TME group of male patients exhibited significantly superior rates of improved local recurrence, as well as mesorectal completeness. These studies once again demonstrated that robotic approach can potentially overcome some technical challenges related to pelvic anatomical differences between sexes compared to laparoscopy. However, our two previously published studies indicated that robotic approach did not provide any benefit in female patients with rectal cancer when compared with laparoscopy [28, 29].
There is a possible bias in the present study between the two groups: (1) since robotic surgery was introduced in our study in 2013, laparoscopic cases performed during the same period were included for comparison; (2) the robotic approach was preferred in male patients; (3) due to the high cost of robotics in Turkey, we opted for the robotic approach in technically difficult cases (narrow pelvis, obese patients).
This study has several limitations. Firstly, it constitutes a retrospective analysis of data collected prospectively from a single surgeon's experience. Secondly, the number of R-TME procedures is lower than L-TME. To validate our results, a large prospective randomized study is necessary. Thirdly, we did not evaluate comparative functional outcomes. Lastly, cost-effectiveness was not investigated in this study. However, the strength of this study lies in its long median follow-up and standardized single surgeon setting, coupled with extensive colorectal expertise.
Conclusion
This study reports a higher rate of mesorectal integrity, no conversions to open and/or abdominoperineal resection, and a lower postoperative complication rate in patients undergoing R-TME. R-TME was associated with a longer operative time. Both R-TME and L-TME showed good OS, DFS, and local recurrence rates.
Data availability
The datasets used or analysed during the current study are available from the corresponding author upon reasonable request.
References
Heald RJ, Ryall RD (1986) Recurrence and survival after total mesorectal excision for rectal cancer. Lancet 1:1479–1482
Guillou PJ, Quirke P, Thorpe H et al (2005) Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet 365(9472):1718–1726
Kang SB, Park JW, Jeong SY, Nam BH, Choi HS, Kim DW et al (2010) Open versus laparoscopic surgery for mid or low rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): short-term outcomes of an open-label randomised controlled trial. Lancet Oncol 11(7):637–645
Huang M-J, Liang J-L, Wang H et al (2011) Laparoscopic assisted versus open surgery for rectal cancer: a meta-analysis of randomized controlled trials on oncologic adequacy of resection and long-term oncologic outcomes. Int J Colorectal Dis 26:415–421
Ng SSM, Lee JFY, Yiu RYC et al (2014) Long-term oncologic outcomes of laparoscopic versus open surgery for rectal cancer: a pooled analysis of 3 randomized controlled trials. Ann Surg 259:139–147
Park EJ, Cho MS, Baek SJ et al (2015) Long-term oncologic outcomes of robotic low anterior resection for rectal cancer: a comparative study with laparoscopic surgery. Ann Surg 261:129–137
Corcione F, Esposito C, Cuccurullo D et al (2005) Advantages and limits of robot-assisted laparoscopic surgery: preliminary experience. Surg Endosc 19:117–119
Bademler S, Koza KB, Ucuncu MZ, Tokmak H, Bakir B, Oral EN et al (2019) Standardized laparoscopic sphincter-preserving total mesorectal excision for rectal cancer: median of 10 years’ long-term oncologic outcome in 217 unselected consecutive patients. Surg Laparosc Endosc Percutan Tech 29:354–361
Aliyev V, Tokmak H, Goksel S, Guven K, Bakir B, Kay H et al (2020) Robotic sphincter-saving total mesorectal excision for rectal cancer treatment: a single-surgeon experience in 103 consecutive male patients. Surg Technol Int 37:93–98
Aliyev V, Tokmak H, Goksel S, Meric S, Acar S, Kaya H et al (2020) The long-term oncological outcomes of the 140 robotic sphincter-saving total mesorectal excision for rectal cancer: a single surgeon experience. J Robot Surg 14:655–661
Weiser MR (2018) AJCC 8th edition: colorectal cancer. Ann Surg Oncol 25:1454–1455
Quirke P, Steele R, Monson J, Grieve R, Khanna S, Couture J et al (2009) Efect of the plane of surgery achieved on local recurrence in patients with operable rectal cancer: a prospective study using data from the MRC CR07 and NCIC-CTG CO16 randomised clinical trial. Lancet 373:821–828
Nagtegaal ID, Marijnen CA, Kranenbarg EK, van de Velde CJ, van Krieken JH, Pathology Review C et al (2002) Circumferential margin involvement is still an important predictor of local recurrence in rectal carcinoma: not one millimeter but two millimeters is the limit. Am J Surg Pathol 26:350–357
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213
Rahbari NN, Weitz J, Hohenberger W, Heald RJ, Moran B, Ulrich A et al (2010) Definition and grading of anastomotic leakage following anterior resection of the rectum: a proposal by the international study group of rectal cancer. Surgery 147:339–351
von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP et al (2008) The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 61:344–349
van der Pas MH, Haglind E, Cuesta MA et al (2013) Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol. https://doi.org/10.1016/S1470-2045(13)70016-0
Fleshman J, Branda ME, Sargent DJ, Boller AM, George VV, Abbas MA et al (2019) Disease-free survival and local recurrence for laparoscopic resection compared with open resection of stage II to III rectal cancer: follow-up results of the ACOSOG Z6051 randomized controlled trial. Ann Surg 269(4):589–595. https://doi.org/10.1097/SLA.0000000000003002
Stevenson ARL, Solomon MJ, Lumley JW et al (2015) Efect of laparoscopic-assisted resection vs open resection on pathological outcomes in rectal cancer: the AlaCart randomized clinical trial. JAMA 314(13):1356–1363
Asoglu O, Kunduz E, Rahmi Serin K et al (2014) Standardized laparoscopic sphincter preserving total mesorectal excision for rectal cancer: long-term oncologic outcome in 217 unselected consecutive patients. Surg Laparosc Endosc Percutan Tech 24:145–152
Aliyev V, Arslan NC, Goksoy B, Guven K, Goksel S, Asoglu O (2022) Is robotic da vinci xi® superior to the da vinci si® for sphincter-preserving total mesorectal excision? Outcomes in 150 mid-low rectal cancer patients. J Robot Surg 16(6):1339–1346
Kapiteijn E, Putter H, van de Velde CJ, Cooperative investigators of the Dutch ColoRectal Cancer Group (2002) Impact of the introduction and training of total mesorectal excision on recurrence and survival in rectal cancer in the Netherlands. Br J Surg 89:1142–1149
Neil C, Helen M, Julie C et al (2018) Exploring and adjusting for potential learning effects in ROLARR: a randomised controlled trial comparing robotic-assisted vs. standard laparoscopic surgery for rectal cancer resection. Trials. https://doi.org/10.1186/s13063-018-2726-0
Baik SH, Kwon HY, Kim JS et al (2009) Robotic versus laparoscopic low anterior resection of rectal cancer: short-term outcome of a prospective comparative study. Ann Surg Oncol 16:1480–1487
Asoglu O, Tokmak H, Bakir B, Aliyev V, Saglam S, Iscan Y, Bademler S, Meric S (2020) Robotic versus laparoscopic sphincter-saving total mesorectal excision for mid or low rectal cancer in male patients after neoadjuvant chemoradiation therapy: comparison of long-term outcomes. J Robot Surg 14(3):393–399
Aliyev V, Goksel S, Bakir B, Guven K, Asoglu O (2021) Sphincter-saving robotic total mesorectal excision provides better mesorectal specimen and good oncological local control compared with laparoscopic total mesorectal excision in male patients with mid-low rectal cancer. Surg Technol Int 20(38):160–166. https://doi.org/10.52198/21.STI.38.CR1391. (PMID: 33537982)
Aliyev V, Piozzi GN, Bulut A, Guven K, Bakir B, Saglam S, Goksel S, Asoglu O (2022) Robotic vs. laparoscopic intersphincteric resection for low rectal cancer: a case matched study reporting a median of 7 year long-term oncological and functional outcomes. Updates Surg. https://doi.org/10.1007/s13304-022-01396-1
Aliyev V, Piozzi GN, Huseynov E, Mustafayev TZ, Kayku V, Goksel S, Asoglu O (2023) Robotic male and laparoscopic female sphincter-preserving total mesorectal excision of mid-low rectal cancer share similar specimen quality, complication rates and long-term oncological outcomes. J Robot Surg 17(4):1637–1644. https://doi.org/10.1007/s11701-023-01558-2. (Epub 2023 Mar 21 PMID: 36943657)
Aliyev V, Piozzi GN, Shadmanov N, Guven K, Bakır B, Goksel S, Asoglu O (2023) Robotic and laparoscopic sphincter-saving resections have similar peri-operative, oncological and functional outcomes in female patients with rectal cancer. Updates Surg 75(8):2201–2209. https://doi.org/10.1007/s13304-023-01686-2. (Epub 2023 Nov 13 PMID: 37955804)
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Conceptualization: Oktar Asoglu, Niyaz Shadmanov, Vusal Aliyev; methodology: Oktar Asoglu; Niyaz Shadmanov; formal analysis and investigation: Niyaz Shadmanov, Oktar Asoglu; writing—original draft preparation: Niyaz Shadmanov, Vusal Aliyev, Oktar Asoglu; writing—review and editing: Niyaz Shadmanov, Vusal Aliyev, Guglielmo Niccolò Piozzi, Barıs Bakır, Suha Goksel, Oktar Asoglu; supervision: Oktar Asogl.
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
This study was conducted in compliance with the Principles of the Declaration of Helsinki. Institutional Review Board (IRB) approval was waived following the retrospective nature of the study.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shadmanov, N., Aliyev, V., Piozzi, G.N. et al. Perioperative and long-term oncological outcomes of robotic versus laparoscopic total mesorectal excision: a retrospective study of 672 patients. J Robotic Surg 18, 144 (2024). https://doi.org/10.1007/s11701-024-01922-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11701-024-01922-w