Abstract
This study analyses the utility of right colectomy as a learning procedure at the beginning of a robotic surgical program. The hypothesis is that right colectomy contains all the technical steps necessary to acquire basic abilities in robotics surgery. The first 23 consecutive robotic right colectomy performed at the beginning of a robotic program were analysed. All surgical times were recorded in the operating room and second checked on a dedicated video-database. Specific robotic times were analysed using CUSUM method to evaluate the learning curve. CUSUM-derived learning phases were compared. Fourteen males and nine females with a mean age of 68.7 (46–84) underwent robotic right colectomy. The mean overall time was 265.3 min (180–320 min), docking time was 7 min (5–12 min), console time was 205.9 min (145–260 min), and anastomotic time was 43.6 (25–60 min). CUSUM analyses identified two learning phases: “starting phase” and “consolidation phase”. Interphase comparison confirmed the significant (p < 0.05) differences between the two phases. Robotic technology facilitates the training process in minimally invasive colorectal surgery. At the beginning of the learning curve, right colectomy could represent a complete procedure to be proficient in robotic colorectal surgery.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Minimally invasive approach in colorectal disease is proven to be safe and feasible [1]. Benefits of a minimally invasive access have been validated since its first application: reduced post-operative pain with short recovery, fewer complications as ileus or pneumonia, low rate of incisional hernias, and better aesthetic results [2, 3]. Recent studies demonstrate that it has also adequate short-term oncological outcomes compared to the open technique [4]. Technological development added new tools and instrumentations, which help surgeon during the procedure. Despite this evolution, minimally invasive approach did not have a widespread adoption even in high-level healthcare countries [5,6,7]. One of the reasons that could explain this phenomenon is the long period needed to complete the learning curve [8, 9]. Long and rigid instruments, bi-dimensional vision, and poor ergonomics are known as the major drawbacks of laparoscopy [10]. After obtaining a leading role in radical prostatectomy, robotic technology has gained popularity in the field of colorectal surgery, allowing increased dexterity while improving operative view [11, 12]. High-resolution 3D camera, magnification, and stable vision combined with flexible instruments created a new standard in minimally invasive colorectal surgery [13, 14]. Robotic surgical program implementation is a long and difficult process that involves different figures [15, 16]. Despite it seems to be intuitive, robotic procedure requires the acquisition of some basic concepts to obtain successful results. Although operating room setup, trocar placement and patient positioning seem slightly similar to the laparoscopic approach, they represent an important key point for the robotic operations and could be affected by time-consuming errors. Manipulation of console master and 3D vision coordination needs to be practiced in real surgical fields to maximize the benefits of tremors filter and stability of the robotic arms. For this reason, several training courses have been created all over the world using simulation and dedicated laboratories [17]. Once the surgical team approaches the clinical setting, a useful starting procedure is needed to consolidate the skills acquired during simulation phase. Increased costs for the robotic surgery might conditionate the choice of a basic procedure to begin the learning curve. Our robotic program started performing right colectomy. We support the idea that right colectomy’s surgical steps represent a complete procedural pattern to understand robotic technology even for an experienced minimally invasive surgeon. Moreover, learning phase in minimally invasive colorectal operations is shortened by robotic applications in contrast to the traditional laparoscopy. The goal of the present study is to analyse our initial experience in robotic colorectal surgery to strengthen the role of right colectomy as the procedure of choice to begin.
Materials and methods
Study design
All the operations were performed at the "Santissima Annunziata Teaching Hospital"-“G. d’ Annunzio University” of Chieti-Pescara. The robotic program in abdominal surgery started in March 2013 after a planned training period. First 23 consecutive patients inserted in the study sample underwent robotic right colectomy for colon cancer. All the procedures were executed by the same surgeon who counts a full-personal experience in colorectal laparoscopic procedures. Demographic, intraoperative, and post-operative data were prospectively collected in a dedicated database. The pathology evaluation as tumour grading, distal margin, and number of harvested lymph nodes was also recorded.
Surgery
The surgical robot da Vinci Si® (Intuitive Surgical Inc., Sunnyvale, CA, USA) was used in every single procedure. Operative room setup and trocar positioning were the same previously described (Fig. 1) [18, 19]. The three-arm technique was adopted. Two robotic 8-mm trocars and two 12-mm trocars were used. The 3D HD camera equipped with a 30° downward lens was positioned in the 12-mm trocar. Air-Seal System® (SurgiQuest Inc., Milford, CT, USA) was used to obtain a stable pneumoperitoneum. The electro-cautery hook was mounted on the right arm and a Maryland bipolar forceps® (Intuitive Surgical Inc., Sunnyvale, CA, USA) was linked to the left one. Large needle-driver® (Intuitive Surgical Inc., Sunnyvale, CA, USA) was additionally used to complete the intervention. During laparoscopic exploration, no other devices were generally used to avoid costs raising. Right colectomy with a medial-to-lateral approach and side-to-side mechanical isoperistaltic ileo-colic intracorporeal anastomosis was executed. The anastomosis was performed using a linear 45-mm stapler and a self-anchoring surgical suture device.
Image recording and time analysis
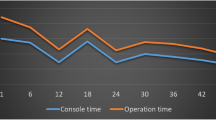
A Blackmagic Design® H.264 Pro Recorder (Blackmagic Design, Freemont, CA, USA) was connected to the surgical robot. All videos were recorded using an Apple® MacBook Pro (Apple Inc., Cupertino, CA, USA) and the Blackmagic® Media Express software (Blackmagic Design, Freemont, CA, USA). The operative times, manually recorded in the operating room, were expressed, as shown in Fig. 2. Overall time (OT) started from the introduction of the first trocar until the completion of skin sutures. Robotic time (RT) was divided into docking time (DT) and console time (CT). The DT represented the time needed to complete the robot positioning on the surgical field, while the CT indicated the time occupied by the robotic surgical procedure. The difference between OT and RT gave as results the laparoscopic time (LT) plus end time (ET). LT was necessary for trocar insertion, cavity exploration, and surgical field preparation. The last step recorded was the ET, which included specimen extraction, control of bleeding, and closure of the surgical wounds. Every video was independently revised by two authors to confirm the CT and to extrapolate the anastomotic time (AT). The AT was defined as the time necessary to perform the intracorporeal anastomosis. It included the enterotomies that enabled mechanical stapling and the closure of the intestinal wound.
CUSUM analysis
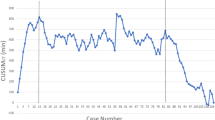
CT and AT were evaluated using the cumulative sum (CUSUM) analysis. CUSUM chart is currently adopted in industrial process control and it is a tool to quantitatively detect small shifts in efficiency of production. CUSUM is the sum of differences between surgical time of a single procedure and the mean of all the surgical times. It has been adopted to evaluate the variation in the CT and AT as an indirect index of skill assessment. CUSUM was calculated for each intervention using Stata SE 10.1 software (StataCorp, College Station, TX, USA) and plotted against case number of our series. Different phases were detected according to the inversion of the CUSUM values on the graph. Interphase parameters were compared using t test analysis. A p value <0.05 was considered significant.
Results
Starting from the adoption of the robotic system in March 2013, 25 patients underwent robotic right colectomy for malignant disease in the first year. The first two patients of our series are not considered in the study, because they were performed under the supervision of a tutor. Our population was composed of 14 males and 9 females, and the mean age was 68.7 (46–84). The mean value of BMI score was 26.24 kg/m2 (19–33 kg/m2), and according to the ASA score system, we had 14 grade II patients (61%) and 9 grade III patients (39%). The mean OT time was 265.3 min (180–320 min), DT was 7 min (5–12 min), and CT was 205.9 min (145–260 min). Mean LT was 17.3 min (10–26 min) and FT was 35 min (20–39 min). Mean AT was 43.6 (25–60 min). All the surgical times were plotted in chronological order to understand the linear evolution of our experience (Fig. 3). One conversion occurred for uncontrolled bleeding and this was the only intraoperative complication reported. The estimated mean blood loss was 87.5 ml (5–400 ml). Only three post-operative complications occurred. According to Clavien–Dindo classification, we had two grade 1 and only one grade 2 complications. The mean hospital stay was 7 days (5–14 days). The mean length of the specimen was 25.3 cm (12.5–45.8 cm) and the mean number of harvested lymph nodes was 19.9 (12–31). All the tumours resulted as adenocarcinoma with R0 marginal status. Nine patients had positive metastatic lymph nodes. We had 5, 9, and 9 patients classified as stage I, II, and III, respectively. We had no hospital readmission. Furthermore, CUSUM-CT and CUSUM-AT results were rendered, as shown in Fig. 4. According to this analysis, two different phases in the learning process were identified. The first 13 cases were named “acquisition phase” and the last 10 patients were defined “consolidation phase”. No significant differences in age, sex, BMI, and ASA score were noted in the two groups belonging to the different phases. Oncological adequacy was confirmed in both phases. The learning curve assessment was underlined by the comparison of OT, CT, and AT which resulted statistically significant between the two phases, as shown in Table 1.
Discussion
The diffusion of the da Vinci® platform and its unique technological potential has met the interest of surgeons all over the world. However, the elevated costs and a closed market are slowing the real clinical validation of this technology [20]. Most of the published studies are stuck trying to make a comparison with laparoscopy, neglecting the potential of this innovation [21, 22]. Actually robotic surgery advantages seem to be poorly understood by the world surgical community and are waiting for a substantial scientific support [23]. In the last years, several efforts were made to validate this technology through well-designed studies [24, 25]. A strictly surgical issue is the acquisition of specific skills that makes robotic technology a new way to perform minimally invasive operations. Wishner et al. [26, 27] stated that 35–50 interventions were required to be proficient in laparoscopic colectomy and these data remain unchanged so far. The intuitiveness of robotic can facilitate the acquisition of basic skills and speed up the learning curve for a colorectal minimally invasive surgeon. Several reports described a clear reduction of number of cases needed to assess proficiency with the adoption of robotic technology [28, 29]. Ahlering et al. underlined the possibility to perform a safe minimally invasive procedure with the robotic platform even for an unexperienced laparoscopic surgeon after only 12 cases [30]. One of the real advantages is the availability of a virtual reality training model [31]. Nevertheless, at the beginning of clinical setting, the surgeon should gradually adopt the new skills starting from basic tasks and continuing with more challenging surgical operations. Usually, in our teaching division, residents and young surgeons are trained in minimally invasive surgery performing laparoscopic cholecystectomy as well as laparoscopic appendectomy. However, higher cost of robotics compared to the traditional laparoscopy does not support the use of the robot to approach those simple operations even for a small number of cases or educational purpose. At the beginning of our robotic experience, considering economic issues and learning needs, we selected right colectomy as training operation. In the present study, that reviews our initial 23 right colon resection using the da Vinci® surgical robot, we tried to identify the key points that can address this procedure as a complete training model for a starting robotic program in colorectal surgery. Indeed, the robotic right colectomy: (1) is a full-robotic procedure; (2) has a linear docking by the right side; (3) is a multi-quadrant surgery; (4) requires at least one vascular dissection with lymphadenectomy; and (5) includes double intestinal resection and intracorporeal anastomosis. Since right colectomy is executed by an integral robotic approach, no other devices are used for laparoscopic exploration. Moreover, the first surgeon sits at the console all the time favouring the familiarity with the surgical controls. Become confident with the robotic console master in a real surgical field is important even for a well-trained laparoscopic surgeon that has to learn a new way to move instruments in the abdomen without excessive strength, avoiding unpleasant bleeding or laceration during tissue manipulation. The docking phase is considered a crucial step for the correct execution of a robotic operation, especially when it comprises multiple ports and angled directions. In some reported series with robot-assisted technology, docking time strongly affected the overall surgical times that resulted longer compared to laparoscopy [32]. The docking time of our series reached a plateau after few cases demonstrating how a standard trocar setup and the position of the patient cart in right colectomy are simple and easy to manage. Learning to dock the robot as well as establish a cohesive surgical team are the keys for achieving best results especially in time-consuming operations. The assistant surgeon could use this phase to understand the docking angles and built the knowledge of the entire team (nurses and anaesthesiologist). The surgical field of right colon included at least two abdominal quadrants [33]. A frequent use of robotic camera implies a continuous alignment of the instruments through the clutch pedal after every change of view. The appropriate use of the camera reducing excessive numbers of movements is important particularly for surgeons that are not skilled in laparoscopic camera control. The time of vascular colic pedicles dissection along with lymphadenectomy and bowel mobilizations represents a useful exercise to understand the Endowrist® technology for both senior and novice surgeons. The lack of the tactile feedback is compensated by the efficiency of the endoscope reaching a semantic translation from the visual inputs to the tactile sensations. Tridimensional view and adequate exposure mediate the perception of the tissue conformation and guarantee a better evaluation of the spatial configuration or tissue warp. Oncological results, in terms of R0 resection, length of specimen, and lymph nodes retrieval, were in line with the previous published articles. If compared to the traditional approach, robotic right colectomy has similar short-term outcomes but longer operative time and higher costs [34]. However, laparoscopic right colectomy often comprises an extracorporeal anastomosis due to the high difficulty of minimally invasive intracorporeal sutures [35]. Higher range of movements of robotic instruments offers the stability and the accuracy to complete the anastomosis in a minimally invasive way even for a not well-trained laparoscopic surgeon. Clinical advantages of intracorporeal anastomosis as less post-operative complications and better surgical long-term results have widely been reported [36, 37]. To support the educational value of right colectomy as starting procedure for a robotic program, we recorded and analysed all the operative times. Many authors have used linear times to express their learning curve eventually using arbitrarily selected splitting phases. D’Annibale et al. reported their first experience with 50 robotic right colectomy [38]. Despite this work represents one of the first works on robotic right colectomy, the authors did not identify a precise number of procedures to complete a learning curve. Similarly, in our series, chronological plots propose a gradual decrease of total and console surgical times. Considering that no criteria for patients’ selection were used, we can assume the reduction of the operative times as indirect values of proficiency [39, 40]. To overcome the limit of a small-size study sample, we applied CUSUM methods to the linear time results, because it is not influenced by the number of procedures and it can detect small shifts in the data series. CUSUM was introduced into the medical language during the seventies and provides a more reliable evaluation of the learning process analysing the starting point and the evolution of the performance during the work-period [41]. Many authors have already performed analysis on the learning curve in robotic surgery using CUSUM method. Bokhary et al. [28] applied the CUSUM method on a mixed sample of benign and malignant disease, generating a wide range of surgical console times. In a recently published paper by Foo et al. CUSUM was adopted to assess the learning curve of the robot-assisted anterior resection for a non-experienced surgeon [42]. The results are similar to the previous studies [43]. Mid-rectal tumours with moderate difficulty were selected in the initial phase, while more complicated cases were faced in the second part. Only Yamaguchi included from the beginning hard cases excluding patient sampling bias from the CUSUM analysis [44]. Our study analyses the learning phase with robotic right colectomy to underline its value as training procedure even for an experienced laparoscopic surgeon. The acquisition of specific abilities is mainly expressed by improvement in CT or in specific tasks like AT. The sums of the distance of a single time from the mean produce a chart that sets the zenith at the case number 13 of the CUSUM-CT. Indeed, the inflection point of the curve draws the end of the “acquisition phase” and the beginning of a “consolidation phase”. In the first phase, basic robotic gestures are quicker acquired thanks to the easiness of the robotic controls that resembling the movements of the hands in the surgical field. Surgeon, once proficient, can consolidate the skills and use them to face more challenging procedures. CUSUM-CT chart outlines a typical course of a training span with an irregular plot that represents the attempts to reach efficacy. CUSUM-AT reveals a trend that is almost overlaid to the CUSUM-CT. This result suggests that basic skills improvement makes the operating surgeon more proficient in difficult steps. Fine skills assessment is mainly expressed by the decrease of anastomotic time, which represents a distinctive factor for the evaluation of the learning progress. Intracorporeal anastomosis is easier and faster with the application of the robotic technology. In our laparoscopic experience, ileo-colic anastomosis is always extracorporeal. Literature reports a learning curve for laparoscopic right colectomy of 55 cases [8]. However, the intent of this analysis is not a comparison with the laparoscopic technique, in particular for the difference in a distinctive element like anastomosis [45]. Ileo-colic intracorporeal anastomosis represents a peculiar element in the minimally invasive abdominal surgery scenario and the possibility to perform suturing movements during robotic right colectomy supports the utility of this operation at the beginning of learning curve. Identification of the two phases is supported by CUSUM ability to understand changes along the procedural times of consecutive cases and to indicate more accurately the point where operative times changed and signed improvement. Accurate evaluation was made to underline the differences between the two periods. CT and AT reduction was confirmed in the interphase comparison and directly affects the concomitant OT. The comparison did not report variations in the characteristics of study population, because difficult cases were faced since the beginning of the learning curve. Considering stable LT and FT, we can assess that the improvements in the difficult tasks are the main reason for total time breakdown. We used laparoscopy exclusively to assess the resectability and to prepare the robot docking. Time spent for peritoneal exploration and trocars placement does not affect significantly the overall time. Residents are involved in all the robotic operations. They are in charge for the specimen extraction and closure of the laparotomy. Training purpose explains the length of this phase that can affect surgical times.
Conclusions
Robotic surgery requires the acquisition of specific abilities and skills. We tried to identify five key points that constitute right colectomy as useful operation for surgeons that are facing this new approach. Intracorporeal anastomosis represents a unique opportunity. Reduction of the robotic and anastomotic times at the CUSUM analysis supports the positive value of right colectomy as learning procedure and the ability of robotic technology to speed up the learning curve in minimally invasive surgery. Once proficiency has been reached, surgeons may progress to more challenging operation where the robotic platform could express major effectiveness.
References
Jayne DG, Thorpe HC, Copeland J, Quirke P, Brown JM, Guillou PJ (2010) Five-year follow-up of the Medical Research Council CLASICC trial of laparoscopically assisted versus open surgery for colorectal cancer. Br J Surg 97(11):1638–1645. doi:10.1002/bjs.7160
Lacy AM, García-Valdecasas JC, Delgado S et al (2002) Laparoscopy-assisted colectomy versus open colectomy for treatment of non-metastatic colon cancer: a randomised trial. Lancet 359(9325):2224–2229. doi:10.1016/s0140-6736(02)09290-5
Veldkamp R, Kuhry E, Hop WC et al (2005) Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomised trial. Lancet Oncol 6(7):477–484. doi:10.1016/S1470-2045(05)70221-7
Bonjer HJ, Deijen CL, Abis GA et al (2015) A randomized trial of laparoscopic versus open surgery for rectal cancer. N Engl J Med 372(14):1324–1332. doi:10.1056/NEJMoa1414882
Kwon S, Billingham R, Farrokhi E et al. (2012) Adoption of laparoscopy for elective colorectal resection: a report from the Surgical Care and Outcomes Assessment Program. J Am Coll Surg 214(6):909–918.e1. doi:10.1016/j.jamcollsurg.2012.03.010
Gruber K, Soliman AS, Schmid K, Rettig B, Ryan J, Watanabe-Galloway S (2015) Disparities in the utilization of laparoscopic surgery for colon cancer in rural nebraska: a call for placement and training of rural general surgeons. J Rural Health 31(4):392–400. doi:10.1111/jrh.12120
Moghadamyeghaneh Z, Carmichael JC, Mills S, Pigazzi A, Nguyen NT, Stamos MJ (2015) Variations in laparoscopic colectomy utilization in the United States. Dis Colon Rectum 58(10):950–956. doi:10.1097/DCR.0000000000000448
Tekkis PP, Senagore AJ, Delaney CP, Fazio VW (2005) Evaluation of the learning curve in laparoscopic colorectal surgery: comparison of right-sided and left-sided resections. Ann Surg 242(1):83–91
Shah PR, Joseph A, Haray PN (2005) Laparoscopic colorectal surgery: learning curve and training implications. Postgrad Med J 81(958):537–540. doi:10.1136/pgmj.2004.028100
Corcione F, Esposito C, Cuccurullo D et al (2005) Advantages and limits of robot-assisted laparoscopic surgery: preliminary experience. Surg Endosc 19(1):117–119. doi:10.1007/s00464-004-9004-9
Casillas MA Jr, Leichtle SW, Wahl WL et al (2014) Improved perioperative and short-term outcomes of robotic versus conventional laparoscopic colorectal operations. Am J Surg 208(1):33–40. doi:10.1016/j.amjsurg.2013.08.028
Baek SK, Carmichael JC, Pigazzi A (2013) Robotic surgery: colon and rectum. Cancer J 19(2):140–146. doi:10.1097/PPO.0b013e31828ba0fd
Peterson CY, Weiser MR (2014) Robotic colorectal surgery. J Gastrointest Surg 18(2):398–403. doi:10.1007/s11605-013-2313-3
Pigazzi A, Ellenhorn JD, Ballantyne GH, Paz IB (2006) Robotic-assisted laparoscopic low anterior resection with total mesorectal excision for rectal cancer. Surg Endosc 20(10):1521–1525. doi:10.1007/s00464-005-0855-5
Palmer KJ, Orvieto MA, Rocco BM, Patel VR (2011) Launching a successful robotic program. In: Patel VR (ed) Robotic Urologic Surgery. Springer, London, pp 11–17. doi:10.1007/978-1-84882-800-1_2
Patel VR (2006) Essential elements to the establishment and design of a successful robotic surgery programme. Int J Med Robot 2(1):28–35. doi:10.1002/rcs.77
Summers S, Anderson J, Petzel A, Tarr M, Kenton K (2015) Development and testing of a robotic surgical training curriculum for novice surgeons. J Robot Surg 9(1):27–35. doi:10.1007/s11701-014-0484-x
Witkiewicz W, Zawadzki M, Rzaca M et al (2013) Robot-assisted right colectomy: surgical technique and review of the literature. Wideochir Inne Tech Maloinwazyjne 8(3):253–257. doi:10.5114/wiitm.2011.33761
Garcea D, Bazzocchi F, Avanzolini A (2015) Right colectomy for cancer: three-arm technique. In: Spinoglio G (ed) Robotic surgery: current application and new trends. Springer-Verlag, Italy, pp 117–123
Park JS, Choi GS, Park SY, Kim HJ, Ryuk JP (2012) Randomized clinical trial of robot-assisted versus standard laparoscopic right colectomy. Br J Surg 99(9):1219–1226. doi:10.1002/bjs.8841
Lim DR, Min BS, Kim MS et al (2013) Robotic versus laparoscopic anterior resection of sigmoid colon cancer: comparative study of long-term oncologic outcomes. Surg Endosc 27(4):1379–1385. doi:10.1007/s00464-012-2619-3
Bhama AR, Obias V, Welch KB, Vandewarker JF, Cleary RK (2015) A comparison of laparoscopic and robotic colorectal surgery outcomes using the American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP) database. Surg Endosc. doi:10.1007/s00464-015-4381-9
Bianchi PP, Pigazzi A, Choi GS (2014) Clinical Robotic Surgery Association Fifth Worldwide Congress, Washington DC, 3–5 October 2013: robotic colorectal surgery. Ecancermedicalscience 8:385. doi:10.3332/ecancer.2014.385
Collinson FJ, Jayne DG, Pigazzi A et al (2012) An international, multicentre, prospective, randomised, controlled, unblinded, parallel-group trial of robotic-assisted versus standard laparoscopic surgery for the curative treatment of rectal cancer. Int J Colorectal Dis 27(2):233–241. doi:10.1007/s00384-011-1313-6
Liao G, Zhao Z, Lin S et al (2014) Robotic-assisted versus laparoscopic colorectal surgery: a meta-analysis of four randomized controlled trials. World J Surg Oncol 12:122. doi:10.1186/1477-7819-12-122
Wishner JD, Baker JW Jr, Hoffman GC et al (1995) Laparoscopic-assisted colectomy. The learning curve. Surg Endosc 9(11):1179–1183
Miskovic D, Ni M, Wyles SM, Tekkis P, Hanna GB (2012) Learning curve and case selection in laparoscopic colorectal surgery: systematic review and international multicenter analysis of 4852 cases. Dis Colon Rectum 55(12):1300–1310. doi:10.1097/DCR.0b013e31826ab4dd
Bokhari MB, Patel CB, Ramos-Valadez DI, Ragupathi M, Haas EM (2011) Learning curve for robotic-assisted laparoscopic colorectal surgery. Surg Endosc 25(3):855–860. doi:10.1007/s00464-010-1281-x
Giulianotti PC, Coratti A, Angelini M et al (2003) Robotics in general surgery: personal experience in a large community hospital. Arch Surg 138(7):777–784. doi:10.1001/archsurg.138.7.777
Ahlering TE, Skarecky D, Lee D, Clayman RV (2003) Successful transfer of open surgical skills to a laparoscopic environment using a robotic interface: initial experience with laparoscopic radical prostatectomy. J Urol 170(5):1738–1741. doi:10.1097/01.ju.0000092881.24608.5e
Bric JD, Lumbard DC, Frelich MJ, Gould JC (2015) Current state of virtual reality simulation in robotic surgery training: a review. Surg Endosc. doi:10.1007/s00464-015-4517-y
Fung AK, Aly EH (2013) Robotic colonic surgery: is it advisable to commence a new learning curve? Dis Colon Rectum 56(6):786–796. doi:10.1097/DCR.0b013e318285b810
Haas EM, Pedraza R (2013) Laparoscopic and robotic colorectal surgery: a comparison and contrast. Semin Colon Rectal Surg 24(1):19–23. doi:10.1053/j.scrs.2012.10.006
deSouza AL, Prasad LM, Park JJ, Marecik SJ, Blumetti J, Abcarian H (2010) Robotic assistance in right hemicolectomy: is there a role? Dis Colon Rectum 53(7):1000–1006. doi:10.1007/DCR.0b013e3181d32096
Jamali FR, Soweid AM, Dimassi H, Bailey C, Leroy J, Marescaux J (2008) Evaluating the degree of difficulty of laparoscopic colorectal surgery. Arch Surg 143(8):762–767. doi:10.1001/archsurg.143.8.762 (discussion 768)
Hanna MH, Hwang GS, Phelan MJ et al (2015) Laparoscopic right hemicolectomy: short- and long-term outcomes of intracorporeal versus extracorporeal anastomosis. Surg Endosc. doi:10.1007/s00464-015-4704-x
Trastulli S, Coratti A, Guarino S et al (2015) Robotic right colectomy with intracorporeal anastomosis compared with laparoscopic right colectomy with extracorporeal and intracorporeal anastomosis: a retrospective multicentre study. Surg Endosc 29(6):1512–1521. doi:10.1007/s00464-014-3835-9
D’Annibale A, Pernazza G, Morpurgo E et al (2010) Robotic right colon resection: evaluation of first 50 consecutive cases for malignant disease. Ann Surg Oncol 17(11):2856–2862. doi:10.1245/s10434-010-1175-0
Hopping JR, Bardakcioglu O (2013) Single-port laparoscopic right hemicolectomy: the learning curve. JSLS 17(2):194–197. doi:10.4293/108680813X13654754534558
Tsai K-Y, Kiu K-T, Huang M-T, Wu C-H, Chang T-C (2016) The learning curve for laparoscopic colectomy in colorectal cancer at a new regional hospital. Asian J Surg 39(1):34–40. doi:10.1016/j.asjsur.2015.03.008
Wohl H (1977) The cusum plot: its utility in the analysis of clinical data. N Engl J Med 296(18):1044–1045. doi:10.1056/NEJM197705052961806
Foo CC, Law WL (2016) The learning curve of robotic-assisted low rectal resection of a novice rectal surgeon. World J Surg 40(2):456–462. doi:10.1007/s00268-015-3251-x
Jimenez-Rodriguez RM, Diaz-Pavon JM, de la Portilla de Juan F, Prendes-Sillero E, Dussort HC, Padillo J (2013) Learning curve for robotic-assisted laparoscopic rectal cancer surgery. Int J Colorectal Dis 28(6):815–821. doi:10.1007/s00384-012-1620-6
Yamaguchi T, Kinugasa Y, Shiomi A et al (2015) Learning curve for robotic-assisted surgery for rectal cancer: use of the cumulative sum method. Surg Endosc 29(7):1679–1685. doi:10.1007/s00464-014-3855-5
Parisi A, Scrucca L, Desiderio J et al (2017) Robotic right hemicolectomy: analysis of 108 consecutive procedures and multidimensional assessment of the learning curve. Surg Oncol 26(1):28–36. doi:10.1016/j.suronc.2016.12.005
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Raimondi, P., Marchegiani, F., Cieri, M. et al. Is right colectomy a complete learning procedure for a robotic surgical program?. J Robotic Surg 12, 147–155 (2018). https://doi.org/10.1007/s11701-017-0711-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11701-017-0711-3