Abstract
Polymeric silicone-based surfactants were prepared by the reaction of polyethylene glycol, maleic anhydride, polydimethylsiloxane, silicone oil, titanium isopropoxide, and sodium hydrogen sulfite. These surfactants showed water-repellent properties when cleaved by different buffer solutions. The synergetic effect of silicone oil-based surfactants and siloxane-based surfactants was studied using different characterization techniques for the surfactants. The hydrophobic properties of cleaved silicone-based surfactants were studied by measuring the contact angle measurement. The polymeric silicone surfactants are stable in nature. The structures of various stages of reaction were confirmed by thin-layer chromatography, Fourier-transform infrared spectroscopy, ultraviolet spectra, and nuclear magnetic resonance analysis. The surface-active properties of surfactants like surface tension and critical micelle concentration as well as foaming and wetting characteristics were also studied. This water-soluble surfactant had low foaming properties and can be used in textile, agriculture, petroleum and paint industries. The cleaved form of silicone surfactant was used for water-repellent application in textile and paint industries.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Surfactants are compounds that lower the surface tension or interfacial tension between two liquids, between a gas and a liquid, or between a liquid and a solid. It is an essential part of the polymerization of an emulsion even though used in low quantity. Surfactants may act as detergents, wetting agents, emulsifiers, foaming agents, and dispersants. A surfactant has distinguished molecular structure comprising of hydrophilic part or a polar portion and a hydrophobic end making them amphiphilic (Attood et al. 1983; Rosen 1978). Generally, the surfactants are stable species, but, in few cases due to their intact nature, it becomes difficult to manipulate them. To overcome this difficulty, various methods have been used to discover a cleavable surfactant. Silicone surfactant is one of them (Hellberg 2002; Jaeger 1995; Hellberg et al. 2000; Jaeger et al. 1988).
The silicone surfactants are a group of small molecules and polymeric surfactants, which comprises of a methylated siloxane unit which is linked to one or more polar groups (Hill 1997). Also, it has small intermolecular interactions between the hydrophobic siloxane groups (Bhattacharjee et al. 2018). Silicone has a fascinating and commercially feasible capability to change the surface properties of polar and non-polar liquids much better than those attained using organic-based traditional surfactants (Petroff and Snow 2012). The other benefit of using a silicone surfactant is that there are various synthetic routes available to produce different structural forms (Hill 1997). And, these different structural forms are also useful in various applications. There are four types of molecular structures of silicone surfactants which include rake type copolymers (also known as graft copolymer or comb copolymers), ABA copolymers (B-silicone portion), trisiloxane surfactants and cyclosiloxane surfactants (Alzobaidi et al. 2018; Hill 1999) as shown in Fig. 1.
Silicone surfactants have a wide range of applications due to their high surface activity and silicon caters favorable performance. They exhibit oleophobic and hydrophobic characteristics as they are soluble in water and organic solvents, thereby decreasing their surface tension. Some basic properties like emulsification, phase behavior, wetting, and foaming are applicable in plastic foam manufacturing as wetting agents and spreading agents, and in cosmetics (Jaeger and Golich 1987). Also, silicone surfactant has unique properties like structural flexibility and ability to reduce surface tension in aqueous as well as nonaqueous system (Aramaki et al. 2019). Hence it can be easy to embed new molecule in surfactant structure.
A surfactant with the weak bond when purposefully inserted into the structure and usually positioned between the polar head (hydrophilic part) and the hydrophobic end is known as a cleavable surfactant (Wang et al. 1993). Such a cleavable surfactant can be degraded chemically into new surface-active species or non-surface active compounds having different properties. This can be done using acid, alkali, ultra violet light, and heat, without disturbing their natural functions (Jaeger 1995; Akamatsu et al. 2019). Nowadays, the degradation property of acid and alkali labile surfactants is controlled by adjusting the pH of the solution. The acid-labile surfactants are generally synthesized by the bonds of significant value like cyclic acrylic acetals, ketals, and orthoesters, in contrast, the ester bonds are present in the alkali labile surfactants (Yue et al. 1996; Hill 2002). The aldehydes and polyethylene glycol are condensed to form noncyclic acetal-lined cleavable surfactants. Such surfactants in acidic pH breakdown into non-surface active compounds because of the acid-sensitive acetal bonds present between the hydrophobic and hydrophilic portion whereas in alkaline or neutral pH they are steady and exhibit surface-active characteristics (Akamatsu et al. 2019).
Cleavable surfactant-containing silicon–oxygen bonds which are affected by both acid and alkaline hydrolysis has been developed by Hellberg (2002) and Jaeger and Golich (1987). In the current study, cleavable silicon surfactants were prepared by reacting polyester with silicon oil and a polysiloxane. The cleavage properties of the surfactant were tested at variable pH values. The cleavage structure of the surfactant was analyzed by UV, FTIR and surface properties like surface tension, foaming, wetting, contact angle, interfacial tension, CMC were studied. The cleaved form of surfactant can be used for water-repellent application in paint as well as on textile fabrics. The water-repellant application was observed my contact angle measurement between fabric or painted material and water (Jaeger 1995; Akamatsu et al. 2019; Lin and Chen 2006).
Materials and methods
Materials
Polyethylene glycol (PEG), maleic anhydride (MA), chloroform, methanol, sodium hydroxide, and sodium hydrogen sulfite were purchased from M/s LOBA Chemicals Pvt Ltd, Mumbai. Titanium isopropoxide and polydimethylsiloxane (PDMS) were obtained from M/s Sigma Aldrich Pvt. Ltd. Mumbai. Silicone oil and potassium bromide were procured from M/s Molychem Pvt. Ltd. Mumbai. Potassium hydrogen phthalate, boric acid, sodium sulfate, acetic anhydride were taken from M/s Thomas Baker Pvt Ltd. Mumbai. Deuterated chloroform (CDCl3) and potassium chloride were purchased from M/s SD Fine Pvt. Ltd. Linear Alkyl Benzene Sulphonic Acid (LABSA) was obtained from M/s Godrej Industries Ltd. Mumbai as a gift sample.
Methods
Synthesis of silicon surfactant
The silicon surfactants were prepared sequentially in three steps: (1) esterification of polymer, (2) conversion of polyester to polysiloxane, (3) sulphonation of polysiloxane (Fig. 2). All the steps were monitored by TLC, FTIR and the structures were confirmed by NMR Spectra.
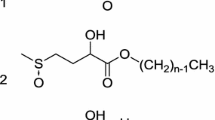
The water-soluble polyester was prepared by polymerization of 2 mol polyethylene glycol (PEG) and 1 mol of maleic anhydride (MA) in the presence of (0.4%) titanium isopropoxide in a round bottom flask. A four-necked round bottom flask fitted with a Dean-stark assembly along with a condenser, overhead stirrer, temperature sensor, and a gas sparger. The reaction temperature was maintained in the range 160–180 °C and continuously stirred at 250 rpm for 7–8 h in presence of nitrogen gas. The reaction was monitored by calculating acid value after every 30 min and also from the amount of water separated in dean stark (Sundararajan et al. 2018). The polyester was treated with sodium sulfate for removing traces of moisture if any.
Two mole of polyester which was formed by PEG and MA was reacted with 1 mol of polydimethylsiloxane (PDMS) or silicone oil or a mixture of silicone oil and PDMS in the presence of titanium isopropoxide as a catalyst in presence of nitrogen gas with continuous stirring at 250 rpm and at 100–120 °C for 3–5 h. The reaction was carried out in 4-necked round bottom flask with a reflux condenser. The formed polysiloxane was passed through sodium sulfate for removing a trace amount of moisture. Sodium hydrogen sulfite was treated with polysiloxane at 90–100 °C with continuous stirring at 250 rpm for 8–13 h with nitrogen purging for the formation of surfactants.
Hydrolysis of surfactant
The prepared surfactants were hydrolyzed in different buffer solutions of pH 4, 7 and 9 with ± 0.01 standard deviation (SD) and kept at 20 °C for 2 h. The buffer solutions were prepared using various salts namely NaOH, potassium hydrogen phthalate, boric acid, potassium hydrogen phosphate, and potassium chloride. Hydrolyzed solutions were used for studying different properties. The structural changes in silicone surfactants with different pH solutions were analyzed by UV spectrometer and FTIR.
Fourier Transform Infrared Spectroscopy (FTIR)
FTIR analysis of each stage of reaction and hydrolyzed product of silicone surfactants was carried out using FTIR IR affinity1 Miracle 10 (Make Shimadzu) using potassium bromide between the wavelength range of 400–4000 cm−1.
UV spectrophotography of hydrolysed surfactants
The hydrolyzed surfactants were observed under UV spectra (Shimadzu 01555) at various pH values of 4, 7 and 9 at 0.1% concentration (w/v).
Thin-layer chromatography (TLC)
Thin-layer chromatography of each stage of polymeric reaction was performed on silica gel aluminum-coated sheets (Make: M/s Sigma Aldrich 60F 254), as a stationary phase. This silica gel was activated by heating at 100 °C for 2 h before use. A TLC protocol for this system was developed using chloroform: methanol (90:10 v/v) and 0.1 ml glacial acetic acid as the mobile phase. Synthesized surfactants were run from the solvent baseline towards the solvent front with the help of this mobile phase. The spots on the stationary phase were visible after treating in iodine chamber. The change in Rf values was observed on TLC plates.
Nuclear magnetic resonance (NMR)
The chemical structure of each step of synthesis was confirmed by 1H NMR Spectrometer in the CDCl3 (1:1, v/v) as a solvent. This analysis was performed on Agilent 600 MHz NMR.
Surface-active properties (SFT and CMC)
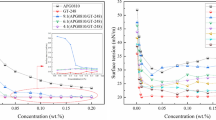
The surface-active properties of the surfactant were measured by Tensiometer (Kyowa K10). The surface tension of synthesized surfactants was analyzed in a 1% solution of surfactants, distilled water as well as in buffer solutions of different pH values 4, 7 and 9 with ± 0.01 SD. The buffer solutions containing surfactants were kept for 2 h at 20 °C for hydrolysis. The surface tension was measured after hydrolysis.
The critical micelle concentration of the surfactant was determined by dissolving it in distilled water. The minimum concentration of the surfactant that was needed to reach CMC was evaluated (Andrade et al. 2018).
Foaming
Foaming was measured using Ross Mile’s instrument. 1% solution of surfactant was prepared of which 50 cm3 solution was poured in the Ross Mile’s tube and 200 cm3 solution was taken in the pipette and then poured from a height of 100 cm into the tube. The foam height and foam stability of the surfactant were observed.
Wetting
Wetting was performed using cotton fabrics in a 1% solution of surfactant. 100 cm3 of the solution was taken in a 250 cm3 beaker and a funnel was placed inverted on to the beaker with cotton fabric attached in its rim. The time for the cloth to sink was noted.
Water-repellent application of cleaved surfactants in paints and cotton fabric
The water-repellent properties of surfactants and its cleaved form for fabric were determined by contact angle measurement between fabric and water. 0.5 g surfactant was hydrolyzed in different buffer solutions having volume 100 cm3, 1 g cotton fabric was placed in a hydrolyzed solution. This was maintained at 29 °C for 1 h in an incubator shaker at 150 rpm. Then the fabric was removed and dried at 140 °C in an oven for 15 min. The change contact angle between treated fabrics and water proved the water-resistance phenomenon. The contact angle for the paint application was also determined. 1 g of surfactant was dissolved in various buffer solutions of pH 4, 7 and 9 (± 0.01 SD). The temperature of the solutions was maintained at 20 °C for 2 h. These cleaved surfactant solutions were mixed with water-based paint which was obtained from the local market and applied on a wooden block and metal plate. The applied material was dried at room temperature for 24 h. The change in contact angle between cleavable surfactant-treated material and water indicates the change in surface morphology.
Results and discussion
The esterification of polymer in the first step of synthesis was monitored by determining the acid value of the reaction after every 30 min. The acid value decreased from 89 to 0.8 in 8 h. (Figure 3).
Water was separated from the reaction mass using a dean stark apparatus. All steps of synthesis were monitored using TLC and FTIR analysis, while the properties of the surfactants were analyzed by tensiometric analysis (SFT and CMC) and foaming-wetting properties. All steps of synthesis and analysis are repeated thrice for reproducibility of the results. Structural analysis was performed using NMR spectroscopy. The synthesized surfactant was cleaved in a buffer solution. This was used for textiles and coating applications followed by contact angle analysis.
TLC
The progress of each step of the reactions was monitored by spotting PEG, polyester, polysiloxane and final surfactant on the TLC plate. The changes in the retention factor (Rf) values of each component in all steps of the reaction indicated the progress of reaction (Table 1).
The Rf values of the final product matched with the standard silicone surfactants. The reaction of PEG 400 and silicone oil gave promising results as compared to PEG 4000. The Rf value of synthesis surfactants from PEG 400 and a mixture of silicone oil and PDMS (silicone oil: PDMS 50:50), gave satisfactory separation of bands on the TLC plate. The Rf values of silicone surfactant with 100% PDMS and partially mixed with silicone oil were 0.55 and 0.61, respectively. The Rf values of the final product matched with the marketed silicone surfactants.
Cleaved surfactants FTIR and UV spectroscopy
The FTIR spectra of the product showed the hydroxyl group at 3470–3480 cm−1, –C–H stretching at 2860–2870 cm−1, carbonyl group of ester at 1731 cm−1, C–O stretching of –OH at 1090–1100 cm−1 and C=C at 1640–1645 cm−1. In FTIR, the peak at 1036 cm−1 indicated the Si–O–Si linkage which confirmed the formation of polysiloxane intermediate. At 1252 cm−1 signified Si–CH3 linkage. All the peaks obtained indicate the progress of the reaction and the formation of the surfactant. The FTIR analysis of surfactant and the cleaved surfactant, which was obtained by hydrolysis when kept for 2 h in pH 4 buffer solution, showed results as given in Fig. 4a, b.
After the surfactant hydrolysis in acidic conditions, it was observed that the absorption band at 1150 and 1750 cm−1 disappeared and the cleaved surfactant showed a broad and strong band at 3200–3600 cm−1. This showed that the ester group of the silicone surfactant was fragmented into the hydroxyl group (OH) and the carboxylic group (–COOH). All the FTIR spectra were compared with the previously reported results by Lin and Chen (2006).
Further, the spectra obtained by UV analysis of surfactant and the cleaved surfactant arrived with two bands at wavelengths 190–220 nm and 220–290 nm, with an abrupt change at 200 nm which indicated the formation of water-insoluble groups, PEG 400 or PEG 4000 and Maleic acid when cleaved in acidic condition (pH 4). The complete cleavage of the ester bond of silicone surfactant was confirmed by UV spectra (Fig. 5). The peak of UV spectra was stable and identical even after 2 h. It indicated the complete cleavage of the ester bond drastically after acid hydrolysis of silicone surfactant. The cleaved surfactant UV spectra were found to be similar to previously mentioned results by Lin and Chen (2006).
Surfactant properties
The surface tension of the prepared surfactant solution was decreased by the addition of surfactant which comprised of the amphiphilic end. In this study, the polar part of the surfactant and the non-polar part (silanol) were connected by weak bonds, which were cleaved into two water-soluble polar parts and one water-insoluble non-polar part. The surface tension changes with a change in the pH of the surfactant solution (Table 2). The hydrolyzed surfactants in the acidic buffer, which was synthesized with PEG 400 and PDMS gave better surface tension. At pH 4, SFT was 33.28 mN/m and increased up to 39.4 mN/m for pH 9. The CMC of PEG400 hydrolyzed surfactant was found to be 3 mg/L. These surfactant properties were equivalent to commercially available silicone surfactants having SFT 28.4 mN/m and CMC 2.1 mg/L. The surface tension of silicone surfactant was found to be on par with previously mentioned results by Akamatsu et al. (2019) (SFT 31 mN/m), Lin and Chen (2006) (SFT 40 mN/m) and Jaeger (1995) (50 mN/m).
Structural analysis
The structure of all steps of reactions and final silicone surfactants was confirmed by 1H NMR analysis and the chemical shifts are represented in Table 3. The chemical shifts observed in the NMR spectra were in accordance with the ABA type of silicone surfactants Fig. 6a–c prove a structural confirmation of all the steps in the reaction. The structure of the silicone surfactant was compared with the previously mentioned data by Akamatsu et al. (2019).
Application of cleaved surfactants in paints industry and textile industry
The treated cotton fabric in different buffer solutions indicated good wetting properties as compared to untreated cotton fabric. The cotton fabric treated with hydrolyzed surfactants at pH 4 took longer wetting time. Silicone surfactants showed water-repellent properties when hydrolyzed in buffer solution. This directly affects the contact angle between the fabric and the water. The wetting time of treated fabric with cleaved surfactants at pH 4 was 6480 s. and at pH 9 it was 2637 s. (Table 4). Contact angles for fabric treated with cleaved surfactant and water were 115° and that for untreated fabric and water was 106° (Table 5). Similar contact angle analysis between nylon fabric and water (Treated nylon 82° and untreated nylon 80°) was reported by Lin and Chen (2006).
The cleaved surfactants also demonstrate the promising application in the paint industry. Cleaved surfactants (at pH 4) mixed with paints displayed better water-repellent properties after their application to the wooden block and metal plate. The contact angle of paint (without surfactants) applied on wooden block and water was 50° while that with surfactant was 119°. Similarly, for metal plate and water when covered with paint containing surfactant was found to be 119 °C and that for without surfactant was 42 °C (Table 5). The contact angle of water with treated fabric material as well as wooden block and metal plate showed good water resistance due to the effect of cleaved silicone surfactants.
Conclusion
The silicone surfactants have good water-repellent properties when hydrolyzed to form the cleaved product. The hydrolyzed surfactants in pH 4 gave promising results for usage in paint and textile industries for water-repellent paint and fabric application, respectively. The structure of intermediates in all the steps of synthesis was confirmed by IR, UV NMR analysis. The surfactant properties have been analyzed with SFT and CMC. The cleaved product showed CMC 3 mg/L which was significantly better than the uncleaved surfactant. All the analysis and change in the contact angle of cleaved surfactants confirmed that cleaved surfactants possessed good water-repellent properties and possibly be useful in shoe cloth, wooden paint, and metal paint which are required to be water-resistant.
Abbreviations
- PEG:
-
Polyethylene glycol
- MA:
-
Maleic anhydride
- PDMS:
-
Polydimethylsiloxane
- CDCl3 :
-
Deuterated chloroform
- TLC:
-
Thin layer chromatography
- FTIR:
-
Fourier-transform infrared spectroscopy
- 1H NMR:
-
Proton nuclear magnetic resonance
- SFT:
-
Surface tension
- CMC:
-
Critical micelle concentration
- LABSA:
-
Linear alkyl benzene sulphonic acid
References
Akamatsu M, Nagai T, Fukuda K, Tsuchiya K, Sakai K, Abe M, Sakai H (2019) Amino acid-type photo-cleavable surfactants: controlled dispersion stability of silica particles and release of active ingredients. Colloids Surf A Physicochem Eng Asp 564(December 2018):108–114. https://doi.org/10.1016/j.colsurfa.2018.12.044
Alzobaidi S, Lee J, Jiries S, Da C, Harris J, Keene K, Enick R (2018) Carbon dioxide-in-oil emulsions stabilized with silicone-alkyl surfactants for waterless hydraulic fracturing. J Colloid Interface Sci 526:253–267. https://doi.org/10.1016/j.jcis.2018.04.056
Andrade RFS, Silva TAL, Ribeaux DR, Rodriguez AF et al (2018) Promising biosurfactant produced by cunninghamella echinulata UCP 1299 using renewable resources and its application in cotton fabric cleaning process. Adv Mater Sci Eng 2018:1–12. https://doi.org/10.1155/2018/1624573
Aramaki K, Fujii M, Sakanishi Y (2019) Rheological properties of silicone-surfactant-based wormlike micellar solution. Colloids Surf A Physicochem Eng Asp 581(August):123841. https://doi.org/10.1016/j.colsurfa.2019.123841
Attood D, Florence AT (1983) Surfactant systems. Chapman & Hall, London. https://doi.org/10.1002/jps.2600741040
Bhattacharjee G, Barmecha V, Kushwaha OS, Kumar R (2018) Kinetic promotion of methane hydrate formation by combining anionic and silicone surfactants: scalability promise of methane storage due to prevention of foam formation. J Chem Thermodyn 117:248–255. https://doi.org/10.1016/j.jct.2017.09.029
Hellberg PE (2002) Ortho ester-based cleavable cationic surfactants. J Surfactants Deterg 5(3):217–227. https://doi.org/10.1007/s11743-002-0221-1
Hellberg PE, Bergström K, Holmberg K (2000) J Surfactant Deterg 3:81. https://doi.org/10.1007/s11743-000-0118-z
Hill RM (1997) Siloxane surfactants. Spec surfactants. https://doi.org/10.1007/978-94-009-1557-2_6
Hill RM (1999) Silicone surfactant. Surfactant science series, vol 86. Marcel Dekker, New York
Hill RM (2002) Silicone surfactants—new developments. Curr Opin Colloid Interface Sci 7(5–6):255–261. https://doi.org/10.1016/s1359-0294(02)00068-7
Jaeger DA (1995) Cleavable surfactants. Supramol Chem 5(1):27–30. https://doi.org/10.1080/10610279508029884
Jaeger DA, Golich TG (1987) Preparation and characterization of double-chain destructible surfactants and derived vesicles. J Am Oil Chem Soc 64(11):1550–1551. https://doi.org/10.1007/bf02609364
Jaeger DA, Ward MD, Dutta AK (1988) Preparation and characterization of cleavable surfactants based on a silicon-oxygen bond. J Org Chem 53(7):1577–1580. https://doi.org/10.1021/jo00242a050
Lin L-H, Chen K-M (2006) Surface activity and water repellency properties of cleavable-modified silicone surfactants. Colloids Surf A 275(1–3):99–106. https://doi.org/10.1016/j.colsurfa.2005.09.032
Petroff LJ, Snow SA (2012) Silicone surfactants. In: Owen M, Dvornic P (eds) Silicone surface science. Advances in silicon science, vol 4. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-3876-8_9
Rosen MJ (1978) Surfactants and interfacial phenomena. Wiley-Interscience, New York. https://doi.org/10.1002/ange.19790910534
Sundararajan S, Kumar A, Chakraborty BC, Samui AB et al (2018) Poly(ethylene glycol) (PEG)-modified epoxy phase-change polymer with dual properties of thermal storage and vibration damping. Sustain Energy Fuels 2(3):688–697. https://doi.org/10.1039/c7se00552k
Wang GW, Lei XG, Liu YC (1993) Preparation and properties of cleavable dianionic surfactants with a 1,3-dioxane ring. J Am Oil Chem Soc 70(7):731–732. https://doi.org/10.1007/bf02641012
Yue C, Harris JM, Hellberg PE, Bergstrom K (1996) Synthesis and characterization of cleavable surfactants derived from poly(ethylene glycol) monomethyl ether. J Am Oil Chem Soc 73(7):841–845. https://doi.org/10.1007/bf02517984
Acknowledgements
Authors are thankful to M/s Godrej Industries Pvt. Ltd. Mumbai for providing gift sample of LABSA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mestri, R.S., Pratap, A.P., Panchal, K.H. et al. Synthesis of cleavable silicone surfactant for water-repellent application. Chem. Pap. 74, 1407–1416 (2020). https://doi.org/10.1007/s11696-019-00961-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-019-00961-0