Abstract
The aim of this paper is to synthesize of Ag-nanoparticles (Ag-NPs) by the pistachio skin extract followed by its application for preconcentration and measurement of ultra-trace levels of Bi(III) by electrothermal atomic absorption spectrometry. Pistachios skin extract was used as a green reducing agent to produce Ag-NPs from silver ion solution. The results of the FT-IR spectroscopy, UV–Vis spectrophotometry, Scanning electron microscopy, Transmission electron microscopy (TEM) and X-ray diffraction spectroscopy show that Ag-NPs with uniform size of 25–40 nm in diameter were synthesized successfully. In order to optimize different parameters affecting the extraction efficiency, Response surface methodology-Box Behnken optimization technique was used. Under the optimum conditions, the calibration curve was linear in the range of 0.5–8 µg L−1 of Bi(III) with a correlation coefficient of 0.9993. The relative standard deviation (%) was 3.5% for five replicate analysis of 1 µg L−1 Bi(III). Also, the limit of detection was 0.09 µg L−1 Bi(III), and the preconcentration factor calculated from the ratio of the volume of sample solution to the desorbent solvent volume after preconcentration step was 100. The accuracy of the method was evaluated by the analysis of certified reference material and spike methods. Finally, this technique was successfully applied for determination of ultra-trace levels of Bi(III) in water, food, human serum and hair samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bismuth (Bi) is a lustrous, silvery-white heavy metal with a tendency to crystallize in coarse grains. It has many applications for the synthesis of alloys, medicine, pharmaceutical products, cosmetic and pigments and superconductor materials (Maile et al. 2005; Ojebuoboh 1992). Due to its wide applications in different industries; its leakage to the environment is also increasing. Although Bi is slightly toxic; however in long times, it can damage the kidney and liver. Therefore, measurement of Bi in real samples has great significance (Pamphlett et al. 2000). Different analytical tools including atomic absorption spectrometry (Vakilzadeh et al. 2015; Shemirani et al. 2005), electrochemical methods (Koper and Grabarczyk 2011; Liu and Wang 2006), ultraviolet–visible spectrophotometry (Afkhami et al. 2006; Madrakian et al. 2003) and inductively coupled plasma-mass spectrometry (Sun and Wu 2011) were used for detection of Bi. Electrothermal atomic absorption spectrometry (ETAAS) is a prevalent instrument for determination of heavy metals with high sensitivity. However, accurate results especially at trace levels of the analyte need a more additional step to reduce or remove interfering ions (Eftekhari et al. 2015). Solid phase extraction (SPE) as a sample preparation technique has advantages including simplicity, the wide range of adsorbents and also high enrichment factor (Tuzen et al. 2008, 2005; Faraji et al. 2010; Sun et al. 2015; Ziaei et al. 2014). Today, metal nanoparticles have considered much attention due to their applications in electronic instruments, sensors, catalytic, etc (Abou El-Nour et al. 2010). Chemically reduction of metal ions by using different reducing agents is one of the synthesize routes for the production of metal nanoparticles (Mulfinger et al. 2007); however, many of them such as sodium borohydride (NaBH4) or hydrazine (N2H4) are toxic and expensive. To overcome these problems, green methods using natural reducing agents have gained much attention.
Pistachio is one of the main export of Iran, so that Iran and USA provide more than 60% of total pistachio in the world. One of the main byproducts of the pistachio industry is its skins which considered as a waste material. However, different researches show that pistachio skin contains lots of phenolic compounds that serve as natural anti-oxidant agents (Tomaino et al. 2010).
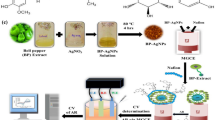
In this work, we have used a green method for the synthesis of Ag nanoparticles (Ag-NPs) followed by its application for efficient preconcentration of Bi(III). Pistachios skin extract as a green reducing agent was used to produce Ag-NPs from silver ion solution. X-ray diffraction spectroscopy (XRD) and transmission electron microscopy (TEM) were employed for characterization of the synthesized Ag-NPs. Also, FT-IR spectrophotometry was used to characterize of the functional groups on the surface of Ag-NPs. The results show that Ag-NPs with the size of 25–40 nm in diameter were synthesized successfully and used as an adsorbent for preconcentration of Bi(III). Response surface methodology-Box Benhken experimental design was used and optimum conditions were determined. Finally, the proposed SPE technique was applied for determination of Bi(III) in water, milk powder, black tea, human serum and hair samples.
Experimental
Regents
A solution of 1000 mg L−1 Bi(III) was made from Bismuth(III) nitrate pentahydrate ( ≥ 98%, Sigma Aldrich, USA) in deionized water. AgNO3 (99–100.5%, Merck, Darmstadt, Germany) was used as a precursor of Ag-NPs. Various concentrations (1, 3 and 5 mol L−1) of HNO3 (65%, Merck, Darmstadt, Germany), HCl (37%, Merck, Darmstadt, Germany), CH3COOH (99.5%, Mojallali, Tehran, Iran) and HCOOH (98–100%, Merck, Darmstadt, Germany) were prepared and used for desorption of Bi(III) from Ag NPs. Concentrated H2O2 (30%, Merck, Darmstadt, Germany) and HNO3 were used for sample digestion.
Instrumentation
Analytic Jena 400P Atomic Absorption Spectrometry was used as a detection system for Bi. A Bismuth HCL lamp (wavelength of 223.1 nm) as a radiation source, argon (Ar) gas as an inert gas with 99.99% purity were used in ETAAS. The electrothermal conditions of the instrument are shown in Table 1. A Metrohm 827 (Switzerland) was used for pH adjustment and Centurion Centrifuge (Hettich D72, Germany) was used for separation of adsorbent from the sample solution. A UV–Vis spectrophotometry (Agilent 8453) with 1 cm quartz cell was used for recording of UV–Vis spectrum. A Leo 912AB Transmission Electron Microscopy (TEM) was used to determine the size of the synthesized Ag NPs and X-Plorer instrument (Italy) was used for recording of the XRD spectrum. The morphology of the synthesize Ag NPs was studied by the Philips S360 SEM (Oxford, UK).
Sample preparation
Water samples
Tap water (Mashhad, Iran), Dijlah (Kadhimiya, Iraq) and Al-Furat River (Karbala, Iraq) were collected and after filtering by filter paper (Whatman No. 42); an aliquot of each sample was diluted to 10 mL and analyzed for determination of Bi(III) based on the preconcentration procedure.
Milk Powder and Black tea
Approximately 1 g of milk powder and powdered black tea samples were transferred into the crucibles. Each sample was heated at 400 ˚C for 2 h followed by addition of 2 mL concentrated HNO3 to it and heating at 70 ˚C for 15 min. Then, the crucible content was diluted to 25 mL with deionized water. Finally, 2 mL of each sample was diluted to 10 mL with deionized water and analyzed for its Bi content based on the preconcentration procedure.
Human serum samples
The human serums were prepared from volunteer and keep at 4˚C before analysis. To the 1 mL of each serum sample, 1 mL of concentrated HNO3 and 100 µL concentrated H2O2 were added and heated at 60–70 ˚C for 10 min. After filtering with No. 40 Whatman paper, it was diluted to 10 mL with deionized water and an aliquot of 2 mL of each sample was diluted to 10 mL and analyzed for its Bi(III) content based on the preconcentration procedure.
Human hair sample
The human hair sample was washed with deionized water and dried at 50 ˚C for 3 h. Then, approximately, 1 g of sample was transferred into the crucible and heated at 400 ˚C for 2 h. Other sample preconcentration steps are similar to the method explained for milk powder and black tea samples.
Preparation of pistachio skin
Pistachio skins were collected from Feyzabad, Torbat Heydarieh and dried at 35–40 ˚C for 2 days. The dried pistachio skins were grounded and used for the green synthesis of Ag-NPs.
Synthesize of Ag-NPs
150 mL of deionized water containing 10 g of powdered pistachio skin was heated at 60–65˚C for 20 min. By filtering of the mixture using No. 40 Whatman filter paper; 50 mL of the filtered solution was added to the 150 mL deionized water containing 0.005 mol L−1 Ag+. The solution was incubated in a dark place for 6 h. Finally, it was centrifuged at 12,000 rpm, washed with deionized water and dried at 50 ˚C for 9 h.
Preconcentration procedure
10 mL of 1 µg L−1 Bi(III) sample solution was adjusted at the pH 10 and transferred into the test beaker followed by adding of 10 mg Ag-NPs to it. The mixture was stirred for 5.8 min at 400 rpm. By its centrifugation at 3500 rpm for 10 min, the supernatant was discarded by a syringe and then 100 µL of 3 mol L−1 HCOOH was added to it. The whole mixture was vortexed for 4.7 min at 2800 rpm. Finally, after centrifugation for another 5 min, 20 µL of the solution was injected into the ETAAS for quantification of Bi.
Results and discussions
Optimization of ETAAS
Drying, ashing and atomization temperatures are three main steps in ETAAS. In order to successfully removal of the extraction solvent; two drying steps (90 and 130 ˚C) were used. Ashing temperature was studied in the range of 400–900 ˚C and based on the results, 600 ˚C was considered as the optimum. Finally, atomization temperature was tested in the range of 1700–2200 ˚C and the results show that the temperature of 2000 ˚C obtained maximum absorbance for determination of Bi. The detailed ETAAS program was presented in Table 1.
Characterization of adsorbent
Different analytical techniques including FT-IR spectrophotometry, TEM images, XRD spectroscopy and UV–Vis spectrophotometry were used for characterization of the synthesized Ag NPs. Figure 1, shows the XRD pattern of the synthesized Ag-NPs. As it shows, the characteristic peaks of Ag-NPs were corresponding to 111, 200, 220 and 311 planes are located at 2θ = 38, 44, 64.5 and 77, respectively. The results from the XRD spectrum clearly show that the Ag-NPs with crystalline phase has been synthesized successfully (Bar et al. 2009).
TEM and SEM images of the synthesized Ag-NPs are shown in Fig. 2a, b, c and d, respectively. As it could be seen, Ag-NPs have an average size of 25–40 nm and spherical in shape. Also, Fig. 3 shows the UV–Vis spectrum of the sample solution containing Ag-NPs. The broad peak at 420–450 nm is due to the surface plasmon of Ag-NPs and shows that the Ag-NPs were successfully synthesized.
FT-IR spectroscopy (Fig. 4) was used for the characterization and identification of the biomolecules adsorbed on the synthesized Ag-NPs. Based on the results, the peaks at 1505 cm−1, 1600 cm−1 and 1650 cm−1 are related to the carboxylate groups, N–H functional groups and aromatic C = C groups, respectively. Also, the alcoholic C–O and aromatic OH– groups are appeared at the wavelength of 1016 and 1196 cm−1, respectively. Finally, the broad peak at 3100–3500 cm−1 is related to the OH– and N–H groups. The obtained results show that the surface of the synthesized Ag-NPs is covered by different functional groups which can interact with Bi(III) to physically adsorb it from sample solution (Bar et al. 2009; Rafiee et al. 2017).
Response surface methodology—BOX Behnken optimization
The critical parameters including sample pH (A), amounts of adsorbent (B), extraction time (C) and desorption time (D) were optimized simultaneously with Response Surface Methodology (RSM)-Box Behnken optimization technique. The centrifuge rate, centrifuge time and stirrer rate were kept constant at 3500 rpm, 10 min and 400 rpm, respectively. These variables and their levels are presented in Table 2.
By the analysis of four models including Linear, 2FI, Quadratic and Cubic; Quadratic experimental model was selected as the optimum. The experimental design for the preparation of quadratic curves is shown in Table 3 and designed by Design Expert 7.0.0 software. The relationship between variables and recovery percentage (RP) was expressed by Eq. 1.
Also, the analysis of variance (ANOVA) for four variables is shown in Table 4. Based on the results, the parameters A, B and A2 had low P values ( < 0.0001) which show that these parameters had highly significant effects on the recovery percentage. However, the parameters C, AB and B2 had significant effects (P < 0.05).
The result of lack of fit P-value, 0.0867, shows that it is not significant and the model F value is 253.2 showing that this model is significant. A normal probability plot of the residuals is illustrated in Fig. 5 provided by the Design Expert software. As the results shown, the points are close to the linear line which confirms that it obeys a normal distribution.
Also, the variables and their values were processed by the full quadratic multiple linear regression model and presented in Fig. 6a–e. Based on the Fig. 6a, pH and amounts of adsorbent (g) have highly significant influence on the RP of Bi(III). As the results shown, by increasing of the pH values, the RP increases. It should be noted that at pH < 7, Bi(III) has Bi(OH)3 and Bi(OH)2+ structures which electrostatically repulse from the Ag-NPs surface (Zeta Potentials analysis show that at pH < 7 the surface of Ag-NPs has positive charge), however, Bi(III) has Bi(OH)3 structure at pH 7–10 which could be adsorbed on the Ag NPs by hydrogen bonding interaction between Bi(OH)3 and phenolic groups of the Ag-NPs. Figure 6b, c show the effects of extraction and desorption time on the RP of Bi(III). As it was predicted by the ANOVA test, extraction time is a significant parameter while desorption time (in the time interval of 1–5 min) is not a significant factor. Also, Fig. 6d shows that the amounts of adsorbent has a high significant effect on RP; and again the extraction time could be considered as a significant factor. Finally, based on the Fig. 6e, f, desorption time was considered as an insignificant factor which means that desorption of analyte from the Ag NPs occurred immediately.
The optimum conditions were determined by RSM-Box Behnken and equal to: pH 10, amounts of adsorbent: 10 mg, extraction time: 5.8 min and desorption time of 4.7 min.
Different desorption solvents such as HCl, HNO3, CH3COOH and HCOOH were tested to find the optimum desorption solvent for the determination of Bi. The results in Fig. 7 show that the solutions of 3 and 5 mol L −1 HCOOH have maximum efficiency for desorption of Bi(III) from Ag NPs. Therefore, 3 mol L−1 HCOOH was selected as the optimum desorption solvent.
Effect of diverse ions
The effect of different ions on the RP of Bi(III) were investigated and presented in Table 5. Interfering ion defined as an ion causes the changes in the recovery of analyte more than ± 5% (Iranzad et al. 2018; Alikhani et al. 2018). Based on the results, the proposed method has an acceptable tolerance limit for determination of Bi(III).
Effect of ionic strength
To study the influence of ionic strength on the RP of Bi(III), different sample solutions containing KNO3 and NaCl (in the range of 0–1% g mL−1) were examined. Based on the results, no significant changes in the RP were observed up to 0.6% (g mL−1) and 0.2% (g mL−1) for KNO3 and NaCl, respectively.
Analytical figures of merit
The calibration curve of Bi(III) was determined in the range of 0.5–8 µg L−1 with the R2 value of 0.9993. Its equation was Absorbance = 0.0655 CBi(III) + 0.0019; where C is the concentration of Bi(III) in µg L−1. The RSD value for the analysis of 1 µg L−1 Bi(III) was 3.5% (n = 5) and LOD was 0.09 µg L−1 Bi(III) (n = 6). The preconcentration factor was 100 which calculated from the ratio of the volume of sample solution (10 mL) to the volume of desorbent solvent (100 µL). Also, the enrichment factor (EF) defined as the ratio of the slope of calibration curve after preconcentration to the direct analysis of analyte (A = 0.0007 CBi(III)–0.002) was 94 (Eftekhari et al. 2018; Al-Kinani et al. 2018; Khodarahmi et al. 2018).
Real samples analysis
Water (tap, river), milk powder, black tea and human serum and hair samples were tested for determination of their Bi content. The results are presented in Tables 6 and 7. Spike analysis was also examined on the samples to certify the validity of the results. The results show very good recoveries for determination of Bi. Also, a certified reference material, trace metal in drinking water (CRM-TMDW) which containing 10 µg L−1 Bi(III) was analyzed. By the analysis of CRM sample, 9.9 ± 0.33 µg L−1 Bi(III) was obtained and shows that the obtained value is in the good agreement with the certified value (student t-test, 95% confidence limit, n = 5).
Comparison with other methods
The SPE technique was compared by the other methods for the measurement of Bi(III) and the results are presented in Table 8. As it shows, the proposed Ag NPs SPE method has acceptable LOD and RSD. Also, this method is green, very rapid and very efficient for preconcentration of Bi(III).
Conclusion
In this research, we have used the plant extract for the green synthesize of Ag-NPs followed by its application for preconcentration and measurement of Bi(III). As Pistachios skin extract contains lots of polyphenol compounds, it could be considered as a natural reducing agent for synthesizing of the Ag-NPs. The results of the XRD spectrum, TEM and SEM images and UV–Vis spectrophotometry show that the uniform Ag-NPs with the average size of 25–40 nm were synthesized successfully and then used for preconcentration of Bi(III) followed by its determination with ETAAS. The critical parameters on the RP of Bi(III) including pH of sample solution, amounts of Ag NPs, extraction time and desorption time were optimized by RSM-Box Behnken experimental design. The main advantages of the proposed SPE technique are the green approach for the synthesizing of Ag NPs, high rapidity (approximately 6 min extraction time), easy to use and high efficiency for determination of ultra-trace levels of Bi(III). Also, the results of the analysis of real samples show that this method could be used for determination of Bi(III) in different real samples.
References
Abou El-Nour KMM, Eftaiha A, Al-Warthan A, Ammar RAA (2010) Synthesis and applications of silver nanoparticles. Arab J Chem 3:135–140
Afkhami A, Madrakian T, Siampour H (2006) Cloud point extraction spectrophotometric determination of trace quantities of Bismuth in urine. J Braz Chem Soc 17:797–802
Alikhani A, Eftekhari M, Chamsaz M, Gheibi M (2018) Paired-ion-based liquid phase microextraction for speciation of iron (Fe2+, Fe3+) followed by flame atomic absorption spectrometry. J Food Measure Charact 12:573–580
Al-Kinani A, Eftekhari M, Gheibi M, Chamsaz M (2018) Polyaniline-coated cerium oxide nanoparticles as an efficient adsorbent for preconcentration of ultra-trace levels of cadmium (II) followed by electrothermal atomic absorption spectrometry. Spectrosc Lett 51:287–296
Bar H, Bhui DK, Sahoo GP, Sarkar P, De SP, Misra A (2009) Green synthesis of silver nanoparticles using latex of Jatropha curcas. Colloids Surf A Physicochem Engin Asp 339:134–139
Eftekhari M, Chamsaz C, Arbab-Zavar MH, Eftekhari A (2015) Vortex-assisted surfactant-enhanced emulsification microextraction based on solidification of floating organic drop followed by electrothermal atomic absorption spectrometry for speciation of antimony (ΙΙΙ, V). Environ Monit Assess 187:4129–4136
Eftekhari M, Gheibi M, Akrami M, Iranzad F (2018) Solid-phase extraction of ultra-trace levels of lead using tannic acid-coated graphene oxide as an efficient adsorbent followed by electrothermal atomic absorption spectrometry; response surface methodology-central composite design. New J Chem 42:1159–1168
Faraji M, Yamini Y, Saleh A, Rezaee M, Ghambarian M, Hassani R (2010) A nanoparticle-based solid-phase extraction procedure followed by flow injection inductively coupled plasma-optical emission spectrometry to determine some heavy metal ions in water samples. Anal Chim Acta 659:172–179
Fayazi M, Afzali D, Mostafavi A (2011) Pre-concentration procedure using dispersive liquid–liquid microextraction for the determination of bismuth by flame atomic absorption spectrometry. J Anal At Spectrom 26:2064–2068
Iranzad F, Gheibi M, Eftekhari M (2018) Synthesis and application of polythiophene-coated Fe3O4 nanoparticles for preconcentration of ultra-trace levels of cadmium in different real samples followed by electrothermal atomic absorption spectrometry. Inter J Environ Anal Chem 98:16–30
Jia X, Han Y, Liu X, Duan T, Chen H (2010) Dispersive liquid–liquid microextraction combined with flow injection inductively coupled plasma mass spectrometry for simultaneous determination of cadmium, lead and bismuth in water samples. Microchim Acta 171:49–56
Khodarahmi M, Eftekhari M, Gheibi M, Chamsaz M (2018) Preconcentration of trace levels of cadmium (II) ion using Descurainia Sophia seeds as a green adsorbent for solid phase extraction followed by its determination by flame atomic absorption spectrometry. J Food Measure Charact 12:1485–1492
Koper A, Grabarczyk M (2011) Electrochemical determination of bismuth with use of a Bi (III)-cupferron complexation system and elimination of interferences connected with the presence of organic substances in natural samples. J Electroanal Chem 663:67–71
Liu L, Wang L (2006) The preparation and application of Bismuth (III) ion-selective electrode based on nanoparticles of bismuth sulfide. Anal Lett 39:879–890
Madrakian T, Afkhami A, Esmaeili A (2003) Spectrophotometric determination of bismuth in water samples after preconcentration of its thiourea-bromide ternary complex on activated carbon. Talanta 60:831–838
Maile FJ, Pfaff G, Reynders P (2005) Effect pigments-past, present and future. Prog Org Coat 54:150–163
Mulfinger L, Solomon SD, Bahadory M, Jeyarajasingam AV, Rutkowsky SA, Boritz C (2007) Synthesis and study of silver nanoparticles. J Chem Educ 84:322–330
Ojebuoboh FK (1992) Bismuth—production, properties and applications. JOM 44:46–49
Pamphlett R, Stoltenberg M, Rungby J, Danscher G (2000) Uptake of bismuth in motor neurons of mice after single oral doses of bismuth compounds. Neurotoxicol Teratol 22:559–563
Rafiee Z, Barzegar M, Sahari MA, Maherani B (2017) Nanoliposomal carriers for improvement the bioavailability of high-valued phenolic compounds of pistachio green hull extract. Food Chem 220:115–122
Rastegarzadeh S, Pourreza N, Larki A (2014) Dispersive liquid–liquid microextraction for the microvolume spectrophotometric determination of bismuth in pharmaceutical and human serum samples. Anal Methods 6:3500–3505
Shemirani F, Baghdadi M, Ramezani M, Jamali MR (2005) Determination of ultra-trace amounts of bismuth in biological and water samples by electrothermal atomic absorption spectrometry (ET-AAS) after cloud point extraction. Anal Chim Acta 534:163–169
Sun J, Liang Q, Han Q, Zhang X, Ding M (2015) One-step synthesis of magnetic graphene oxide nanocomposite and its application in magnetic solid phase extraction of heavy metal ions from biological samples. Talanta 132:557–563
Sun M, Wu Q (2011) Determination of trace bismuth in human serum by cloud point extraction coupled flow injection inductively coupled plasma optical emission spectrometry. J Hazard Mater 192:935–939
Tomaino A, Martorana M, Arcoraci T, Monteleone D, Giovinazzo C, Saija A (2010) Antioxidant activity and phenolic profile of pistachio (Pistacia vera L., variety Bronte) seeds and skins. Biochimie 92:1115–1122
Tuzen M, Soylak M, Elci L (2005) Multi-element pre-concentration of heavy metal ions by solid phase extraction on Chromosorb 108. Anal Chim Acta 548:101–108
Tuzen M, Saygi KO, Soylak M (2008) Solid phase extraction of heavy metal ions in environmental samples on multiwalled carbon nanotubes. J Hazard Mater 152:632–639
Vakilzadeh S, Eftekhari M, Chamsaz M, Javedani-Asleh F (2015) Ion pair based dispersive liquid–liquid microextraction for the preconcentration of ultra-trace levels of bismuth (III) and its determination by electrothermal atomic absorption spectroscopy. Anal Methods 7:7653–7658
Wen X, Zhao Y, Deng Q, Ji S, Zhao X, Guo J (2012) Investigation of novel rapidly synergistic cloud point extraction pattern for bismuth in water and geological samples coupling with flame atomic absorption spectrometry determination. Spectrochim Acta Part A 89:1–6
Wen X, Yang S, Zhang H, Wang J (2014) Determination of trace bismuth by using a portable spectrometer after ultrasound-assisted dispersive liquid–liquid microextraction. Anal Methods 6:8773–8779
Ziaei E, Mehdinia A, Jabbari A (2014) A novel hierarchical nanobiocomposite of graphene oxide-magnetic chitosan grafted with mercapto as a solid phase extraction sorbent for the determination of mercury ions in environmental water samples. Anal Chim Acta 850:49–56
Acknowledgements
The authors wish to thank the Ferdowsi University of Mashhad, Iran for the financial support of the project of Mr. Mustafa Alawadi (MSc thesis).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alawadi, M., Eftekhari, M., Gheibi, M. et al. Synthesis of silver nanoparticles by pistachio skin extract and its application for solid phase extraction of Bi(III) followed by electrothermal atomic absorption spectrometry. Chem. Pap. 73, 2041–2051 (2019). https://doi.org/10.1007/s11696-019-00765-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-019-00765-2