Abstract
For the first time, Descurainia Sophia (DS) seeds as an efficient and green adsorbent were used in solid phase extraction for preconcentration of trace levels of cadmium prior to its determination by flame atomic absorption spectrometry (FAAS). By using a batch method, Descurainia Sophia seeds were used as adsorbent to retain cadmium (ІІ) ions in the sample solution. After eluting the adsorbent with 3 mol L−1 HCl, the retained cadmium (ІІ) was determined by flame atomic absorption spectrometry. Different parameters affecting the extraction efficiency such as pH, amounts of adsorbent, type and concentration of eluent solvent, extraction and desorption time were investigated and optimized. Under the optimum conditions, the calibration curve was linear in the range of 5–300 µg L−1 cadmium (ІІ) with a correlation coefficient of 0.998. The limit of detection (LOD) was 1.0 µg L−1 and the relative standard deviation (RSD, %) based on seven replicate analysis of 25 µg L−1 cadmium (ІІ) was 3.2%. The accuracy of the proposed method was checked by the analysis of certified reference material (CRM) and spike methods. The results show a good agreement with certified values. The proposed method was successfully applied to determination of trace levels of cadmium in different water and rice flour samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cadmium (Cd) is one of the environmental contaminant that can be accumulating in human body causing lung and pancreatic cancer [1]. Cadmium is widely used in industry, especially in electroplating, rechargeable batteries, paint pigments and alloys [2]. As the use of cadmium in different industrial activities has increased, its presence in the environment is growing and therefore, its determination at trace levels is highly demanded. Flame atomic absorption spectrometry (FAAS) shown to be a standard analytical tool for determination of metal ions in various matrices owing to its wide application range, simplicity in operation and low cost of analysis; however because of low sensitivity of FAAS, preconcentration steps are applied to concentrate the analyte prior to its determination.
Solid phase extraction is one of the most common preconcentration steps which has the advantages of simplicity, rapidity and high enrichment factor. Different adsorbents such as modified nanoparticles [3,4,5], multiwall carbon nanotubes [6] and synthetic polymers [7, 8] have been used to preconcentrate heavy metals. Although the results show that these adsorbents are very efficient, however, most of them are naturally toxic or their modification process introduce hazardous material to environment. Therefore, the use of green and non-toxic adsorbent for preconcentration of heavy metals is an interesting subject. Many natural materials such as active carbons from leaves [9], fly ash [10], green coconut shell powder [11], spherical cellulose [12], lignocellulosic fibers [13] and walnut shell [14, 15] have been used as green adsorbents for removal or preconcentration of trace heavy metals because these biosorbents are economical, non-toxic, environmental friendly and have high adsorption capacity.
Descurainia Sophia (DS) is the member of mustard family and the seeds of this plant have been used in Traditional Chinese Medicine (TCM) to relieve cough, prevent asthma, reduce edema, and can be used in the treatment of some types of cancer [16]. The seeds of DS contain over 25% protein, 22–44% oil, 3.5–4% ash and around 7.6% fiber [17, 18]. Owing to natural functional groups such as hydroxyl, carboxyl, carbonyl and ester groups in its structure, DS seeds have a strong potential for adsorption and preconcentration of heavy metals without the need of any complicated and hazardous modification process.
For the first time, in this paper, DS seeds have been used as an adsorbent for preconcentration of trace levels of Cd (ІІ) in different real samples. Based on our knowledge there is not any publication to investigate the use of DS seeds in the field of solid phase extraction. The experimental parameters affecting the extraction efficiency such as pH, amounts of adsorbent, extraction and desorption time and type and concentration of eluent solvent were investigated and optimized conditions were selected.
Experimental
Instrumentation
A Shimadzu AA-670 (Shimadzu, Japan) flame atomic absorption spectrometer equipped with a 100-mm burner head, deuterium background correction and an air-acetylene (flow rate of 8.5:5.2 L min− 1 air:acetylene) was utilized. A cadmium hollow-cathode lamp (Hamamatsu Photonics, Shizuoka, Japan) at a wavelength of 228.8 nm operated at 4 mA with a monochromator spectral bandpass of 0.3 nm was used as a radiation source. The pH values were measured with a pH meter (Metrohm 827 pH lab, Switzerland) supplied with a glass-combined electrode. Phase separation was assisted using Centurion Scientific Centrifuge (Model Andreas HettichD72, Tuttlingen, Germany). A vortex Gilson mixer (Villiers Le Bel, France) was used to desorb the analyte from adsorbent. Laboratory Oven (Azmon Tajhiz Gostar Company, Iran) was used for drying of DS seeds.
Reagents
All reagents were of analytical reagent grade and deionized water was used throughout. A stock solution of 1000 mg L− 1 cadmium (II) ion was prepared by dissolving appropriate amounts of cadmium nitrate tetra-hydrate (Merck, Darmstadt, Germany) in 2% (v/v) HNO3. Working solutions were prepared by dilution of stock solution with deionized water. HCl (37%), HNO3 (65%) and CH3COOH (98–99.5%) as eluent solvents were purchased from Merck Company (Merck, Darmstadt, Germany). Ions are used as interfering were prepared from their salts as listed in Table 1 and provided from Merck, Darmstadt, Germany.
Preparation of adsorbent
The seeds of DS with uniform size were purchased from Alborz, Iran, and characterized by Department of Agricultural of Ferdowsi University of Mashhad, Iran. Before analysis, DS seeds were stirred in deionized water for 30 min to remove impurities and subsequently, collected through a 400 mesh sieve (with 37 µm pore size) and dried in oven at 40 °C for 24 h.
Batch method in preconcentration procedure
Fifteen millilitre of sample solution containing 100 µg L−1 Cd (ІІ) was adjusted at pH 5–6 and transferred into the vial; by addition of 0.1500 g of DS seeds as adsorbent to the sample solution, the magnetic stirrer was turned on and stirred for 25 min. After extraction, the mixture was centrifuged for 5 min by 3000 rpm. The supernatant was removed by decanting followed by addition of 500 µL of 3 mol L−1 HCl to the adsorbent. The separated solid was vortex for 1 min at 2800 rpm to desorb Cd (ІІ) and centrifuged for another 5 min at 3000 rpm. Finally, the solution containing Cd (ІІ) was sprayed into the FAAS for cadmium quantification.
Sample preparation
Different water samples including tap (Obtained from Mashhad, Iran), spring (Nowchah, Mashhad, Iran) and river (Kashaf Rood, Mashhad, Iran) waters were collected from their local sources and filtered through 0.45 µm pore size membrane filter to remove suspended particles. The samples were acidified with diluted nitric acid and stored at 5 °C. Then, 15 mL of each sample was adjusted at pH 5–6 and analyzed for Cd (ІІ) content according to the preconcentration procedure.
Rice flour sample was purchased from local supermarket in Mashhad, Iran. 2.000 g of sample was weighted and transferred into the crucible followed by heating for 2 h at 400 °C. By addition of 2 mL concentrated nitric acid to the crucible; it was heated again at 60–70 °C for 10 min. The content of crucible was filtered by Whatman No.42 filter paper and diluted to 25 mL with deionzied water. Finally, 10 mL of the prepared sample was adjusted at desired pH value and after dilution to 15 mL; it was analyzed for its Cd (ІІ) content according to the preconcentration procedure.
Results and discussions
Different parameters affecting the extraction efficiency such as pH, amounts of DS seeds, type and concentration of eluent solvent, desorption time and ionic strength were completely investigated and optimized.
Characterization of functional groups
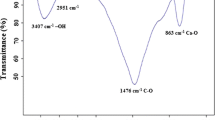
DS seeds have a strong adsorption potential for preoncentration of Cd (II) owing to various functional groups such as hydroxyl, carbonyl, sulfur, ether and ester groups on its surface. The presences of these functional groups were reported previously by other researchers [19,20,21]. Figure 1, shows the IR spectrum of powdered DS seeds. Different peaks were observed at 3441 cm−1 (the stretching vibration of O–H), 1729–1746 cm−1 (the stretching vibration of C=O), 1056 cm−1 (the stretching vibrations of C–O–C groups). 1631 cm−1 (the asymmetric vibrations of –COO−), 1369 cm 1 (the symmetric stretching vibrations of –COO−), 1240 cm−1 (the stretching vibration of C–O in carboxylic acids). These results indicate that the electrostatic and coordinative interactions between different functional groups specially –COO− group and Cd (ІІ) ions may play a major role.
Effect of pH
pH has an important effect on the absorbance of heavy metals in solid phase extraction [15]. The effect of pH on the absorbance of Cd (ІІ) was studied in the range of 2–9. Based on the results shown in Fig. 2, the absorbance increased from pH 2 to 5, leveled off at pH 5–6 and decreased gradually at higher pH values than 6. It can be concluded that at low pH values, the functional groups on the surface of DS seeds have been protonated which electrostatically repulse the positive cadmium ion. However, at high pH values cadmium hydroxide was formed in solution which can decrease the extraction efficiency. Therefore, pH 5–6 was selected as the optimum value.
Effect of amounts of DS seeds
The effect of amounts of DS seeds on the absorbance of Cd (ІІ) was studied in the range of 0.05–0.30 g. According to the results shown in Fig. 3, the absorbance of Cd (ІІ) increases by increasing the amounts of adsorbent up to 0.1500 g and with a slight decrease it remained constant afterwards. This decrease may be related to the aggregation of DS seeds and decreasing its available surface. Therefore, 0.1500 g of adsorbent was selected as the optimum value.
Effect of extraction time
The extraction time is an important parameter in solid phase extraction [15]. The effect of extraction time on the absorbance of Cd (ІІ) ion was studied in the range of 5–45 min and presented in Fig. 4. According to the results, 25 min extraction time is the optimum value.
Effect of type, concentration of eluent solvent and desorption time
In order to choose the most effective eluent for quantitative stripping of the Cd (ІІ) ion retained by the adsorbent, HNO3, HCl and CH3COOH at different concentrations, 1 mol L−1 (pH = 0 for HCl and HNO3 and pH = 2.3 for CH3COOH), 3 mol L−1 (pH= − 0.47 for HCl and HNO3 and pH = 2.1 for CH3COOH) and 5 mol L−1 (pH= − 0.70 for HCl and HNO3 and pH = 2.0 for CH3COOH) were examined. According to the results shown in Fig. 5, HCl is more efficient to desorb the Cd (ІІ) from the adsorbent. Therefore, 3.0 mol L−1 HCl (pH= − 0.47) was selected as the optimum eluent solvent. The effect of desorption time on the absorbance of Cd (ІІ) was studied by the vortex of solid phase in the range of 1–10 min at 2800 rpm. According to the results (Fig. 6), 1 min vortex time at 2800 rpm provided the maximum absorbance for determination of Cd (II) ion.
Effect of ionic strength
The effect of ionic strength on the absorbance of Cd (II) ion was studied in the range of 0.1–3% (w/v) KNO3 solution. The results in Fig. 7 show that, the absorbance remains constant up to 0.5% (w/v) and it gradually decreases at higher concentrations of KNO3.
Effect of interfering ions
The effect of different cations and anions on the adsorption of Cd (ІІ) ion on the DS seeds was completely investigated and the results are shown in Table 1. Ions are considered as interfering when their presence cause a variation in the absorbance of the sample greater than ± 5%. The results show that the proposed preconcentration method has a good potential for microextraction of Cd (ІІ) ion in different water samples.
Adsorption isotherm
For the sorption isotherms, initial metal ion concentration was varied while the pH of solution and adsorbent weight in each sample was held constant. The sorption isotherms were realized with 0.1500 g of DS seeds in 15 mL of sample solution. The effects of Cd (II) ion concentrations on the sorbent were also analyzed in terms of Langmuir [(Ce/Qe) = (1/Qm)b + (Ce/Qm)] and Freundlich isotherm (Qe=KfCen) equations, where Qe is the amount of metal ions sorbed per unit mass of the sorbent (mg g−1) and Ce (mg L−1) is the amount of metal ions in the liquid phase at equilibrium. Qm and b are the capacity parameter (mg g−1) and affinity parameter or Langmuir constant (L g−1) and Kf and n are the coefficients of Freundlich equation [15]. By dividing of Langmuir equation to Ce, the following equation [1/Qe = (1/CeQm) b + 1/Qm] was obtained. Figure 8, shows the Langmuir graph which shows that it is linear (R2 = 0.9862). Langmuir monolayer capacity, Qm, for this sorbent is 11.9 mg g−1 and therefore the seeds of DS can take up an acceptable amount of cadmium ions. Also, the Langmuir constant (b) had the values of 65.83 L g−1. The empirical Freundlich isotherm (Fig. 9) yielded a linear plot (R2 = 0.9966) and values of the coefficients n (0.61) and Kf (0.44) indicated that the sorbent had a good potential to be used as an adsorbent for Cd (II) [15]. According to these results both the Freundlich and Langmuir models are suitable for describing the biosorption equilibrium of Cd (ІІ) by DS seeds.
Analytical figures of merit
The analytical characteristics of the proposed SPE method including enrichment factor (EF), linear range, limit of detection (LOD) and relative standard deviation (RSD, %) were examined for the extraction of Cd (ІІ) from aqueous solution. Under the optimum conditions, the calibration curve was linear in the range of 5–300 µg L−1 Cd (ІІ), with a correlation coefficient of 0.998. The calibration equation was determined to be A = 0.0021C + 0.0016, where A is the analytical signal measured as absorbance and C is the concentration of Cd (II) in micrograms per liter. The limit of detection (LOD) based on three times of standard deviation of blank (n = 8) was 1.0 µg L−1 and the relative standard deviation (RSD, %) for seven replicate analysis of 25 µg L−1 Cd (ІІ) was 3.2%. The enrichment factor (EF) was calculated as the ratio of the slope of a calibration curve prepared from aqueous solutions submitted to the recommended preconcentration procedure, and that obtained without the preconcentration and found to be 20. Preconcentration factor (PF), calculated as the ratio between the volume of the aqueous phase (15 mL) and the final volume of the extraction phase (500 µL), was 30.
Analysis of real samples
In order to check the applicability of the proposed method, it was applied to determine Cd (ІІ) in different water samples. To check the accuracy of the obtained results, spike method was also examined on the samples. The results are shown in Table 2. Also, the accuracy of the method was validated by the analysis of WatR™ Supply Metals 697 (Lot number: S198-697) water sample. The observed value for the analysis of CRM was 38.6 µg L−1, which is in good agreement with the certified value of 39.8 µg L−1 Cd (II).
Comparison to other methods
Table 3 shows the comparison of the proposed method with some reported methods for determination of Cd (II). As can be seen, the proposed method has advantages such as simplicity and environmentally friendly. Also, the linear range and limit of detection of the proposed method is comparable to other reported methods.
Conclusion
For the first time, Descurainia Sophia (DS) seeds as a novel, green and efficient adsorbent was used for preconcentration of Cd (ІІ) followed by its determination by FAAS. By the use of DS seeds as adsorbent different parameters affecting the extraction efficiency were determined. The obtained results show that DS seed have good potential to remove of Cd (ІІ) from water samples which makes it a green and efficient adsorbent in solid phase extraction. Also, the results of adsorption behavior show that DS seeds obey both Freundlich and Longmuir equation for determination of Cd (ІІ).
References
G.G. Schwartz, I.M. Reis, Cancer Epidemiol. Biomark. Prev. 9(2), 139–145 (2000)
G. Buxbaum, G. Pfaff, Industrial Inorganic Pigments (Wiley-VCH, Weinheim, 1990). https://doi.org/10.1002/3527603735
N. Baghban, A.M. Haji Shabani, S. Dadfarnia, Inter. J. Environ. Anal. Chem. 93(13), 1367–1380 (2013)
A.E. Karatapanis, Y. Fiamegos, C.D. Stalikas, Talanta. 84(3), 834–839 (2011)
Y. Wang, T. Tian, L. Wang, X. Hu, Microchim. Acta. 180(3–4), 235–242 (2013)
A. Duran, M. Tuzen, M. Soylak, J. Hazard. Mater. 169(1–3), 466–471 (2009)
E. Melek, M. Tuzen, M. Soylak, Anal. Chim. Acta. 578(2), 213–219 (2006)
Y. Liu, X. Chang, S. Wang, Y. Guo, B. Din, S. Meng, Anal. Chim. Acta. 519(2), 173–179 (2004)
K. Ranganathan, Bioresour. Tech. 73, 99–103 (2000)
E. Pehlivan, S. Cetin, B.H. Yanik, J. Hazard. Mater. B135, 193–199 (2006)
G. HuamanPino, L.M.S. de Mesquita, M.L. Torem, G.A. Pinto, Miner. Engin. 19, 380–387 (2006)
M. Liu, Y. Deng, H. Zhan, X. Zhang, J. Appl. Poly. Sci. 84, 478–485 (2002)
B.G. Lee, R.M. Rowell, J. Nat. Fibers. 1, 97–108 (2004)
Y. Li, J. Yang, Y. Jiang, J. Agri. Food Chem. 60, 3033–3041 (2012)
M.H. Baki, F. Shemirani, Anal. Methods. 5, 3255–3263 (2013)
M. Khan, Y. Xiao, B. Yo, N. Wang, A. Rasul, F. Yi, L. Yang, H. Yang, T. Ma, J. Med. Plants Res. 61, 3754–3765 (2012)
J.A. Duke, E.S. Ayensu, Medicinal Plants of China. (Reference Publications Inc., Algonac, 1985), p. 705, ISBN 0-917256-20-4
N.P. Bekker, N.T. Ulchenko, A.I. Glushenkova, Chem. Nat. Comp. 41, 346–347 (2005)
R. Tavakoli, M. Mohadjerani, R. Hosseinzadeh, M. Tajbakhsh, A. Naqinezhad, Anal. Chem. Lett. 2(5), 269–274 (2012)
W.S. Feng, C.G. Li, X.K. Zheng, L.L. Li, W.J. Chen, Y.L. Zhang, Y.G. Cao, J.H. Gong, H.Z. Kuang, Nat. Prod. Res. 30(15), 1675–1681 (2016)
J.H. Gong, Y.L. Zhang, J.L. He, X.K. Zheng, W.S. Feng, X.L. Wang, H.X. Kuang, C.G. Li, Y.G. Cao, Molecules. 20, 13296–13312 (2015)
M. Chamsaz, A. Atarodi, M. Eftekhari, S. Asadpour, M. Adibi, J. Adv. Res. 4, 35–41 (2013)
H. Parham, N. Pourreza, N. Rahbar, J. Hazard. Mater. 163, 588–592 (2009)
M.R. Nabid, R. Sedghi, A. Bagheri, M. Behbahani, M. Taghizadeh, H. Abdi Oskooie, M.M. Heravi, J. Hazard. Mater. 203–204, 93–100 (2012)
A.M. Haji Shabani, S. Dadfarnia, Z. Dehghani, Talanta. 79, 1066–1070 (2009)
Acknowledgements
This work was financially supported by Ferdowsi University of Mashhad, Iran.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khodarahmi, M., Eftekhari, M., Gheibi, M. et al. Preconcentration of trace levels of cadmium (ІІ) ion using Descurainia Sophia seeds as a green adsorbent for solid phase extraction followed by its determination by flame atomic absorption spectrometry. Food Measure 12, 1485–1492 (2018). https://doi.org/10.1007/s11694-018-9763-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11694-018-9763-y