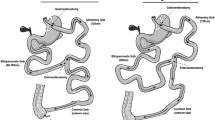
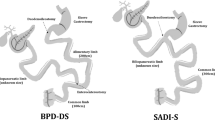
Abstract
Purpose
There are no formal guidelines for choosing among different bariatric surgery procedures for obesity treatment. So, our aim was to evaluate whether post-absorptive metabolite and hormone profiles could aid the surgeon decision when considering bariatric surgery interventions.
Materials and Methods
Subjects (N=38) previously submitted to biliopancreatic diversion with duodenal switch (BPD-DS, n=9), single anastomosis duodenal–ileum bypass with sleeve gastrectomy (SADI-S, n= 9), long biliopancreatic limb Roux-en-Y gastric bypass (RYGB-M, n= 11), and classic RYGB (RYGB-C, n= 9) underwent a mixed meal test to evaluate post-absorptive glucose, total amino acid (AA), insulin, and GLP-1 profiles.
Results
Glucose, AA, insulin, and GLP-1 excursions were lower after BPD-DS when compared to other surgeries. SADI-S resulted in lower glucose but similar AA and insulin excursions when compared to RYGB-M. The highest GLP-1 excursion was observed after RYGB-M. There were no significant differences in glucose or AA post-prandial excursions between RYGB procedures, yet insulin excursion was higher after RYGB-C when compared to RYGB-M.
Conclusion
Post-prandial metabolite excursions diverge across bariatric procedures being lowest after BPD-DS, intermediate after SADI-S, and highest after RYGB, in parallel with the anti-diabetic efficacy and malabsorption risk reported for each type of intervention. SADI-S and RYGB-M seem to elicit similar post-prandial hormonal profiles, with potentially lower risk of protein malnutrition when compared to BPD-DS. Post-absorptive metabolite and hormone profiles could provide a rationale as decision-aid when choosing among bariatric surgery interventions, as long as these findings are validated in future trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bariatric surgery is the most effective treatment for severe obesity and obesity-related comorbid conditions [1]. Surgery has demonstrated to be particularly effective and even superior to conventional medical interventions when treating obesity-related type 2 diabetes (T2D). The sequence of events leading to overt diabetes are characterized by a vicious cycle of glucotoxicity, lipotoxicity, and systemic inflammation, which is responsible for worsening insulin resistance and triggers progressive beta cell failure with decreased insulin secretion [2].
Several different bariatric surgery procedures are currently performed worldwide. However, it is still controversial whether any bariatric surgery intervention significantly outperforms the others within acceptable risk, when the primary aim is to target diabesity [3]. One of the main reasons for this knowledge gap is the fact that there are no data from randomized clinical trials comparing the outcomes of homogeneous patient groups submitted to different bariatric techniques. Moreover, it is unlikely that such solid data to enable conclusions will be available in the near future, given the difficulties inherent in conducting a large controlled clinical trial in this area. Therefore, it is understandable that no formal patient-tailored guidelines are yet available to guide the choice of a bariatric procedure. Rather the choice remains at the surgeon’s discretion in agreement with patient consent.
Thus, our aim was to evaluate whether a comparison of the post-absorptive metabolite and entero-pancreatic profiles after different bariatric procedures could aid the surgeon decision when selecting surgical technique for the treatment of patients with obesity.
Materials and Methods
Participants
Subjects (n=38) previously submitted to bariatric surgery interventions were selected among the post-bariatric surgery cohort under routine follow-up at a single bariatric center in a public hospital. Patients without T2D diagnosis (Hb1Ac < 6.5% and fasting blood glucose < 126 mg/dL), with identical post-operative BMI, and who were weight-stable (defined as a body weight variation under 5% of total body weight) during the previous 6 months were included in this study (Table 1). Pre-diabetes was defined as Hb1Ac of 5.7–6.4% or/and a fasting blood glucose of 100–125 mg/dL.
The study protocol and the patient information leaflet were approved by the Institutional Ethical Review Board. Written informed consent was obtained from all participants before enrolment and the study was conducted according to Data Protection Regulations.
Study Design
Patients submitted to 4 different bariatric surgery techniques: biliopancreatic diversion with duodenal switch (BPD-DS) (n=9), single anastomosis duodenal–ileum bypass with sleeve gastrectomy (SADI-S) (n=9), long biliopancreatic limb RYGB (RYGB-M) (n=11), and classic Roux-en-Y gastric bypass (RYGB-C) (n=9), were subjected to a liquid mixed meal test (Fresubin Energy Drink, 200 mL, 300 kcal; Fresenius Kabi Deutschland, Bad Homburg, Germany). Blood samples were collected before the meal and at 15, 30, 60, 90, and 120 min after the start of the mixed meal intake. Whole blood glucose was measured during the MMTT, using a glucometer (Freestyle Precision Neo Glucose meter, Abbott, USA).
Plasma separation was performed by centrifugation at 2500g during 12min. Plasma insulin, GLP-1, and amino acid levels were quantified. Insulin was measured by an electrochemiluminescence sandwich immunoassay (ECLIA) on a Cobas 8000 e602 module (Roche Diagnostics, Mannheim, GmbH), following the manufacturer instructions. The coefficient of variation was below 5%, using liquid human serum-based controls (Liquichek™ Immunoassay Plus Control: Level 1 #361 and Level 3 #363, Bio-Rad).
Total glucagon-like peptide-1 (GLP-1) levels were measured by radioimmunoassay (RIA) targeting the C-terminal of GLP-1 (antiserum 89390), after extraction using 70% ethanol. Hormone moieties were separated with plasma-coated charcoal (E. Merck, Darmstadt, Germany).
Total amino acid levels were quantified using an enzyme-linked immunosorbent assay (ELISA) kit (catalog no. ab65347; Abcam, Cambridge, UK), following the manufacturer instructions.
Surgical Procedures
All four bariatric surgical procedures were performed by the same team of bariatric surgeons in a standardized manner, as previously described [4, 5]. RYGB techniques were performed with a constant 120-cm alimentary limb and a 15-mL gastric pouch. RYGB variants only differed in the biliopancreatic limb length: 60–100 cm for the standard RYGB-C and 200 cm for the RYGB-M variant. SADI-S procedure was performed with a common channel of 300cm, while BPD-DS was performed with a 100 cm common limb and an alimentary limb of 200 cm.
Statistical Analysis
Incremental area under the curve was calculated using the trapezoid rule and subtracting the basal values.
Nominal variables are expressed as number of cases and percentage (%), and the continuous variables are expressed as mean ± standard deviation. D’Agostinho and Pearson test was used to evaluate variable normality. For variables that passed this test, one-way ANOVA with the post hoc Tukey test was used. For the variables that did not pass the normality test, the Kruskal–Wallis with a post hoc Dunn’s test was used. MMTT result analysis was performed using a two-way analysis of variance (ANOVA) with Sidak’s post hoc test. Statistical analysis was performed using the GraphPad Prism (GraphPad Software, La Jolla, CA, USA). Differences resulting in p < 0.05 were considered significant.
Results
In patients submitted to malabsorptive surgeries (SADI-S and BPD-DS), pre-operative BMI was higher compared to patients submitted to gastric bypass. Nevertheless, all study groups had similar post-operative BMI (Table 1). None of the study subjects has had a prior T2D diagnosis, although study groups included patients with pre-diabetes, with the sole exception of the RYGB-C group. This distribution was entirely random as this was not a pre-specified exclusion criterion included in the study design. Still, pre-diabetes status after bariatric surgery reverted in every single patient with prior pre-diabetes criteria.
Different bariatric surgery procedures elicit a peculiar post-operative hormonal and metabolite profile (Table 2).
After BPD-DS, glucose and amino acid (AA) post-prandial excursions, as well as insulin and GLP-1 levels, were lower when compared to all other bariatric surgery procedures (Fig. 1 and Table 2). BPD-DS showed significantly lower glucose levels at 30 min after the meal when compared to SADI-S, as well as lower glucose levels at t=15min, t=30min, and t=45min when compared to both RYGB procedures (Fig. 1A). Post-prandial levels of total amino acids were also significantly lower in the BPD-DS group as compared to both RYGB procedures at 15, 30, 45, and 60 min after the meal and compared to SADI-S at t=30 and t=45min (Fig. 1B). BPD-DS showed significantly lower insulin levels at t=45min as compared to RYGB-M and at t = 15min, t=30min, and t=45min, when compared with the RYGB-C procedure (Fig. 1C). GLP-1 levels were significantly lower at t=30 min post-meal after BPD-DS as compared to SADI-S and both RYGB procedures (Fig. 1D).
Glycemia (A), total amino acids (B), insulin (C), and GLP-1 levels (D) in 38 non-diabetic weight-stable subjects previously submitted to classical RYGB (RYGB-C, n=9), long BPL RYGB (RYGB-M, n=11), BPD-DS (n=9), or SADI-S (n=9) after ingestion of a standard mixed meal served at t = 0 min. aBPD-DS vs RYGB-C: p<0.05; bBPD-DS vs RYGB-M: p<0.05; cSADI-S vs RYGB-C: p<0.05; dSADI-S vs RYGB-M: p<0.05; eBPD-DS vs SADI-S: p<0.05; fRYGB-M vs RYGB-C: p<0.05; two-way ANOVA
SADI-S showed significantly lower plasma glucose levels at t=15min and t=30min after the meal, as compared to both RYGB procedures. AA, GLP-1, and insulin post-prandial excursions after SADI-S and RYGB-M were not significantly different (Fig. 1A, B). The highest GLP-1 response was elicited by RYGB-M surgery (Table 2).
No significant differences in glucose or AA post-prandial excursions were observed between RYGB-C and RYGB-M procedures, yet insulin levels observed at t=30min and t=45min after RYGB-C were significantly higher when compared to RYGB-M (Fig. 1A–C).
Discussion
This study compared the post-absorptive metabolite and entero-pancreatic hormone profiles of patients previously submitted to BPD-DS, SADI-S, RYGB-M, and RYGB-C, weight stable and with similar post-operative BMI.
The post-surgical hormonal and absorption patterns of patients submitted either to malabsorptive surgeries (BPD-DS vs SADI-S) or two RYGB variants (RYGB-M vs RYGB-C) were already reported by our group [5,6,7,8]. Nonetheless, the post-absorptive profiles after malabsorptive surgeries versus RYGB have not been previously compared and are herein reported for the first time. Although the variables presented in this current study are the same, the goal of the analysis was completely different. In this study, our aim is to compare the post-operative endocrine profile after four different surgical interventions: BPD-DS vs SADI-S vs RYGB-M vs RYGB-C. Hormonal and metabolic responses elicited by the surgeries are key determinants of the bariatric surgeries’ success [9]. Therefore, we hypothesized that instead of focusing on the malabsorptive component of the surgical technique and patient BMI for choosing a given bariatric procedure, a decision could also be based on the predicted endocrine profile aiming to improve metabolic outcomes.
Our findings show that BPD-DS stands out with a lower post-prandial glycemic excursion along with lower insulin secretion, while the classic RYGB occupies the opposite extreme, with the greater post-prandial glycemic excursion and exacerbated insulin response. This post-prandial glycemic profile observed after BPD-DS is easily recognized in the clinic with the highest long-term T2D remission rate, while reactive hyperinsulinemic hypoglycemia is seldomly reported [10]. After intestinal bypass procedures, the modification of upper gastrointestinal tract anatomy leads to rapid glucose absorption with a subsequent insulin spike, as observed after RYGB-C. This contrasts with the consistently lower glucose and insulin excursions after an oral glucose challenge or mixed meal test observed after BPD-DS, but not RYGB [11]. In addition, BPD-DS patients present the lowest post-prandial AA excursion and so greater risk of protein malabsorption.
SADI-S and RYGB-M are two bariatric procedures that present post-absorptive profiles that seem to lie in the middle of the spectrum and to stand out from both BPD-DS and RYGB-C. Post-prandial glucose excursions after SADI-S are greater than those observed after BPD-DS and lower when compared to those of patients submitted to RYGB, while no significant differences in glucose excursion were observed between the RYGB variants. Yet, the post-prandial insulin secretion profiles observed after SADI-S and RYGB-M were significantly lower when compared to RYGB-C. Thus, the distinctive post-prandial glucose excursion and insulin secretion patterns suggest that a greater post-prandial glycemic variability is more likely to be observed after RYGB-C as compared to SADI-S.
As far as the entero-pancreatic hormone response is concerned, BPD-DS was characterized by the least pronounced post-prandial GLP-1 response, which clearly distinguishes this bariatric procedure from the other techniques. This is in agreement with earlier studies where RYGB consistently is reported to enhance post-prandial GLP-1 responses which are less affected by BPD-DS. Although post-prandial GLP-1 responses after BPD-DS are twofold enhanced, the increase is much greater after RYGB [12].
Overall, the post-prandial hormone profiles observed after different bariatric surgery procedures probably reflect the different mechanisms underlying weight loss and glucose-lowering effects, which seem to be dependent on the anatomical gut rearrangement associated with each procedure. GLP-1 is a gut hormone known to decrease appetite and promote weight loss. Although SADI-S leads to a higher GLP-1 excursion as compared to BPD-DS, the highest GLP-1 response is elicited by RYGB-M.
In the quest for a decision-aid rationale to guide the surgeon when choosing between procedures, we sought to compare the post-prandial metabolite and hormone profiles after four different commonly performed bariatric surgery techniques.
In patients with presumably preserved pancreatic endocrine function, these bariatric surgery techniques can be separated according to insulin and GLP-1 post-prandial response into 3 classes: a first including the RYGB-C, with a greater post-prandial insulin excursion curve; a second including RYGB-M and SADI-S, which share similar hormonal post-prandial responses; and a third comprising BPD-DS that can be distinguished from the other procedures by a lower insulin and GLP-1 response along with significantly lower plasma glucose excursion. Post-absorptive plasma AA levels can be used as malabsorption surrogates, which also allows to separate the surgical procedures into 2 different classes: one that includes the two RYGB variants and SADI-S with a lesser degree of protein malabsorption, since post-prandial AA excursions are higher after SADI-S and the two RYGB variants, and BPD-DS with the lowest post-prandial AA excursion and greater protein malabsorption risk.
Patients submitted to malabsorptive surgeries (SADI-S and BPD-DS) had a higher pre-operative BMI as compared to patients submitted to gastric bypass, which is an inevitable consequence of the fact that currently the choice between surgical procedures is largely grounded on patients’ pre-operative BMI.
The BPD-DS group has a higher percentage of males when compared with other groups; however, it does not represent a bias for our conclusion since the statistical analysis performed in the female patient’s subgroup yielded similar results (data not shown).
Since our surgical team has a longer experience performing RYGB procedures when compared with SADI-S and BPD-DS, the post-operative time of patients submitted to SADI-S and BPD-DS was shorter. Nevertheless, only patients with stable weight and in the same current BMI range were included to limit patient weight as a confounding factor.
Nonetheless, it should be highlighted that this data alone does not provide enough evidence to aid the bariatric surgeon decision in the choice among different procedures for each individual patient. However, despite the limitations previously acknowledged, this study still yielded important findings that could be used as preliminary data and rationale to design controlled randomized clinical trials in order to gain further understanding of the post-absorptive metabolite and hormone profile contribution as decision-aid when choosing among bariatric surgery interventions.
In conclusion, our data supports that excluding weight loss from the algorithm, BPD-DS is the bariatric procedure with the highest probability of achieving euglycemia without risk of post-prandial hyperinsulinemia, although at the expense of a higher risk of nutrient malabsorption. In contrast, both SADI-S and RYGB-M seem to elicit a similar and favorable post-prandial hormonal profile, with a potentially lower risk of protein malnutrition when compared to BPD-DS and lower risk of post-prandial hyperinsulinemia as compared to RYGB-C. In the future, post-absorptive metabolite and hormone profiles could provide a rationale as decision-aid when choosing among bariatric surgery interventions, as long as these findings are validated in future trials.
References
Cummings DE, Rubino F. Metabolic surgery for the treatment of type 2 diabetes in obese individuals. Diabetologia. 2018 Feb;61(2):257–64.
Jezek P, Jaburek M, Holendova B, Plecita-Hlavata L. Fatty acid-stimulated insulin secretion vs. lipotoxicity. Molecules. 2018 19;23(6).
El Khoury L, Chouillard E, Chahine E, et al. Metabolic surgery and diabesity: a systematic review. Obes Surg. 2018;28(7):2069–77.
Nora M, Morais T, Almeida R, et al. Should Roux-en-Y gastric bypass biliopancreatic limb length be tailored to achieve improved diabetes outcomes? Medicine (Baltimore). 2017;96(48):e8859.
Pereira SS, Guimaraes M, Almeida R, et al. Biliopancreatic diversion with duodenal switch (BPD-DS) and single-anastomosis duodeno-ileal bypass with sleeve gastrectomy (SADI-S) result in distinct post-prandial hormone profiles. Int J Obes. 2019;43(12):2518–27.
Jarak I, Pereira SS, Carvalho RA, et al. Gastric bypass with different biliopancreatic limb lengths results in similar post-absorptive metabolomics profiles. Obes Surg. 2020;30(3):1068–78.
Patricio BG, Morais T, Guimaraes M, et al. Gut hormone release after gastric bypass depends on the length of the biliopancreatic limb. Int J Obes. 2019;43(5):1009–18.
Pereira SS, Jarak I, Carvalho RA, Oliveira PF, Alves MG, Guimarães M, et al. Different malabsorptive obesity surgery interventions result in distinct postprandial amino acid metabolomic signatures. Obes Surg. 2020.
Guimarães M, Pereira SS, Monteiro MP. From entero-endocrine cell biology to surgical interventional therapies for type 2 diabetes. Adv Exp Med Biol. 2021;1307:273–97.
Sudan R, Jain-Spangler K. Tailoring bariatric surgery: sleeve gastrectomy, Roux-en-Y gastric bypass and biliopancreatic diversion with duodenal switch. J Laparoendosc Adv Surg Tech A. 2018;28(8):956–61.
Pontiroli AE, Gniuli D, Mingrone G. Early effects of gastric banding (LGB) and of biliopancreatic diversion (BPD) on insulin sensitivity and on glucose and insulin response after OGTT. Obes Surg. 2010;20(4):474–9.
Bradley D, Magkos F, Klein S. Effects of bariatric surgery on glucose homeostasis and type 2 diabetes. Gastroenterology. 2012;143(4):897–912.
Acknowledgements
The authors would like to thank Bárbara Patrício (UMIB, Porto, Portugal), Ivana Jarak (UMIB, Porto, Portugal), and Nicolai J. Wewer Albrechtsen (Faculty of Health and Medical Sciences, University of Copenhagen, Denmark), for their help with the hormones and metabolomic assays.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Guimarães, M., Pereira, S.S., Holst, J.J. et al. Can Metabolite and Hormone Profiles Provide a Rationale for Choosing Between Bariatric Procedures?. OBES SURG 31, 2174–2179 (2021). https://doi.org/10.1007/s11695-021-05246-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-021-05246-8