Abstract
Background
This study evaluated changes in serum high-density lipoprotein cholesterol (HDL-C) induced by laparoscopic bariatric surgery (BS) in non-diabetic obese subjects with low (L-HDL-C) or normal (N-HDL-C) levels of HDL-C. We assessed whether increased HDL-C is associated with weight loss, serum non-HDL cholesterol (non-HDL-C), serum triglycerides (TG), and physical activity (PA) before and 6 and 15 months after BS.
Methods
In this prospective cohort study, 71 subjects undergoing BS (42 by Roux-en-Y gastric bypass and 29 by sleeve gastrectomy) were evaluated for the % Excess Weight Loss (%EWL), waist circumference (WC), serum levels of glucose, glycosylated hemoglobin, TG, HDL-C, non-HDL-C, and LDL-C, and the degree, time, and energy expenditure related to PA. The short version of the International Physical Activity Questionnaire was used to assess PA.
Results
Levels of HDL-C significantly increased 15 months after BS (p < 0.001) in subjects with low (p < 0.001) or normal (p = 0.017) values at baseline. A similar %EWL, decrease in WC, glucose, non-HDL-C, and LDL-C levels and increase in energy expenditure related to PA, was observed in both groups (L-HDL-C and N-HDL-C) at 15 months after BS. However, the L-HDL-C group presented greater decrease in TG levels compared with N-HDL-C group (p = 0.004). In subjects with increased HDL-C 15 months after BS, there was an association between this increase and the %EWL (p = 0.030), but there was no association with the change in PA.
Conclusions
Irrespective of PA after BS, subjects with low and normal HDL-C levels at baseline showed an increase in HDL-C after BS, and this increase was associated with %EWL induced by BS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One of the key risk factors used by clinicians to assess cardiovascular risk (CVR) is the serum level of high-density lipoprotein cholesterol (HDL-C) [1]. According to large-scale studies, a low concentration of serum HDL-C increases CVR [2], and for each 1 mg/dL increase in HDL-C, there is a 2–3% decrease in the risk of coronary artery disease (CAD) [3]. There are several well-known risk factors for low HDL-C, including a high body mass index (BMI), alcoholism, smoking, type 2 diabetes mellitus (T2DM), the use of certain drugs like beta blockers and anabolic steroids, a sedentary lifestyle [3, 4], and a genetic predisposition [5].
There is a positive correlation between BMI and non-HDL cholesterol (non-HDL-C) and triglyceride (TG) levels [6], and it has also been reported that a higher BMI increases the risk for hypertriglyceridemia, high LDL cholesterol (LDL-C), and low HDL-C [7]. Physical activity (PA), as well as certain drugs and interventions, is known to decrease non-HDL-C, which could lead to an increase in serum HDL-C [8].
It has been estimated that about 40–60% of the variability in HDL-C can be explained by heritability [5]. Loss-of-function mutations in some genes cause conditions with extremely low HDL-C and, in contrast, loss-of-function mutation variants in other genes are associated with extremely high HDL-C [9, 10]. Because of this, the response of HDL-C levels to dietary changes, exercise, and medications are not homogeneous, and the extent of the response varies with different baseline levels of serum HDL-C.
In subjects with severe obesity, bariatric surgery (BS) results in significant and sustained weight loss, in addition to reductions in all-cause mortality and cause-specific mortality associated with CAD [11]. Furthermore, BS is considered safe and effective for treating these patients. In our country, it is currently recommended for patients with a BMI ≥ 40 kg/m2 or for those with a BMI between 35 and 39.9 kg/m2 who have at least one comorbidity [12]. In addition, BS has been shown to decrease LDL-C, TG, and non-HDL-C levels and increase HDL-C [13, 14].
An increase in HDL-C could be one of the factors related to the decrease in mortality associated with BS. In subjects with low HDL-C, efforts to use statins, niacin, and cholesteryl ester transfer protein inhibitors to raise HDL-C levels and to protect against cardiovascular disease (CVD) have been disappointing [15]. Thus, it seems of great importance to determine the magnitude of the effect of the HDL-C serum response to the weight reduction induced by BS. As exercise-induced weight loss has been shown to affect HDL-C concentrations in men to a different extent depending on their baseline level [16], we decided to evaluate the response to BS in subjects with low HDL-C serum levels prior to BS.
In patients undergoing BS, an increased level of PA is always precociously prescribed as an auxiliary measure to lose weight and then maintain weight loss. It is also expected that patients who lose a higher percentage of weight will have greater mobility, thus an increase in PA will probably occur irrespective of counseling, which could also influence HDL-C levels.
There were three main aims of the present study: (1) to determine whether serum HDL-C is increased 15 months after BS; (2) to assess whether the changes in HDL-C levels after BS are related to weight loss, increased PA or decreased serum non-HDL-C, and TG levels; and (3) if the increase in HDL-C induced by BS differs between subjects whose HDL-C levels prior to BS were normal or low.
Materials and Methods
Study Design and Population
A prospective cohort study was conducted in 71 obese subjects undergoing laparoscopic BS, performed using the Roux-en-Y gastric bypass (RYGB) or sleeve gastrectomy (SG) techniques. All participants were evaluated for PA and energy expenditure preoperatively (T0), then again at 6 (T1) and 15 (T2) months postoperative. The study was conducted from April 2016 to June 2019. This study used data from a cohort of patients that looked to evaluate the incidence and progression of peripheral polyneuropathy after BS. Participants with a follow up of 15 months after BS were chosen to participate. The subjects were evaluated by two evaluators during routine consultations at the hospital. Subjects of both sexes were included if they were older than 18 years of age, met the criteria for BS, and had HDL-C levels at baseline and 6 and 15 months postoperatively. The exclusion criteria were T2DM, cancer, decompensated hypothyroidism, pregnancy, infants, alcoholism, smoking, or the use of statins, fibrates, beta blockers, or steroids.
From 132 initial subjects, 61 were excluded. Of the excluded subjects, 12 had T2DM, three had decompensated hypothyroidism, seven were smokers, two were alcoholic, eight used beta blockers, nine used statins, one was HIV positive, and 19 had no serum HDL-C measurements.
Diet and Physical Activity Recommendations
In the pre- and postoperative periods, all subjects received a prescribed balanced diet in addition to other recommendations such as to eat 5 to 6 meals per day and to increase their PA by completing at least 150 min of walking per week. All subjects received multivitamin supplementation in the postoperative periods. After BS, outpatient visits were scheduled for every 3 months.
Anthropometric, Clinical, and Laboratory Data
Body weight, height, BMI, and waist circumference (WC) were measured and health history data were obtained from medical records in the basal and postoperative periods. Serum levels of glucose, glycosylated hemoglobin (HbA1c), HDL-C, LDL-C, non-HDL-C, and TG were also collected. The excess weight (EW) was calculated from the difference between pre- or postoperative body weights, with the ideal weight corresponding to a BMI of 25 kg/m2, and the % Excess Weight Loss (%EWL) was obtained by the difference between the subject’s pre- and postoperative body weight divided by EW. We also calculated % Total Weight Loss (%TWL) and % Adjustable Weight Loss (%AWL) according to van de Laar et al. [17].
Physical Activity and Energy Expenditure
To evaluate PA, the short version of the International Physical Activity Questionnaire (IPAQ) [18] was used, which evaluates daily activities over the last 7 days. The participants were classified according to the frequency and duration of the different types of PA. The time spent sitting on weekdays and the weekend was also recorded. In addition, patients were evaluated for whether they completed ≥ 150 min/week of PA (sum of PA from IPAQ), as recommended by the World Health Organization and the American College of Sports Medicine.
Using data collected in the IPAQ, energy expenditure was quantified and defined as the metabolic equivalent of task (MET) in min/week. The calculation of energy expenditure was performed using the Ainsworth formula [19].
HDL Cholesterol Group Definition
Subjects were divided into two groups according to their serum HDL-C levels before BS: those who had low HDL-C (L-HDL-C group, < 40 mg/dL for men and < 50 mg/dL for women), and those who had normal HDL-C (N-HDL-C group, ≥ 40 mg/dL for men and ≥ 50 mg/dL for women). An increase in HDL-C 15 months after BS was defined as a percentage equal to or greater than the median obtained for each group.
Statistical Analysis
Quantitative data are presented as the median and interquartile range. Normality was defined by the Shapiro-Wilk test. To compare the L-HDL-C and N-HDL-C groups and groups with no change or increased HDL-C at T2, we used the Mann-Whitney U test. For comparing T0 and T2 in each group, Wilcoxon’s test was used. Categorical data are presented as absolute values and frequencies. Yates’ continuity correction or Pearson’s chi-square test were used to compare L-HDL-C and N-HDL-C groups and groups with no change or increased HDL-C at T2. The McNemar test was used to compare T0 and T2 in each group. Data were analyzed using SPSS version 18.0, and values with p ≤ 0.05 were considered statistically significant.
For sample calculation, we used data from the 2017 study by Praveen Raj et al. [20], which investigated changes in the lipid profile 1 year after RYGB and SG. They found an increase in serum HDL-C levels from 41.40 ± 9.98 to 52.06 ± 16.16 mg/dL after 1 year. Considering a power of 80% and a confidence interval of 5%, 74 subjects would be needed, with 37 in each group. Assuming a loss of 10%, there would be 82 subjects. The Sealed Envelope website was used for the sample calculation [21].
Results
Table 1 presents the demographic, anthropometric, and PA data of the 71 non-diabetic obese subjects at baseline. Before BS, when the L-HDL-C group was compared with the N-HDL-C group, the number of females, subject age, BMI, WC, EW, serum glucose, HbA1c and LDL-C levels, and PA and energy expenditure measures were similar. The serum TG and non-HDL-C levels were higher in the L-HDL-C group compared with the N-HDL-C group (p = 0.001 and p = 0.013, respectively), and, as expected, differences between groups were observed for HDL-C levels (p < 0.001).
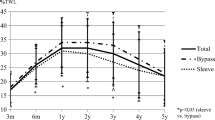
Fifteen months after BS, patients had a mean %EWL of 90.5 ± 20.4%, correspondingly to a mean reduction in BMI of 14.7 ± 5.1 kg/m2, and there was a significant reduction in CVR indicated by a change in the total cholesterol:HDL-C ratio from 4.3 to 2.8 (p < 0.001; data not shown).
Changes in anthropometric, laboratory, and PA measures at 6 (T1) and 15 (T2) months post-BS for the L-HDL-C and N-HDL-C groups are shown in Table 2. BS was associated with a similar decrease in the L-HDL-C and N-HDL-C groups at 15 months for body weight (37.9% versus 34.4%, p = 0.080), BMI (37.9% versus 33.9%, p = 0.078), WC (26.8% versus 25.9%, p = 0.190), and serum LDL-C (26.6% versus 29.5%, p = 0.498) and non-HDL-C 39.5% versus 35.0%, p = 0.781) levels. However, higher decrease in serum TG levels (52.6% versus 40.6%, p = 0.014) and higher increase in serum HDL-C levels (27.4% versus 14.6%, p = 0.043) were found in the L-HDL-C group compared with N-HDL-C group. Six months after BS, neither group showed an increase in HDL-C from baseline, but a significant increase appeared 15 months after BS. Walking time, PA, energy expenditure, and the number of subjects completing ≥ 150 min/week of PA increased from T0 to T2 in both groups, with no differences observed between the two groups for these parameters.
Table 3 presents data looking for association between increased HDL-C with changes in body weight, BMI, %EWL, %TWL, %AWL, WC, glucose, HbA1c, TG, non-HDL-C, LDL-C, walking time, total PA, total sitting time, PA ≥ 150 min/week, and energy expenditure and abnormal values of serum glucose, HbA1c, HDL-C, Non-HDL-C, and TG levels before BS. Only %EWL was associated with an increase in HDL-C (p = 0.030), but only in subjects who presented an increase in HDL-C 15 months after BS. Of the 31 subjects with low HDL-C at baseline, 16 (51.6%) increased their levels at T2, and of the 40 subjects with initially normal HDL-c levels, 20 (50.0%) increased their levels after 15 months of BS. In the L-HDL-C group, 71.0% (n = 22) normalized their levels at T2, and in the N-HDL-C group, 87.5% (n = 35) maintained their normal levels.
Discussion
The observation that there was a correlation between low serum levels of HDL-C with increased mortality from CVD led researchers to seek strategies to increase the levels of HDL-C in people with low HDL-C [22]. In the search for these treatments, it was seen that subjects’ responses could vary depending on their baseline HDL-C levels, where individuals with low HDL-C levels presented greater resistance to response to different therapeutic proposals [23].
Among the treatments proposed to increase the HDL-C levels of individuals at increased risk for atherosclerotic disease, the most noteworthy include a diet low in saturated fat, physical exercise, and the use of drugs such as statins and nicotinic acid [15]. As a consequence of this change in diet, it is common to observe weight loss and even an increase in PA; however, it is not clear whether the effect on HDL-C is due to the low-calorie diet, restriction of fats (with a consequent decrease in the influx of lipoproteins from the intestine to circulation), increased PA, or weight loss.
In the present study, we sought to clarify the effect of BS on HDL-C levels in individuals with low or normal serum HDL-C levels before BS. We observed a mean increase in HDL-C level post-BS of 10.0 mg/dL. The increase in HDL-C occurred when the baseline values were low or normal, and both groups simultaneously showed decreases in non-HDL-C, TG, LDL-C, and fasting plasma glucose, along with an increase in PA. In the subjects who showed increased HDL-C after BS, there was an association between this increase and the %EWL.
The typical dyslipidaemia described in obese patients consists of elevated fasting and postprandial TGs, reduced HDL-C, and normal or slightly elevated LDL-C [24]. However, for each patient, the serum HDL-C level depends on their genetic predisposition, degree of obesity, diet, non-HDL-C levels, daily PA, and the presence of other co-morbidities that could decrease HDL-C, such as T2DM, hypothyroidism, smoking, and alcoholism [22].
In patients submitted to BS, both a decrease in the influx of lipoproteins from the diet and an increase in fatty acid turnover (due to a loss of fat mass associated with greater PA and/or use of reserves due to caloric restriction) are predicted, and these effects could be related to an increase in HDL-C levels. In fact, studies that assessed the effect of BS have shown no change or increase in HDL-C after 1 year of follow up [20]. In an attempt to elucidate this discrepancy, the present study is the first longitudinal evaluation with 15 months of follow up and the first to look into the effect of BS on patients with low HDL-C levels at baseline.
In order to decrease the impact of co-morbidities that could influence HDL-C levels on the metabolic results observed after the surgery, patients with these conditions were excluded from our sample. Taking into account these exclusions, we believe that the results for lipid measurements and glycaemic control can be predominantly attributed to the effects of the surgery and the subsequent change in behavior, as well as the genetic predisposition.
In the present study, we clearly show that in the majority of patients submitted to BS, 15 months after BS, HDL-C increases and the increase occurs in patients with and without abnormalities on their HDL-C serum levels. The mean HDL-C increased to 11.3 mg/dL in the L-HDL-C group and 9.0 mg/dL in the N-HDL group. Given that for every 1 mg/dL increase in HDL-C, there is a 2–3% decrease in the risk of CAD [2]; the percentage decrease in the risk of CVD in our subjects was 18.0–33.9%.
The strengths of our study include the evaluation of subjects with low HDL-C levels at baseline and the subsequent follow up at 15 months. A limitation of the study was that the subjects evaluated did not have a very high risk for CVD according to the Framingham score [25].
Conclusion
Subjects with low and normal HDL-C levels before BS showed an increase in serum HDL-C levels after 15 months, and this increase was associated with the %EWL. In addition, it was possible to show that BS decreased the CVR of subjects, even when they had low HDL-C. In order to evaluate the benefits of the observed HDL-C increase, it will be important to include subjects with a higher CVR in future studies.
References
Gordon T, Castelli WP, Hjortland MC, et al. High density lipoprotein as a protective factor against coronary heart disease. The Framingham. Study Am J Med. 1977;62(5):707–14.
Gordon DJ, Probstfield JL, Garrison RJ, et al. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation. 1989;79(1):8–15.
Chapman MJ, Assmann G, Fruchart JC, et al. Raising high-density lipoprotein cholesterol with reduction of cardiovascular risk: the role of nicotinic acid--a position paper developed by the European consensus panel on HDL-C. Curr Med Res Opin. 2004;20(8):1253–68.
Jung UJ, Choi MS. Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int J Mol Sci. 2014;15(4):6184–223.
Weissglas-Volkov D, Pajukanta P. Genetic causes of high and low serum HDL-cholesterol. J Lipid Res. 2010;51(8):2032–57.
Cong L, Zhan JQ, Yang L, et al. Overweight and obesity among low-income Muslim Uyghur women in far western China: correlations of body mass index with blood lipids and implications in preventive public health. PLoS One. 2014;9(2):e90262.
Yang Z, Ding X, Liu J, et al. Associations between anthropometric parameters and lipid profiles in Chinese individuals with age ≥ 40 years and BMI <28kg/m2. PLoS One. 2017;12(6):e0178343.
Rye KA, Bursill CA, Lambert G, et al. The metabolism and anti-atherogenic properties of HDL. J Lipid Res. 2009;50:S195–200.
Hegele RA. Plasma lipoproteins: genetic influences and clinical implications. Nat Rev Genet. 2009;10(2):109–21.
Larach DB, Cuchel M, Rader DJ. Monogenic causes of elevated HDL cholesterol and implications for development of new therapeutics. Clin Lipidol. 2013;8(6):635–48.
Adams TD, Mehta TS, Davidson LE, et al. All-cause and cause-specific mortality associated with bariatric surgery: a review. Curr Atheroscler Rep. 2015;17(12):74.
Conselho Federal de Medicina. Resolução CFM n° 2.131/2015. Diário Oficial da União. 2016;8(1):66.
Kjellmo CA, Karlsson H, Nestvold TK, et al. Bariatric surgery improves lipoprotein profile in morbidly obese patients by reducing LDL cholesterol, apoB, and SAA/PON1 ratio, increasing HDL cholesterol, but has no effect on cholesterol efflux capacity. J Clin Lipidol. 2018;12(1):193–202.
Cunha FM, Oliveira J, Preto J, et al. The effect of bariatric surgery type on lipid profile: an age, sex, body mass index and excess weight loss matched study. Obes Surg. 2016;26(5):1041–7.
Parhofer KG. Increasing HDL-cholesterol and prevention of atherosclerosis: a critical perspective. Atheroscler Suppl. 2015;18:109–11.
Williams PT, Stefanick ML, Vranizan KM, et al. The effects of weight loss by exercise or by dieting on plasma high-density lipoprotein (HDL) levels in men with low, intermediate, and normal-to-high HDL at baseline. Metabolism. 1994;43(7):917–24.
van de Laar AW, Dollé MH, de Brauw LM, et al. Validating the alterable weight loss (AWL) metric with 2-year weight loss outcome of 500 patients after gastric bypass. Obes Surg. 2014;24(7):1085–9.
Pardini R, Matsudo S, Araújo T, et al. Validação do questionário internacional de nível de atividade física (IPAQ - versão 6): estudo piloto em adultos jovens brasileiros. Rev Bras Ciên e Mov. 2001;9(3):45–51.
Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sport Exerc. 2000;32(9):S498–516.
Praveen Raj P, Bhattacharya S, Saravana Kumar S, et al. Comparison of effects of sleeve gastrectomy and gastric bypass on lipid profile parameters in Indian obese: a case matched analysis. Obes Surg. 2017;27(10):2606–12.
Sealed Envelope Ltd. 2012. Power calculator for continuous outcome superiority trial. [Online] Available from: https://www.sealedenvelope.com/power/continuous-superiority/ [Accessed Jan 18 2019].
Rondanelli M, Giacosa A, Morazzoni P, et al. MediterrAsian diet products that could raise HDL-cholesterol: a systematic review. Biomed Res Int. 2016;2016:1–15.
März W, Kleber ME, Scharnagl H, et al. HDL cholesterol: reappraisal of its clinical relevance. Clin Res Cardiol. 2017;106(9):663–75.
Klop B, Elte JWF, Cabezas MC. Dyslipidemia in obesity: mechanisms and potential targets. Nutrients. 2013;5(4):1218–40.
D'Agostino Sr RB, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham heart study. Circulation. 2008;117(6):743–53.
Acknowledgments
The authors would like to thank the team of the Centro de Tratamento da Obesidade of the Hospital Santa Rita from Complexo Hospitalar Irmandade Santa Casa de Misericórdia de Porto Alegre for providing conditions to execute the study and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for providing the PhD fellowships.
Author information
Authors and Affiliations
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
The study was approved by the Ethical Committee of the Hospital (Approval number 51843515.1.0000.5335).
Informed Consent
Written informed consent was obtained for each participant in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nienov, O.H., Machado, F.D., Dias, L.S. et al. Effect of Bariatric Surgery on High-Density Lipoprotein (HDL) Cholesterol in Non-diabetic Patients with Severe Obesity. OBES SURG 30, 154–160 (2020). https://doi.org/10.1007/s11695-019-04126-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-019-04126-6