Abstract
Background
Laparoscopic greater curvature plication (LGP) has recently emerged as a new bariatric procedure. This surgery provides gastric restriction without resection, which could potentially provide a lower risk alternative, with fewer complications. The real benefit of this technique in the short and long term is unknown. This systematic review aims to compare laparoscopic gastric plication and laparoscopic sleeve gastrectomy for obesity treatment.
Methods
Clinical trials were identified in MEDLINE, Embase, Cochrane, LILACS, BVS, SCOPUS, and CINAHL databases. Comparison of LGP and laparoscopic sleeve gastrectomy (SG) included hospital stay, operative time, loss of hunger feeling, body mass index loss (BMIL), percentage of excess weight loss (%EWL), complications, symptoms in the postoperative period, and comorbidity remission or improvement.
Results
This systematic review search included 17,423 records. Eight studies were selected for meta-analysis. There is no difference in operative time, hospital stay, and complications. Patients in the SG group had improved loss of hunger feeling. BMIL was better in the SG group at 12 and 24 months [mean difference (MD) − 2.19, 95% confidence interval (CI) − 3.10 to − 1.28, and MD − 4.59, 95% CI − 5.55 to − 3.63, respectively]. SG showed improved %EWL compared with gastric plication in 3, 6, 12, and 24 months. However, no difference was found in %EWL long-term results (24 and 36 months). Patients who underwent LGP had more sialorrhea. SG showed better results in diabetes remission.
Conclusions
SG showed improved weight loss when compared with LGP, with better satiety, fewer symptoms in the postoperative period, and improved diabetes remission.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity is a growing global epidemic affecting more than 1.9 billion adults worldwide [1]. The increasing global burden of obesity and its associated comorbidities has created an urgent need for additional treatment options to fight this pandemic [2]. Bariatric surgery remains the gold standard of treatment for sustainable weight loss and reduction of comorbidities in morbidly obese patients when compared with other nonsurgical options, including behavior modification, diet modification, drug therapy, and exercise [3].
Surgical treatment is an effective therapy for weight loss in patients with obesity [4]. The need for a safer and less expensive treatment is imperative, since many patients decline surgical treatment based on fear of surgery, costs, and concerns about results and complications [5, 6]. For this reason, researchers continue to look for an ideal surgery with minimal complications and lower costs. There are many reports of minimally invasive procedures with different strategies for obesity treatment [7, 8].
Gastric plication was first reported in animal models in 1969 by Kirk as a procedure for weight loss [9]. This surgery consists of a restrictive technique that reduces the gastric volume by plication of the greater curvature [10]. In recent years, laparoscopic greater curvature plication has emerged as a new bariatric procedure [11]. The rationale for this surgery is to provide a gastric restriction without gastric resection, intestinal bypass, or placement of a foreign body. This could potentially provide a lower risk alternative, with fewer complications [12].
Laparoscopic greater curvature plication is still considered experimental by the primary bariatric surgery societies, and the true benefits of this surgery in the short and long term are unknown [11]. Prospective studies comparing gastric plication to other well-established bariatric procedures were recently published and were not included in a recent meta-analysis [13, 14]. Our systematic review aims to compare the outcomes of clinical trials that compared laparoscopic greater curvature plication and laparoscopic sleeve gastrectomy for obesity treatment.
Material and Methods
This systematic review was conducted in accordance with the preferred reporting items for systematic reviews and meta-analyses (PRISMA) recommendations and registered on the PROSPERO international database (CRD42017056733) [15, 16].
Eligibility Criteria
The inclusion criteria were as follows: only complete published clinical trials (randomized and prospective nonrandomized) comparing laparoscopic greater curvature plication and laparoscopic sleeve gastrectomy. No restrictions for language or year of publication were applied. We excluded abstracts and studies including patients for revisional surgery.
The following outcome measures were used for comparison: hospital stay, operative time, body mass index loss (BMIL) (6, 12, and 24 months), percentage of excess weight loss (%EWL) (3, 6, 12, 18, 24, and 36 months), loss of hunger feeling (6 and 12 months), complications (bleeding, leak, stenosis, and thromboembolism), symptoms after surgery (nausea, vomiting, sialorrhea, and abdominal pain), and comorbidity improvement or remission (hypertension, diabetes, and sleep apnea).
Search and Information Sources
Studies were identified by searching electronic databases (MEDLINE, Embase, Cochrane, Scopus, LILACS, BVS, and CINAHL). The gray literature search included chapters of endoscopy and gastroenterology books, theses, ResearchGate, and references in the selected articles and in published systematic reviews. The last search was run on October 22, 2017.
Search terms included in MEDLINE were (Gastric plication OR Gastric plicature OR Great curvature plication OR Gastric vertical plication OR Laparoscopic gastric greater curvature plication OR Laparoscopic gastric plication OR Gastric imbrication OR Stomach Sparing Gastric Sleeve) OR (Sleeve gastrectomy OR Vertical gastrectomy). In the other databases, the same strategy was used with a few modifications.
Study Selection and Data Collection Process
Two reviewers performed eligibility assessment and selection of screened records independently in an unblinded, standardized manner. Disagreements were resolved by consensus. In case of duplicated publications, the most complete and recent was selected. The same authors extracted data from selected studies using a standardized form (Supplementary Information Sheet). Disagreements were resolved by consensus.
Data Items
The following information was extracted from each trial: (1) characteristics of the trial’s participants and the trial’s inclusion and exclusion criteria, (2) type of intervention and control groups, and (3) type of outcome measures.
The operative time is measured in minutes, and the hospital stay was recorded in days. Complications were considered as reported. Gastric outlet obstruction was considered as stenosis in one case. Symptoms in the postoperative time were not considered complications. Each symptom was reported and analyzed individually.
Risk of Bias of Individual Studies
Two reviewers analyzed together the quality of the studies with the Jadad scale for randomized trials and the Newcastle-Ottawa scale for nonrandomized trials [17, 18], to certify the risk of bias and the quality of the studies.
Summary Measures and Planned Methods of Analysis
The meta-analyses were performed with Review Manager 5.3 software (RevMan), which was obtained from the Cochrane Informatics and Knowledge Management Department (http://tech.cochrane.org/revman). Continuous data were analyzed by computing mean difference (MD), with the inverse variance method and fixed effect. Dichotomous data were analyzed by computing risk differences (RD) with a fixed effect model, Mantel-Haenszel test, and intention-to-treat analysis (ITT). We calculated 95% confidence intervals (95% CI) and number necessary to treat (NNT) or to harm (NNH) for each outcome and study.
Graphical analyses with a funnel plot and a forest plot were generated. Inconsistency (heterogeneity) was calculated using the chi-square test and the Higgins method (I2). The advantages of the Higgins method are that it does not depend on the number of studies and it is accompanied by an uncertainty interval. A cutoff point of I2 < 50% was established as acceptable [19].
Risk of Bias Across Studies
A graphical method was used (forest plots) to evaluate the relation between sample size and effect size for each outcome. Funnel plots were used to evaluate the risk of publication bias across the studies’ outcomes. The graphical method analysis involved a plot of the trials’ mean differences and search for asymmetry.
Quantification of heterogeneity is another component of the investigation of variability across studies. Considering the clinical implications of the observed degree of inconsistency across studies, the cutoff value of 50% was considered adequate for this meta-analysis. If the heterogeneity of the results of a meta-analysis (I2) was greater than 50%, a sensitivity analysis was conducted, excluding the reports located outside the funnel (outliers) if possible; another meta-analysis without the given report was then performed. In case of persistent high heterogeneity after this process or if we could not detect outliers, true heterogeneity was presumed and a random model was assumed.
We acknowledge that other factors could produce asymmetry in funnel plots leading to a high heterogeneity (true study heterogeneity), such as differences in the population studied, differences in trial quality, or even different techniques applied for the same surgery.
Additional Analyses
No subgroup analysis was generated.
Results
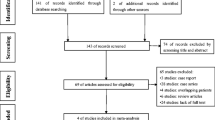
The literature search resulted in 3807 records in MEDLINE and 13,614 in the other databases. Two studies were identified in the gray literature search. This systematic review included 17,423 records. Ten trials were included in the initial selection of articles [20,21,22,23,24,25,26,27,28,29]. One study that compared comorbidity outcomes was excluded because it had losses greater than 20% [25]. The other excluded trial aimed to evaluate food tolerance and quality of life [26]. One randomized clinical trial was not clear whether the gastric plication and sleeve gastrectomy were both laparoscopic [27]. It was necessary to contact the author to confirm that a laparoscopic technique was used. Eight studies were considered for the meta-analysis, with a total of 422 patients [20,21,22,23,24, 27,28,29]. The study selection process is illustrated in Fig. 1. The study characteristics, risk of bias, and individual results are reported in Tables 1, 2, 3, and 4. The detailed surgical technique used in each procedure is described in Tables 5 and 6 (Supplementary Material).
Hospital Stay
Five studies reported this outcome [20, 22,23,24, 29], but one could not be included because of lack of standard deviation [20]. A high heterogeneity was noted in the initial analysis. After identifying and excluding the outlier [24], the heterogeneity remained. A random effect was used for analysis, and no significant difference was found (MD − 0.49, 95% CI [− 1.52, 0.54], I2 70%) (Fig. 2).
Operative Time
Four trials included this outcome for meta-analysis [20, 22,23,24], but one was excluded because of lack of standard deviation [20]. A high heterogeneity was noted, and it was not possible to exclude outliers. There is no difference for both surgical therapies with random effect (MD − 16.62, 95% CI [− 43.32, 10.08], I2 95%) (Fig. 3).
BMIL
This outcome was evaluated at 6, 12, and 24 months. Two studies reported BMIL for 6 and 12 months only [22, 23]. Two other trials included these outcomes for 24 months [20, 22]. There was no difference between interventions at 6 months (MD − 1.18, 95% CI [− 3.23, 0.88], I2 82%). A random effect was used due to high heterogeneity in the 6-month analysis (Fig. 4). The results were favorable for sleeve gastrectomy at 12 and 24 months, considering fixed effect (MD − 2.19, 95% CI [− 3.10, − 1.28], I2 0% and MD − 4.59, 95% CI [− 5.55, − 3.63], I2 0%, respectively) (Figs. 5 and 6).
EWL
This outcome was evaluated after 3, 6, 12, 18, 24, and 36 months. Three studies included EWL at 3 months in the analysis [23, 28, 29]. Five trials had EWL at 6 and 12 months in the analysis [21,22,23, 28, 29]. Two trials compared 18- and 24-month [22, 29] and 36-month [21, 22] results. Sleeve gastrectomy had better results compared with gastric plication at 3 months (MD − 7.42, 95% CI [− 10.58, − 4.26], I2 0%), 6 months (MD − 8.50, 95% CI [− 12.15, − 4.85], I2 38%), 12 months (MD − 11.55, 95% CI [− 15.72, − 7.39], I2 20%), and 18 months (MD − 6.98, 95% CI [− 12.47, − 1.49], I2 43%), but there was no difference in 24 (MD − 17.69, 95% CI [− 54.05, 18.68], I2 97%) and 36 months (MD − 31.41, 95% CI [− 72.37, 9.56], I2 95%). High heterogeneity was noted in the 24- and 36-month analyses. Random effect was used, because it was not possible to exclude outliers (Figs. 7, 8, 9, 10, 11, and 12).
Loss of Hunger Feeling
This outcome was evaluated at 6 and 12 months. Two studies were used for meta-analysis [22, 23], with better results in the sleeve gastrectomy group (RD − 0.31, 95% CI [− 0.49, − 0.12], I2 0%, NNH 3, and RD − 0.38, 95% CI [− 0.63, − 0.13], I2 50%, NNH 3, respectively). Random effect was used for the 12-month outcome, due to high heterogeneity (Figs. 13 and 14).
Complications
Three studies evaluated thromboembolism [22, 23, 29], stenosis [20, 21, 23], and bleeding [22, 23, 27]. Five studies compared leaks, and no differences were found (RD − 0.02, 95% CI [− 0.06, 0.03], I2 0%) [20,21,22,23, 29]. There are no differences between gastric plication and sleeve gastrectomy with regard to bleeding (RD 0.00, 95% CI [− 0.07, 0.07], I2 0%), stenosis (RD 0.03, 95% CI [− 0.05, 0.12], I2 12%), and thromboembolism (RD 0.01, 95% CI [− 0.04, 0.06], I2 0%) (Figs. 15, 16, 17, and 18 of Supplementary Material).
Symptoms After Surgery
The main reported symptoms after surgical treatment were nausea, vomiting, abdominal pain, and sialorrhea. Two trials reported all those symptoms [22, 23]. Vomiting was reported in three studies [22, 23, 27]. No difference was noted with regard to nausea (RD 0.12, 95% CI [− 0.02, 0.26], I2 0%), vomiting (RD 0.09, 95% CI [− 0.01, 0.18], I2 0%), and abdominal pain (RD 0.09, 95% CI [− 0.02, 0.21], I2 0%). Patients submitted to laparoscopic gastric plication had more sialorrhea (RD 0.23, 95% CI [0.09, 0.37], I2 10%, NNH 4) (Figs. 19, 20, 21, and 22 of Supplementary Material).
Comorbidity Improvement and Remission
Hypertension, diabetes, and sleep apnea were evaluated in this meta-analysis. Three trials compared hypertension improvement [20, 22, 23], hypertension remission [22, 23, 27], and diabetes outcomes [22, 23, 27]. Two studies evaluated sleep apnea [22, 23]. Hypertension improvement and remission showed high heterogeneity; after using a random effect, no difference was noted for both outcomes (RD 0.08, 95% CI [− 0.47, 0.64], I2 65% and RD − 0.14, 95% CI [− 0.57, 0.29], I2 55%, respectively). For diabetes, there was no difference in improvement (RD 0.19, 95% CI [− 0.05, 0.44], I2 0%). Laparoscopic gastric plication showed inferior results compared with laparoscopic sleeve gastrectomy considering diabetes remission (RD − 0.29, 95% CI [− 0.58, − 0.01], I2 0%, NNH 4). Sleep apnea also showed no significant difference in improvement (RD 0.45, 95% CI [− 0.10, 1.00], I2 17%) and remission (RD − 0.45, 95% CI [− 1.00, 0.10], I2 17%) (Figs. 23, 24, 25, 26, 27, and 28 of Supplementary Material).
Additional Analyses
No additional analyses were performed.
Discussion
As laparoscopic gastric plication is still an experimental procedure, there are just a few previous published systematic reviews without meta-analysis [11, 12]. The previous publications do not include all prospective trials available now [14, 27, 30], and the most recent meta-analysis showed several methodological issues [30]. We aimed to correct these deficiencies in our work, trying to shed more light on this procedure.
Our meta-analysis noted no difference between both surgeries when comparing mean operative time. The expected result for analysis is a shorter operative time for sleeve gastrectomy. One trial showed the opposite [24]. Maybe this is the reason for a high heterogeneity in the meta-analysis. Further, the complete analysis of this outcome must consider the surgical technique. Some authors used reinforcement sutures in the stapling line in the sleeve gastrectomy group, which can increase the operative time and minimize the mean difference between procedures.
Laparoscopic gastric plication was assumed to be a less invasive procedure, so it was expected to be better tolerated than sleeve gastrectomy. Laparoscopic greater curvature plication studies reported a higher number of patients with symptoms in the postoperative period than sleeve gastrectomy in all studies. However, there was no significant difference in the meta-analysis of pain, nausea, and vomiting. Generally, vomiting, nausea, and abdominal pain are expected in the first days after laparoscopic greater curvature plication surgery, but it may persist for weeks [31, 32]. Sialorrhea was the only symptom that had a significant difference in the meta-analysis. Every four patients who undergo laparoscopic greater curvature plication will result in one more case of sialorrhea symptoms compared with sleeve gastrectomy.
One of the main reasons to justify laparoscopic greater curvature plication is the idea of a less invasive procedure. The meta-analysis failed to demonstrate any benefit in reducing complications with the gastric plication. There was no difference in bleeding, stenosis, and thromboembolism outcomes. The rationale was to expect fewer cases of leaks with this new procedure. Only four cases of leaks were reported in all studies, with no significant difference between groups. Also, in one of the selected studies, leakage was only reported after laparoscopic gastric plication [21]. As leaks are the Achilles’ heel of sleeve gastrectomy, it is important to demonstrate that with laparoscopic gastric plication, complications were not reduced. Still, we can consider laparoscopic gastric plication a safe procedure, with a low number of major complications.
Hospital stay was equivalent between the two groups. However, this analysis had a high heterogeneity, and outliers had to be excluded. Because the major complications were similar between the groups, we expect no difference in the hospital stay.
The most important outcomes to consider are the BMIL and EWL. Sleeve gastrectomy showed improved BMIL after 12 and 24 months. Further, EWL was superior in the sleeve gastrectomy group after 3, 6, 12, and 18 months. Long-term results (24 and 36 months) from our data are not valuable, due to the low number of patients included and high heterogeneity. In addition, the loss of hunger feeling was better in the sleeve gastrectomy group after 6 and 12 months. One possible explanation for these results is the atrophy of the in-folded mucosa, which may result in a relative increase of gastric volume after 6 months in gastric plication patients [31]. Both procedures involve gastric restriction, but the possible mechanism that could explain improved satiety and weight loss is the gastric fundus resection. Several studies found reduced levels of ghrelin after sleeve gastrectomy, acting as a metabolic effect [33, 34]. In an overall view, weight loss seems to be superior in the sleeve gastrectomy patients, but due to lack of long-term results, we cannot make strong conclusions.
The weight loss results have to be considered with caution. There is scarce long-term evidence about follow-up of 5 or more years for plication. Only Talebpour and Dolezalova-Kormanova had long-term results [32, 35]. Both articles come from single-center series and retrospective reviews, but we do not have results from long-term comparative prospective trials. In fact, Talebpour showed concerns after a 4-year period following laparoscopic gastric plication, due to atrophy of the in-folded mucosa, gastric enlargement, and consequent weight regain.
No difference was noted in hypertension and sleep apnea improvement and remission after different interventions. Those outcomes were not reported in all included studies, and just a few patients were available for analysis. Sleeve gastrectomy showed improved diabetes remission compared with gastric plication. For this outcome interpretation, we must consider that BMIL and EWL were greater in the laparoscopic gastric plication patients. To the present, no studies classified results of laparoscopic gastric plication according to BMI categories. Perhaps the analysis of this data would show a specific group with better results for this technique. Prediction methods would help to estimate the results of surgery for obesity [36]. With that in mind, the decision to choose a technique that fits a patient would be easier.
The strengths of this systematic review are the broad search for prospective studies. One limitation is the number of studies and included patients. Moreover, most of those articles did not report long-term results. Some outcomes could not be compared, because of lack of uniformity and standard deviation. It would have been interesting to have more randomized trials comparing those surgical therapies with long-term follow-up. It is important to mention that due to these reasons, the results of laparoscopic gastric plication may be overestimated. Maybe those long-term results were not published due to losses to follow-up or publication bias.
Conclusion
Laparoscopic sleeve gastrectomy showed better results for obesity treatment than did laparoscopic gastric plication. Vertical gastrectomy had improved results when considering weight loss, satiety, and diabetes remission and had fewer symptoms in the postoperative period. No difference was noted when comparing operative time, hospital stay, complications, hypertension, and sleep apnea improvement and remission. Thus, laparoscopic gastric plication seems that it will not be the new procedure to replace sleeve gastrectomy. It continues to be a low morbidity and mortality procedure, but its weight loss results have not accomplished the presumed expectations; therefore, its real role in the bariatric surgery armamentarium may be compromised.
References
WHO. Obesity and overweight. 2016. Available from: http://www.who.int/mediacentre/factsheets/fs311/en/
Smith KB, Smith MS. Obesity statistics. Prim Care. 2016;43:121–35. Available from: https://www.ncbi.nlm.nih.gov/pubmed/26896205
Tran DD, Nwokeabia ID, Purnell S, et al. Revision of Roux-en-Y gastric bypass for weight regain: a systematic review of techniques and outcomes. Obes Surg. 2016;26:1627–34. Available from: https://www.ncbi.nlm.nih.gov/pubmed/27138603
Colquitt JL, Pickett K, Loveman E, et al. Surgery for weight loss in adults. In: Colquitt JL, editor. Cochrane Database Syst. Rev. [Internet]. Chichester: Wiley; 2014. [cited 2015 Jul 21]. Available from: http://www.ncbi.nlm.nih.gov/m/pubmed/25105982/?i=2&from=/19726018/related.
Afonso BB, Rosenthal R, Li KM, Zapatier J, Szomstein S. Perceived barriers to bariatric surgery among morbidly obese patients. Surg Obes Relat Dis. 2010;6:16–21. Available from: https://www.ncbi.nlm.nih.gov/pubmed/20005784
Fung M, Wharton S, Macpherson A, et al. Receptivity to bariatric surgery in qualified patients. J Obes. 2016;2016:1–6. Available from: https://www.ncbi.nlm.nih.gov/pubmed/27516900
de Moura EGH, Lopes GS, da Costa Martins B, et al. Effects of duodenal-jejunal bypass liner (EndoBarrier®) on gastric emptying in obese and type 2 diabetic patients. Obes Surg. 2015;25:1618–25. Available from: https://www.ncbi.nlm.nih.gov/pubmed/25691349
Choi HS, Chun HJ. Recent trends in endoscopic bariatric therapies. Clin Endosc. 2017;50:11–6. Available from: https://www.ncbi.nlm.nih.gov/pubmed/28147471
Kirk RM. An experimental trial of gastric plication as a means of weight reduction in the rat. Br J Surg. 1969;56:930–3.
Talebpour M, Amoli BS. Laparoscopic total gastric vertical plication in morbid obesity. J Laparoendosc Adv Surg Tech A. 2007;17:793–8. [cited 2011 Jul 21];Available from: http://www.ncbi.nlm.nih.gov/pubmed/18158812
Abdelbaki TN, Huang C-K, Ramos A, et al. Gastric plication for morbid obesity: a systematic review. Obes Surg. 2012;22:1633–9. Available from: https://www.ncbi.nlm.nih.gov/pubmed/22960951
Committee CI. ASMBS policy statement on gastric plication. Surg Obes Relat Dis. 2011;7:262. Elsevier Inc.; [cited 2012 Aug 31];Available from: http://www.ncbi.nlm.nih.gov/pubmed/21621164
Ji Y, Wang Y, Zhu J, et al. A systematic review of gastric plication for the treatment of obesity, Surg Obes Relat Dis. 10:1226–32. [Internet]. [cited 2015 Apr 22];Available from: http://www.ncbi.nlm.nih.gov/pubmed/24582413
Tang Y, Tang S, Hu S. Comparative efficacy and safety of laparoscopic greater curvature plication and laparoscopic sleeve gastrectomy: a meta-analysis. Obes Surg. 2015;25:2169–75. Available from: https://www.ncbi.nlm.nih.gov/pubmed/26311494
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ [Internet]. 2009;339:b2700. Available from: https://www.ncbi.nlm.nih.gov/pubmed/19622552
Davies S. The importance of PROSPERO to the National Institute for Health Research. Syst Rev. 2012;1:5. [cited 2018 Jan 15] Available from: http://www.ncbi.nlm.nih.gov/pubmed/22587962
Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. [cited 2018 Jan 15]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/8721797
GA Wells, B Shea, D O’Connell, J Peterson, V Welch, M Losos PT. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [Internet]. [cited 2018 Jan 15]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. [cited 2018 Jan 15]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/12958120
Abouzeid M, Taha O. Laparoscopic sleeve gastrectomy versus laparoscopic gastric greater curvature plication: a prospective randomized comparative study. Egypt J Surg. 2015;34:41. Available from: http://www.ejs.eg.net/article.asp?issn=1110-1121
Sharma S, Narwaria M, Cottam DR, et al. Randomized double-blinded trial of laparoscopic gastric imbrication v laparoscopic sleeve gastrectomy at a single Indian institution. Obes Surg. 2015;25:800–4. Available from: http://springerlink.bibliotecabuap.elogim.com/10.1007/s11695-014-1497-2
Grubnik V V, Ospanov OB, Namaeva KA, Medvedev O V, Kresyun MS. Randomized controlled trial comparing laparoscopic greater curvature plication versus laparoscopic sleeve gastrectomy. Surg Endosc [Internet]. 2016;30:2186–2191. Available from: http://springerlink.bibliotecabuap.elogim.com/10.1007/s00464-015-4373-9
Shen D, Ye H, Wang Y, et al. Comparison of short-term outcomes between laparoscopic greater curvature plication and laparoscopic sleeve gastrectomy. Surg Endosc. 2013;27:2768–74. [cited 2015 Apr 26] Available from: http://www.ncbi.nlm.nih.gov/pubmed/23443480
Morshed G, Abdalla H. Laparoscopic gastric plication versus laparoscopic sleeve gastrectomy. Med J Cairo Univ. 2011;79:4. Available from: http://www.medicaljournalofcairouniversity.com
Kuca K, Buzga M, Maresova P, et al. The influence of methods of bariatric surgery for treatment of type 2 diabetes mellitus. Ther Clin Risk Manag. 2016;12:599. Available from: https://www.ncbi.nlm.nih.gov/pubmed/27143901
Bin KS, Kim SM. Short-term analysis of food tolerance and quality of life after laparoscopic greater curvature plication. Yonsei Med J. 2016;57:–430. Available from: https://synapse.koreamed.org/DOIx.php?id=10.3349/ymj.2016.57.2.430
Casajoana A, Pujol J, Garcia A, et al. Predictive value of gut peptides in T2D remission: randomized controlled trial comparing metabolic gastric bypass, sleeve gastrectomy and greater curvature plication. Obes Surg. 2017; Available from: http://www.ncbi.nlm.nih.gov/pubmed/28451931;27:2235–45.
Bužga M, Švagera Z, Tomášková H, et al. Metabolic effects of sleeve gastrectomy and laparoscopic greater curvature plication: an 18-month prospective, observational, open-label study. Obes Surg. 27:3258–66. [Internet]. 2017 [cited 2018 Jan 15] Available from: http://springerlink.bibliotecabuap.elogim.com/10.1007/s11695-017-2779-2
Talebpour M, Sadid D, Talebpour A, et al. Comparison of short-term effectiveness and postoperative complications: laparoscopic gastric plication vs laparoscopic sleeve gastrectomy. Obes Surg. 2017 [cited 2018 Jan 15]; Available from: http://springerlink.bibliotecabuap.elogim.com/10.1007/s11695-017-2951-8;28:996–1001.
Ye Q, Chen Y, Zhan X, et al. Comparison of laparoscopic sleeve gastrectomy and laparoscopic greater curvature plication regarding efficacy and safety: a meta-analysis. Obes Surg. 2017;27:1358–64. Available from: https://www.ncbi.nlm.nih.gov/pubmed/28281232
Abdelbaki TN. An insight on the superior outcome of sleeve gastrectomy over gastric plication. Surg Obes Relat Dis. 2015;11:733–4. [cited 2015 Jun 27]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/25868839
Talebpour M, Motamedi SMK, Talebpour A, et al. Twelve year experience of laparoscopic gastric plication in morbid obesity: development of the technique and patient outcomes. Ann Surg Innov Res. 2012;6:7. [cited 2012 Aug 31];Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3444326&tool=pmcentrez&rendertype=abstract
Wang Y, Liu J. Plasma ghrelin modulation in gastric band operation and sleeve gastrectomy. Obes Surg. 2009;19:357–62. Available from: https://www.ncbi.nlm.nih.gov/pubmed/18841429
Langer FB, Reza Hoda MA, Bohdjalian A, et al. Sleeve gastrectomy and gastric banding: effects on plasma ghrelin levels. Obes Surg. 2005;15:1024–9. Available from: https://www.ncbi.nlm.nih.gov/pubmed/16105401
Doležalova-Kormanova K, Buchwald JN, Skochova D, et al. Five-year outcomes: laparoscopic greater curvature plication for treatment of morbid obesity. Obes Surg. 2017;27:2818–28. cited 2018 Apr 10] Available from: http://www.ncbi.nlm.nih.gov/pubmed/28560523
Slotman GJ. Prospectively validated preoperative prediction of weight and co-morbidity resolution in individual patients comparing five bariatric operations. Surg Obes Relat Dis. 2017;13:1590–7. Available from: https://www.ncbi.nlm.nih.gov/pubmed/28583814
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Manoel Galvao Neto, MD, is consultant for these companies: Ethicon Endosurgery®, Apollo Endosurgery®, GI Windows®, and GI Dynamics®. All the other authors have nothing to declare.
Ethical Approval Statement
For this type of study, formal consent is not required.
Informed Consent Statement
This is not applicable to this study.
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Barrichello, S., Minata, M.K., García Ruiz de Gordejuela, A. et al. Laparoscopic Greater Curvature Plication and Laparoscopic Sleeve Gastrectomy Treatments for Obesity: Systematic Review and Meta-Analysis of Short- and Mid-Term Results. OBES SURG 28, 3199–3212 (2018). https://doi.org/10.1007/s11695-018-3330-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-018-3330-9