Abstract
Background
The aim of the study was to investigate the role of the gut microbiota in weight regain or suboptimal weight loss following Roux-en-Y gastric bypass (RYGB).
Materials and Methods
The gut microbiota composition in post-RYGB patients who experienced successful weight loss (SWL, n = 6), post-RYGB patients who experienced poor weight loss (PWL, n = 6), and non-surgical controls (NSC, n = 6) who were age- and BMI-matched to the SWL group (NSC, n = 6) were characterized through 16S rRNA gene sequencing. To further investigate the impact of the gut microbiota on weight profile, human fecal samples were transplanted into antibiotic-treated mice.
Results
Orders of Micrococcales and Lactobacillales were enriched in SWL and PWL groups compared to the NSC group. No significant difference was observed in the gut microbiota composition between PWL and SWL patients. However, transfer of the gut microbiota from human patients into antibiotic-treated mice resulted in significantly greater weight gain in PWL recipient mice compared to SWL recipient mice. A few genera that were effectively transferred from humans to mice were associated with weight gain in mice. Among them, Barnesiella was significantly higher in PWL recipient mice compared to SWL and NSC recipient mice.
Conclusion
These results indicate that the gut microbiota are at least functionally, if not compositionally, different between PWL and SWL patients. Some taxa may contribute to weight gain after surgery. Future studies will need to determine the molecular mechanisms behind the effects of the gut bacteria on weight regain after RYGB.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Weight regain or insufficient weight loss can be a major concern in a considerable number of patients following Roux-en-Y gastric bypass surgery (RYGB). In a retrospective study, 17.1% of post-RYGB patients experienced weight regain after initial successful weight loss [1]. Mechanisms for weight regain are not well understood but are likely multifactorial. Surgical-related factors (e.g., increased gastric pouch size), behavioral factors (e.g., non-adherence to dietary and physical activity recommendations), and biological factors (e.g., gut hormones) have all been suggested as contributors to weight regain after surgery [2].
Human and animal studies have shown that the gut microbiota composition is different between lean and obese states [3,4,5,6], although this observation has not been consistent across all cohorts [7, 8]. Transplanting the gut microbiota from obese donors to germ-free (GF) mice (i.e., mice living in the absence of any microbial associates) increased fat mass in recipient mice [9, 10], suggesting that the gut microbiota plays a role in the regulation of adiposity. Recent studies have also demonstrated that RYGB is associated with significant changes in the gut microbiota composition and these changes appear to play a role in surgically induced weight loss and metabolic improvements [11]. Colonization of GF mice with the post-RYGB microbiota from human donors has been reported to result in lower fat accumulation and reduced respiratory quotient compared to mice colonized with the microbiota of non-surgical obese controls [12]. In addition, microbiota transfer from a mouse model of RYGB into GF mice resulted in a reduction in body weight and fat mass [13].
Although there are some data to suggest that altered gut microbiota might have a functional impact on weight profile and metabolism after surgery [12, 13], there is a current lack of information on the gut microbiota composition in patients who have experienced insufficient weight loss or weight regain over time. Therefore, using a combined human and murine model in this study, we aimed to explore whether weight change after surgery could be related to the gut microbiota.
Materials and Methods
There were two inter-related phases associated with this study. First, a clinical study was conducted in which three groups of volunteers were recruited, including post-RYGB patients who had sustained weight loss, those who had suboptimal weight loss, and non-surgical controls. This was followed by an animal study, in which a humanized mouse model was generated through colonization with human donor microbiota. Given our limited sample size, in an effort to exclude potential hormonally mediated differences in outcome data, female participants were enrolled and female mice were utilized.
Clinical Study
Participants and Sample Collection
Three groups of female participants (n = 6 per group) were included; two groups of patients who were 2–5 years post-RYGB, one group with successful weight loss (SWL) and the other with poor weight loss (PWL), and a group of non-surgical control (NSC) participants age and BMI matched to the SWL group’s post-surgery weight. Patients were allocated to the SWL group if they had lost and maintained at least 50% excess weight loss (%EWL) and to the PWL group if they had lost < 50% EWL at the time of study enrollment. Pre-surgical weight was ascertained through participant self-report and the enrollment weight was obtained using a Tanita® scale. Participants were excluded from participation if they took antibiotics, prebiotics, or probiotics in the 3 months prior to study enrollment; were currently taking a drug with a known major impact on gastrointestinal transit time; were pregnant or lactating, smoking, or using tobacco in the prior 3 months; had diabetes or a psychotic or bipolar spectrum disorder; or underwent major gastrointestinal surgery aside from a primary obesity and metabolic surgery (RYGB) or commonly performed procedures, such as a cholecystectomy.
Participants recorded their dietary intake through the Automated Self-Administered 24-h Recall (ASA24, National Cancer Institute) for three consecutive days. On the third (± 1 day) study day, fecal samples were collected and frozen at − 80 °C.
Animal Study
Mice
Female wild-type C57BL/6 mice between the ages of 9 and 10 weeks were purchased from Charles River Laboratories and allowed to acclimate to the laboratory for at least 1 week prior to the start of the experiment. Mice were individually housed in sterile and disposable microisolator cages and were provided with ad libitum access to irradiated food and sterile water throughout the experiment.
Antibiotic Treatment and Fecal Transplant
Mice were orally gavaged for a total of 17 days with an antifungal and for 14 days with an antibiotic cocktail in an attempt to sterilize the gastrointestinal tract in preparation for colonization. The previously published protocol by Reikvam and colleagues (2011) was used to determine dosing and duration of the antibiotic and antifungal cocktail, which included amphotericin-B (0.1 mg/ml), vancomycin (5 mg/ml), neomycin (10 mg/ml), and metronidazole (10 mg/ml). Additionally, ampicillin (1 mg/ml) was added to drinking water [14].
Twelve hours after antibiotic treatment, mice were colonized with the fecal microbiota from human donors once daily for 5 days. Five fecal samples from each group of human donors were transferred to 15 antibiotic-treated mice (3 mice per human donor). For each colonization, 0.5 g of the frozen human fecal sample was vortexed in 5 ml sterile phosphate-buffered saline, which had been purged with nitrogen gas to remove dissolved oxygen. Three mice were colonized from the same fecal suspension and a total of 0.2 ml of fecal suspension was administered through oral gavage to each mouse once a day. Fecal samples at pre-antibiotics, post-antibiotics, 1 day, and 4 weeks post-colonization were collected and stored at − 80 °C until downstream analysis.
DNA Extraction and 16S rRNA Sequencing: Clinical and Animal Microbiota
DNA was extracted from fecal samples using phenol-chloroform extraction combined with physical disruption of bacterial cells and then followed by a DNA clean-up step using QIAamp DNA Stool Mini Kit (Qiagen, Valencia, CA), as previously described [15, 16]. Fecal microbiota was characterized by 16S rRNA gene sequencing according to published methods [17] and can be found in the Supplementary Material. Sequencing was performed on an Illumina MiSeq benchtop sequencer (Illumina, San Diego, CA) by the High-Throughput Sequencing Facility in the Carolina Center for Genome Sciences at the University of North Carolina at Chapel Hill, School of Medicine. All sequences have been deposited in NBCI SRA (https://www.ncbi.nlm.nih.gov/sra under project PRJNA505687). The 16S rRNA sequences were demultiplexed through an automated metagenomics pipeline (https://github.com/msioda/BioLockJ). Primers were removed from forward reads by using Java and then forward reads were clustered into amplicon sequence variants (ASV) using DADA2 1.8 [18]. To further confirm the results from DADA2, the 16S rRNA sequences were additionally classified using the Ribosomal Database Project (RDP) classifier [19].
ASV and operational taxonomic units (OTU) tables at different taxonomic classifications were normalized using a published method to account for differences in the number of raw sequences between samples [20].
Statistical Analysis
Demographics and Clinical Sample Comparisons
Non-parametric tests, including Kruskal-Wallis and Mann-Whitney tests (SPSS Version 23 (SPSS Inc.)) were used to compare age, BMI, years after surgery, %EWL, and food intake with an α level of 0.05 among the clinical groups. All other statistical testing was performed using R.
Comparisons Between Mouse Groups on Weight and Food Intake
A two-way ANOVA was used to determine whether there were group differences in weight gain and food intake in recipient mice during the month following colonization. Weight gain and food intake at each time point was compared between groups using one-way ANOVA Tukey’s Honestly Significant Difference (HSD) post hoc test with an α level of 0.05.
Microbiota Analyses
One-way ANOVA with Tukey’s HSD post hoc test was used to identify bacterial taxa that had different abundance between PWL, SWL, and NSC patients. Differences in the abundance of bacteria classified at the genus taxonomic level relative to weight gain in recipient mice at 1 week and 4 weeks post-colonization were determined by the following univariate regression models:
-
Abundance (4 weeks post-colonization) = Weight gain (4 weeks post-colonization) + e
-
Abundance (1 day post-colonization) = Weight gain (1 week post-colonization) + e
-
Change in abundance from 1 day to 4 weeks = Weight change from 1 to 4 weeks + e
For all the statistical models, rare taxa that were present in less 25% of samples were removed prior to analysis. The Benjamini-Hochberg (BH) procedure with a false discovery rate (FDR) of p < 0.10 was used for multiple testing corrections.
Multidimensional scaling was performed on the genus table using the “capscale” function of the R statistical package “vegan” with Bray-Curtis dissimilarity. The ADONIS test, a permutation-based multivariate analysis of variance using Bray-Curtis dissimilarity matrices, was used to test whether the gut microbiota composition was significantly different among the groups. The Shannon diversity index was calculated to determine the within-sample species diversity of the gut microbial community.
Results
Clinical Study
Patient Characteristics
All participants were female with an age range of 38–45 years. Pre-surgical BMI did not differ significantly between the PWL and SWL patients. However, PWL patients had the highest BMI following surgery compared to SWL patients and NSC subjects (χ2(2) = 8.57, p < 0.05). The average %TWL in PWL and SWL patients was 24.35 ± 1.45% and 33.99 ± 2.36%, respectively (Table 1).
Dietary Intake
Patients recorded their dietary intake for three consecutive days through the ASA24 online dietary recall system. Daily total energy intake, total fat, protein, and carbohydrate did not differ between SWL, PWL, and NSC patients (data not shown).
Fecal Microbiota Composition
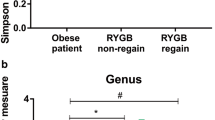
Bacterial orders Micrococcales within the Actinobacteria phylum and Lactobacillales within the Firmicutes phylum were enriched in both SWL and PWL patients compared to NSC participants at a 10% FDR. Higher abundance of Verrucomicrobiales and Enterobacteriales in PWL patients reached significance compared to the NSC group (Fig. 1). At the genus level, a greater abundance of Rothia within the Actinobacteria phylum and Streptococcus within the Firmicutes phylum were observed in both SWL and PWL patients compared to NSC participants at a 10% FDR. The abundances of Oscillibacter, Lactobacillus, Enterobacter, and Akkermansia were higher in the PWL group compared to the NSC group at a 10% FDR (Table S1).
a Relative abundance of bacterial orders in SWL, PWL, and NSC humans. Box plots show the abundance of Lactobacillales (b), Micrococcales (c), Enterobacteriales (d), and Verrucomicrobiales (e) in SWL, PWL, and NSC humans. Statistical differences were analyzed by one-way ANOVA with post hoc Tukey’s HSD test. The number sign indicates BH-corrected p < 0.1, asterisk indicates BH-corrected p < 0.05, double asterisks indicate BH-corrected p < 0.01, triple asterisks indicate BH-corrected p < 0.001. PWL, poor weight loss; SWL, successful weight loss; NSC, non-surgical controls
Fecal Microbiota Diversity
Within-sample microbiota diversity tended to be higher in the surgical groups compared to NSC and the difference was statistically significant when comparing PWL to the NSC group (Fig. 2).
Animal Study
Weight Profile in Recipient Mice
Mice colonized with human fecal suspensions were weighed weekly for 1 month following colonization. Baseline body weight prior to colonization was not significantly different between groups of mice colonized with PWL, SWL, and NSC microbiota (F(2,41) = 1.53, p = 0.229). Two-way ANOVA revealed that the experimental group (F(2,164) = 13.26, p < 0.0001) and time (F(3,164) = 25.25, p < 0.0001) significantly influenced body weight gain, while their interaction was not significant. Post hoc analyses showed that mice colonized with PWL microbiota gained more weight than mice colonized with SWL microbiota, which was sustained at 2 weeks (p = 0.0086), 3 weeks (p = 0.017), and 4 weeks (p = 0.020) following colonization (Fig. 3a).
a Weight gain in SWL, PWL, and NSC recipient mice at 1 week, 2 weeks, 3 weeks, and 4 weeks following colonization. Data are presented as means ± standard errors. Statistical differences are analyzed by one-way ANOVA with post hoc Tukey’s HSD test. Asterisk indicates p < 0.05 PWL versus SWL. b Cumulative food intake in recipient mice at 1 week, 2 weeks, 3 weeks, and 4 weeks after colonization. PWL, poor weight loss; SWL, successful weight loss; NSC, non-surgical controls
Food Intake in Recipient Mice
Cumulative food intake was measured at weekly intervals following colonization. Cumulative food intake was not significantly different between mice that were colonized by PWL, SWL, and NSC microbiota (F(2,158) = 0.25, p = 0.78; Fig. 3b).
Fecal Microbiota in Recipient Mice
To determine the efficiency of fecal microbiota transfer from human fecal samples into antibiotic-treated mice through oral gavage, the clustering patterns of the fecal microbiota from humans and mice before and after colonization were visualized using multidimensional analysis. There is clear clustering, with the pre- and post-antibiotic groups, the human donor samples, and the 1- and 4-week post-humanization time-points each forming distinct clusters (Fig. 4). Antibiotic treatment resulted in a shift in the gut microbiota which demonstrated clustering on the first axis (MDS1), explaining 35.3% of total variation in the data. The ADONIS test revealed that the gut microbial composition was different between groups (F(4,151) = 59.90 p = 0.001). The gut microbiota composition was different between pre- and post-antibiotic groups, the human donors and mice groups, and the 1- and 4-week post-humanization groups (p = 0.01) with the lowest variation between 1-day and 4-week post-colonization (r2 = 0.15) and highest variation between humans and pre-antibiotic mice (r2 = 0.61). Using the RDP classifier resulted in a comparable clustering pattern of the gut microbiota (Fig. S1).
Relation of Fecal Microbiota to Body Weight
To determine how the fecal microbiota were associated with weight gain in recipient mice, a series of univariate linear regression models were performed as described in the “Methods” section. We found that weight gain at 4 weeks post-colonization was positively associated with genus Barnesiella (p < 0.01, BH-adjusted p = 0.09) and negatively associated with genus Gordonibacter (p < 0.001, BH-adjusted p = 0.05). At 1 week post-colonization, weight gain was positively associated with genus Anaerotruncus (p < 0.001, BH-adjusted p = 0.05). These bacteria had zero abundance after antibiotics, indicating that colonization with human fecal samples after antibiotic treatment transplanted these bacteria into recipient mice (Fig. S2). Among these bacteria, Barnesiella tended to be higher in PWL patients compared to SWL and NSC patients, although this was not significant after controlling for multiple comparisons (Fig. 5b, p = 0.03, BH-corrected p = 0.30). Colonization with human fecal samples resulted in significantly higher abundance of Barnesiella in PWL recipient mice compared to SWL and NSC recipient mice at 4 weeks post-colonization (Fig. 5e).
a Association between mouse weight gain at 1 and 4 weeks post-colonization and log-normalized count of Barnesiella at 1 day and 4 weeks post-colonization. b–e Abundance of Barnesiella in humans and mice at pre-antibiotics, 1 day post-colonization, and 4 weeks post-colonization. Asterisk indicates BH-corrected p < 0.05, double asterisks indicate BH-corrected p < 0.01
In order to generate a finer taxonomic resolution, we used the results of the DADA2 clustering algorithm to compare sequence variants at a 100% identity threshold, allowing us to examine whether the variants observed in mice were transferred from their corresponding donors (Fig. 6). Human donors carried four different variants of Barnesiella (variants 1 and 3 were observed in PWL patients and variants 2 and 4 were observed in NSC or PWL patients). Fecal transplantation resulted in mice receiving the same variant of Barnesiella from their donors, except for one mouse in the SWL group (Fig. 6b). Taken together, these data demonstrate that Barnesiella is associated with weight outcome in mice and that the source of the Barnesiella was the human donor.
aBarnesiella variants in PWL human donors (left plots) and in their corresponding recipient mice at 1 day and 4 weeks post-colonization (right plots). bBarnesiella variants in SWL human donors (left plots) and in their corresponding recipient mice at 1 day and 4 weeks post-colonization (right plots). cBarnesiella variants in NSC human donor (left plot) and in their corresponding recipient mice at 1 day and 4 weeks post-colonization (right plot). Donors and mice that did not have any variants of Barnesiella are not shown in this figure. Values on the y axis represent log-normalized count for human donors and average log-normalized count for recipient mice. “n” represents the number of mice having the specified Barnesiella variant at 1 day and 4 weeks post-colonization. In figure c, six mice had Barnesiella variant 2 and five mice had Barnesiella variant 4
Discussion
To our knowledge, this is the first study which investigated whether the gut microbiota in post-RYGB patients who experienced PWL was associated with insufficient weight loss or weight regain. For this purpose, we characterized the fecal microbiota from SWL, PWL, and NSC human participants and transplanted these into antibiotic-treated wild-type mice. The results of this study demonstrated that the fecal microbiota in the surgical groups were enriched in the orders Micrococcales and Lactobacillales and tended to be more diverse compared to the NSC group. This is consistent with previous studies showing increased richness and bacterial diversity following RYGB [21,22,23]. Anatomical changes induced by RYGB are likely the major driving force for the increase in bacterial diversity, since anatomical and physiological changes in the gut environment allow for the overgrowth of acid-sensitive bacteria, facultative anaerobic microbes, and bile-tolerant microbes [24, 25].
Our study did not find any differences in gut microbiota composition between SWL and PWL patients. This could be due to our small sample size or due to the major shift that RYGB surgery produces in the gut microbiota. It is also worth noting that PWL and SWL microbiota may have different functions despite having similar composition. Therefore, a humanized mouse model was used to further investigate any potential difference in the composition and function of the gut microbiota between PWL, SWL, and NSC participants.
Mice that received the gut microbiota from PWL patients gained more weight compared to the mice that received the gut microbiota from SWL patients, suggesting that the gut microbiota from PWL patients may contribute to weight regain after surgery. Similar food intake among the groups of recipient mice further support the hypothesis that the PWL microbiota would contribute to weight gain through mechanisms independent of food intake. Increasing energy absorption from diet, increasing fat accumulation in adipose tissues, and inducing low-grade inflammation are potential mechanisms by which the gut microbiota can affect the host metabolism and body weight and composition [25].
We observed that a few bacterial genera that were effectively transplanted from human patients into antibiotic-treated mice were associated with weight changes in the recipient mice. These results suggest that some bacteria originating in human patients may contribute to weight gain. Interestingly, genus Barnesiella presented in higher abundance in PWL recipient mice compared to SWL and NSC recipient mice, which mirrored the trend observed in their human donors. This was associated with the transfer of the same variants from donors to recipient, further confirming the transfer of these species from humans to mice. Previous literature has shown that the genus Barnesiella and its family Porphyromonadaceae were associated with hyperglycemia, insulin resistance, hepatic steatosis, and inflammation in mice [26, 27]. Future studies are needed to determine whether Barnesiella could play a causal role in weight regain after RYGB and whether different variants of Barnesiella would have different functions. A replication cohort will be especially important to establish reproducibility as our sample size in this pilot study was modest.
Our study has some limitations. First, the clinical study included a small sample size and only female participants, some of whom had undergone procedures such as cholecystectomy which could impact the gut microbiota. Second, due to the cross-sectional nature of the clinical study, data on the percentage of weight gain prospectively after surgery could not be obtained. Therefore, it was not possible to accurately determine whether participants initially experienced successful weight loss followed by weight regain, or whether they never achieved 50% EWL following surgery. Third, the use of antibiotic-treated mice instead of GF mice might have limited the efficiency of microbiota transfer from humans to mice as a result of competition with residual commensal enteric microbes in mice or carry-over effects of antibiotics [28]. Fourth, our study detected only a few human-derived taxa associated with weight gain in mice and was underpowered to uncover the potential effects of other taxa on weight profile. Finally, it is worth noting that the observed association of the taxa and weight gain in humanized mice may be dependent on the reshaped microbial community in mice, which may not exactly resemble the complexity of the human gut microbiota. Therefore, future studies need to evaluate if these findings could be translated in humans.
In summary, by using a humanized mouse model, we showed that the gut microbiota in PWL patients were more obesogenic and can contribute to weight regain after surgery compared to the SWL microbiota. Our study revealed that some members of the gut microbial community may account for the greater weight gain in recipient mice. Future studies are warranted to replicate and confirm these results in germ-free mice with larger clinical sample sizes and to determine the molecular mechanisms by which gut microbiota could induce weight regain. Metagenomic and transcriptomic studies are also needed to determine the functional impact of the PWL and SWL gut microbiome on body weight and metabolism.
References
Shantavasinkul PC, Omotosho P, Corsino L, et al. Predictors of weight regain in patients who underwent Roux-en-Y gastric bypass surgery. Surg Obes Relat Dis. 2016;12(9):1640–5. https://doi.org/10.1016/j.soard.2016.08.028.
Maleckas A, Gudaitytė R, Petereit R, et al. Weight regain after gastric bypass: etiology and treatment options. Gland Surg. 2016;5(6):617–24. https://doi.org/10.21037/gs.2016.12.02.
Ley RE, Bäckhed F, Turnbaugh P, et al. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005;102:11070–5.
Ley RE, Turnbaugh PJ, Klein S, et al. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–3.
Million M, Maraninchi M, Henry M, et al. Obesity-associated gut microbiota is enriched in Lactobacillus reuteri and depleted in Bifidobacterium animalis and Methanobrevibacter smithii. Int J Obes. 2012;36(6):817–25. https://doi.org/10.1038/ijo.2011.153.
Turnbaugh PJ, Hamady M, Yatsunenko T, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–4.
Walters WA, Xu Z, Knight R. Meta-analyses of human gut microbes associated with obesity and IBD. FEBS Lett. 2014;588(22):4223–33. https://doi.org/10.1016/j.febslet.2014.09.039.
Sze MA, Schloss PD. Looking for a signal in the noise: revisiting obesity and the microbiome. MBio. 2016;7(4) https://doi.org/10.1128/mBio.01018-16.
Ridaura VK, Faith JJ, Rey FE, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341(6150):1241214.
Turnbaugh PJ, Ley RE, Mahowald MA, et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–31.
Peat CM, Kleiman SC, Bulik CM, et al. The intestinal microbiome in bariatric surgery patients. Eur Eat Disord Rev. 2015;23(6):496–503. https://doi.org/10.1002/erv.2400.
Tremaroli V, Karlsson F, Werling M, et al. Roux-en-Y gastric bypass and vertical banded gastroplasty induce long-term changes on the human gut microbiome contributing to fat mass regulation. Cell Metab. 2015;22(2):228–38. https://doi.org/10.1016/j.cmet.2015.07.009.
Liou AP, Paziuk M, Luevano Jr JM, et al. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci Transl Med. 2013;5(178):178ra41. https://doi.org/10.1126/scitranslmed.3005687.
Reikvam DH, Erofeev A, Sandvik A, et al. Depletion of murine intestinal microbiota: effects on gut mucosa and epithelial gene expression. PLoS One. 2011;6(3):e17996. https://doi.org/10.1371/journal.pone.0017996.
Carroll IM, Ringel-Kulka T, Keku TO, et al. Molecular analysis of the luminal- and mucosal-associated intestinal microbiota in diarrhea-predominant irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2011;301(5):G799–807. https://doi.org/10.1152/ajpgi.00154.2011.
Carroll IM, Ringel-Kulka T, Siddle JP, et al. Alterations in composition and diversity of the intestinal microbiota in patients with diarrhea-predominant irritable bowel syndrome. Neurogastroenterol Motil. 2012;24(6):521–e248. https://doi.org/10.1111/j.1365-2982.2012.01891.x.
Kleiman SC, Bulik-Sullivan EC, Glenny EM, et al. The gut-brain axis in healthy females: lack of significant association between microbial composition and diversity with psychiatric measures. PLoS One. 2017;12(1):e0170208. https://doi.org/10.1371/journal.pone.0170208.
Callahan BJ, McMurdie PJ, Rosen MJ, et al. DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods. 2016;13(7):581–3. https://doi.org/10.1038/nmeth.3869.
Wang Q, Garrity GM, Tiedje JM, et al. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73(16):5261–7. https://doi.org/10.1128/aem.00062-07.
Noble EE, Hsu TM, Jones RB, et al. Early-life sugar consumption affects the rat microbiome independently of obesity. J Nutr. 2017;147(1):20–8. https://doi.org/10.3945/jn.116.238816.
Kong LC, Tap J, Aron-Wisnewsky J, et al. Gut microbiota after gastric bypass in human obesity: increased richness and associations of bacterial genera with adipose tissue genes. Am J Clin Nutr. 2013;98(1):16–24. https://doi.org/10.3945/ajcn.113.058743.
Palleja A, Kashani A, Allin KH, et al. Roux-en-Y gastric bypass surgery of morbidly obese patients induces swift and persistent changes of the individual gut microbiota. Genome Med. 2016;8(1):67. https://doi.org/10.1186/s13073-016-0312-1.
Ilhan ZE, DiBaise JK, Isern NG, et al. Distinctive microbiomes and metabolites linked with weight loss after gastric bypass, but not gastric banding. ISME J. 2017;11(9):2047–58. https://doi.org/10.1038/ismej.2017.71.
Sweeney TE, Morton JM. Metabolic surgery: action via hormonal milieu changes, changes in bile acids or gut microbiota? A summary of the literature. Best Pract Res Clin Gastroenterol. 2014;28(4):727–40. https://doi.org/10.1016/j.bpg.2014.07.016.
Seganfredo FB, Blume CA, Moehlecke M, et al. Weight-loss interventions and gut microbiota changes in overweight and obese patients: a systematic review. Obes Rev. 2017;18(8):832–51. https://doi.org/10.1111/obr.12541.
Henao-Mejia J, Elinav E, Jin C, et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482:179–85.
Le Roy T, Llopis M, Lepage P, et al. Intestinal microbiota determines development of non-alcoholic fatty liver disease in mice. Gut. 2013;62(12):1787–94. https://doi.org/10.1136/gutjnl-2012-303816.
Lundberg R, Toft MF, August B, et al. Antibiotic-treated versus germ-free rodents for microbiota transplantation studies. Gut Microbes. 2016;7(1):68–74. https://doi.org/10.1080/19490976.2015.1127463.
Acknowledgments
We would like to acknowledge Ross Crosby, Ph.D., Carrie Nelson, RN, and Jacob Shreffler for their input and help with study design, coordination, and execution.
Funding
This study is funded by the Neuropsychiatric Research Institute (Fargo, North Dakota) and from the Core Synthesis and Analytical Services Facility which was made possible by NIH Grant Number P30GM103332 from the National Institute of General Medicine (NIGMS).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Ian M. Carroll reports personal fees from Vivelix and Salix pharmaceuticals for being consultant outside the submitted work. Kristine J. Steffen reports grants from the Center for Protease Research - NIH COBRE Pilot Grant through NDSU during the conduct of the study; grants from NIH, grants from Sanford Profile/NDSU, grants from Shire Pharmaceuticals, outside the submitted work. Amanda E. Brooks reports grants from NIH IDEA Center, during the conduct of the study. Anthony A. Fodor reports grants from NIH during the conduct of the study; personal fees from Second Genome, Salix Corporation, and the NKarta corporation outside the submitted work. Farnaz Fouladi, Emily C. Bulik-Sullivan, Matthew C. B. Tsilimigras, and Michael Sioda have no conflict of interest.
Ethical Approval Statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the University of North Dakota and North Dakota State University (NDSU) Institutional Review Boards. The study was registered at www.clinicaltrials.gov (NCT02654496). All Institutional Animal Care and Use Committee of North Dakota State University guidelines for the care and use of animals were followed.
Informed Consent Statement
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fouladi, F., Brooks, A.E., Fodor, A.A. et al. The Role of the Gut Microbiota in Sustained Weight Loss Following Roux-en-Y Gastric Bypass Surgery. OBES SURG 29, 1259–1267 (2019). https://doi.org/10.1007/s11695-018-03653-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-018-03653-y