Abstract
Background
Single-anastomosis duodeno-ileal bypass with sleeve gastrectomy (SADI-S) has launched a huge challenge to classic Roux-en-Y gastric bypass (RYGB). Our objective was to compare diabetes remission and micronutrient deficiency in a mildly obese diabetic rat model undergoing SADI-S versus RYGB.
Methods
Thirty adult male mildly obese diabetic rats were randomly assigned to sham (S), SADI-S, and RYGB groups. Body weight, food intake, fasting plasma glucose (FPG), oral glucose tolerance test (OGTT), plasma insulin, GLP-1, and ghrelin levels were measured at indicated time points. Meanwhile, insulin sensitivity and pancreatic β cell function were assessed during OGTT. Finally, plasma micronutrient evaluation and islet β cell mass analysis were performed after all animals were sacrificed.
Results
As compared to sham, the SADI-S and RYGB groups achieved almost equivalent efficacy in caloric restriction and FPG control without excessive weight loss. During OGTT, the SADI-S and RYGB groups also provided comparable effects on glycemic excursion, insulin sensitivity, and β cell function; however, only rats in the RYGB group showed significant changes in gut hormones, whereas the three groups were found to exhibit no significant difference in β cell mass. In addition, only vitamin E in the RYGB group was deficient as compared with the SADI-S and S groups.
Conclusion
In mildly obese diabetic rat, SADI-S and RYGB procedures have comparable efficacy in diabetes remission and risk of micronutrient deficiency. These data show that each of the surgery accomplishes diabetes improvements through both overlapping and distinct mechanisms requiring further investigation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes is one of the most common chronic conditions. In particular, type 2 diabetes’ prevalence has increased continuously over the past decades. Rapid economic growth, prolonged life expectancy, and lifestyle changes have significantly increased the prevalence of type 2 diabetes (T2D) [1,2,3], which highlights the significance of research and development of new therapies. Compared with pharmaceutical and behavioral approaches, bariatric surgery has a better outcome in T2D treatment. Therefore, it was adopted within the treatment modalities for T2D by international diabetes organizations [4].
Roux-en-Y gastric bypass (RYGB), one of the most commonly performed bariatric surgeries, has demonstrated a favorable effect on remission, or even resolution of comorbid disease states in obese patients, particularly those with T2D. However, this surgery can lead to miscellaneous postoperative complications, which are associated with the changes of gastrointestinal anatomy, including marginal ulcers at the top anastomosis, internal hernia, dumping syndrome, and excluded stomach cannot be checked in a regular examination, resulting in the tumorigenesis in the stomach which cannot be easily found punctually. As a result, these hurdles impair the popularization of this surgery in areas with high incidence of gastric cancer, such as Asia.
Single-anastomosis duodeno-ileal bypass with sleeve gastrectomy (SADI-S) was first described in 2007 by Sanchez-Pernaute, Torres et al. [5] as a simplification of the biliopancreatic diversion with duodenal switch (BPD/DS), which is a kind of procedure with technical complexity and high risk of long-term nutritional deficiencies. SADI-S begins with the creation of a sleeve gastrectomy (SG) (over a 54 F bougie) and preserving the pylorus, but replaces the Roux-en-Y reconstruction with one anastomosis comprised of a single-anastomosis duodeno-ileal bypass with a longer 250- to 300-cm common channel [6]. The rationale for this procedure is being able to address certain limitations of RYGB. First of all, the preservation of the pyloric valve prevents occurrence of dumping syndrome associated with RYGB; On the other hand, the loop structure maintains the contact between pancreatic enzymes, bile salts, and food, eliminating the formation of ulcers and strictures at anastomosis and even inner hernia related to RYGB.
Short mid-term follow-up data have shown that SADI-S surgery is a safe procedure with favorable weight loss outcomes, fewer anastomotic complications, and better correction rate on T2D with morbid obesity as compared with RYGB [6,7,8,9]. However, the majority of diabetic patients in China are overweight (BMI between 24 and 28 kg/m2) [2], and the effect of SADI-S on low BMI diabetic subjects remains unclear. Due to the differences in surgical design, we hypothesize that the effects of diabetes remission and the postoperative malnutrition may be different and achieved through different mechanisms in mildly obese diabetic subjects.
To test this hypothesis, we measured glucose fluctuation, hormone response, insulin sensitivity, pancreas β cell function, residual β cell mass, and micronutrient level in a high-fat diet (HFD) and streptozotocin (STZ)-induced mildly diabetic rat model undergoing SADI-S or RYGB, in order to provide experimental data about treatment effect and potential mechanism.
Materials and Methods
Animals
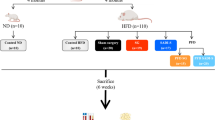
Male Wistar rats (age, 8 weeks; weight, 160–180 g), adopted from the Shanghai Laboratory Animal Research Center (Shanghai, China), were housed in individual cages with controlled temperature (24 ± 2 °C), humidity (40–70%), and light/dark cycle (12 h light/12 h dark, with light on at 7:00 a.m.). The rats were fed an HFD (40% of calories as fat) rodent chow and tap water for a period of 8 weeks, and then injected with STZ intraperitoneally (40 mg/kg). After 72 h of injection, animals with the non-fasting basal plasma glucose of ≥ 16.7 mmol/L were considered diabetic and selected for further studies [10].
Thirty HFD and STZ-induced mildly obese diabetic rats were randomly assigned into three groups: the SADI-S group (n = 10), RYGB group (n = 10), and sham (S) group (n = 10), according to the procedure undergone. The study protocol was reviewed and approved by the Institutional Animal Care and Utilization Committee (IACUC) of Fudan University Pudong Medical Center.
Surgical Procedures
Preoperative preparation and postoperative care are the same as we reported previously [11]. SADI-S surgery (Fig. 1) [12] was performed by simulating the procedure which was completed on humans. We selected 10 rats for the pilot study and measured the length of the small intestine as 80 ± 5.7 cm, so we chose 40 cm as a common channel. For sleeve gastrectomy, we just followed the protocol referring to Xu B [13]. First, the gastrocolic and gastrosplenic ligaments were carefully separated for exposure of the whole stomach and duodenum. Then, two thirds to three quarters of the stomach on the greater curvature were removed and the cutting line was closed by running suture (Fig. 2a). Next, we performed an intermittent embedding suture of the cutting line. And then, the duodenum was transected just distal to the pylorus. Duodenal stump was sutured and ligated using a 5-0 silk suture, followed by a 3-mm longitudinal incision on the jejunum 40 cm up from the ileocecal junction. An end-to-side anastomosis was made to connect the jejunum to the proximal duodenal (Fig. 2b).
Surgical procedures for SADI-S and RYGB in the rat model. a In the SADI-S group, two thirds to three quarters of the stomach was excised and the duodenum was transected just distal to the pylorus. The arrow shows cutting line. b And then, a 3-mm enterotomy was performed 40 cm up from the ileocecal junction and an end-to-side anastomosis (yellow arrow) was made to connect the jejunum to the proximal duodenal. c In the RYGB group, the jejunum was transected 15 cm from the Treitz ligament. Then, a 4-mm enterotomy was performed 15 cm down from the distal jejunum incision and connected to the biliopancreatic limb by a side-to-side anastomosis (yellow arrow). d Next, the rat stomach was divided cambered about 3 mm below the gastroesophageal junction and gastrojejunal anastomosis (yellow arrow) was performed
RYGB surgery was completed using a rat protocol referring to Xiong Zhang [14]. In brief, the jejunum was transected 15 cm from the Treitz ligament to create the biliopancreatic limb. Then, a 4-mm enterotomy was performed 15 cm down from the distal jejunum incision and connected to the biliopancreatic limb by a side-to-side anastomosis (Fig. 2c). The rat stomach was divided cambered about 3 mm below the gastroesophageal junction to create a small stomach pouch. The stomach remnant was subsequently closed by continuous running suture. Last, the gastrojejunal anastomosis was performed (Fig. 2d).
In the S group, rats underwent the same abdominal incisions and transections of the small bowel followed by re-suturing. And as a result, the anatomic structure was not changed.
Body weight, food intake, fasting plasma glucose, and oral glucose tolerance test
In all groups, body weight, daily food intake, and fasting plasma glucose (FPG) after a 6-h fast were measured 1 week before and after STZ administration, and 1, 4, and 8 weeks after surgery. A handheld glucometer (Roche One Touch®Ultra, Germany) was employed to directly measure the FPG level with a drop of blood obtained by tail nipping.
Oral glucose tolerance test (OGTT) was done in each individual rat 1 week before and after STZ administration, 4 and 8 weeks after surgery. Before OGTT, the rats were deprived of food (but not water) for 12 h. During OGTT, each rat was given a bolus of 2 g/kg hypertonic glucose (50% w/v) by oral gavage. Blood samples were collected by tail nipping, and glucose concentration in the samples were measured directly by the handheld glucometer, as described above, before and at 15, 30, 60, 120, and 180 min after receiving the hypertonic glucose solution.
Hormone, Vitamin, and Mineral Determination
In order to measure serum insulin, ghrelin, and GLP-1 levels, additional blood samples (400 μl) were taken from each animal before and at 15 and 30 min after giving an oral glucose bolus during OGTT 1 week after STZ administration and 8 weeks after surgery. These blood samples were also collected by tail nipping into chilled 1.5-mL Eppendorf tubes (Axygen Scientific Inc., Union City, CA, USA) containing a dipeptidyl peptidase IV (DPP-IV) inhibitor (EMD Millipore, Billerica, MA, USA) in an ethylene diamine tetraacetic acid (EDTA) solution. Blood samples for micronutrient analysis were collected from the abdominal aorta at 8th week postoperatively after euthanizing rats and stored in EDTA tubes. After centrifugation at 4 °C for 10 min at 3000 rpm, plasma was immediately extracted and stored at − 80 °C until further analysis. Enzyme-linked immunosorbent assay (ELISA) kits were used for measuring serum insulin (Mercodia Corporation, Sweden), ghrelin (Phoenix Pharmaceuticals, USA), active GLP-1 (EMD Millipore, Billerica, MA, USA), and vitamin (BIOSH, Shanghai, China). Mineral were detected by atomic absorption spectrophotometer (Thermo, MA, USA).
Insulin Sensitivity and β cell Function
Insulin sensitivity and β cell function were analyzed during OGTT. Insulin sensitivity was indicated by homoeostasis model assessment-IR (HOMA-IR) and ISI M, calculated as [Ins0 (μIU/ml) × Glu0 (mmol/l)/22.5] and [10,000/(Glu0 (mg/dl) × Ins0 (μIU/ml) × average glucose OGTT (mg/dl) × average insulin (μU/ml) OGTT)1/2], respectively. β cell function was assessed by the HOMA-B calculated as [20 × Ins0 (μIU/ml)]/[Glu0 (mmol/l)- 3.5], Disposition Index 0 min (DI 0), 15 min (DI 15), and 30 min (DI 30), calculated as HOMA-B×(1/HOMA-IR), (InsAUC30/GluAUC30) × ISIM and (InsAUC120/GluAUC120) × ISI M, respectively.
Pancreatic Islet Immunohistochemistry Staining
Pancreatic β cells were quantified by insulin immunostaining. After euthanizing rats at 8 weeks after surgery, the whole pancreas was excised and rinsed in phosphate-buffered saline (PBS), and then fixed in 4% paraformaldehyde solution for 24 h. The fixed tissues were embedded in paraffin and sectioned at a thickness of 2 mm using a microtome before being mounted onto slides. When doing the immunohistochemistry staining, we rehydrated, permeabilized, and blocked the tissues slides for 10 min in normal goat serum (CWBiotech, Beijing, China). We incubated them overnight at 4 °C in a primary rabbit anti-rat insulin antibody (Cell Signaling Technology, Danvers, MA, USA) at 1:400 dilution, and then incubated them with Boost IHC Detection Reagent (HRP, Rabbit) (Cell Signaling Technology, Danvers, MA, USA) at 37 °C for 30 min. Sections then were stained with 3,3-diaminobenzidine (DAB) for 1–10 min, and immersed in distilled water to terminate DAB staining, counterstained with hematoxylin for 2–5 min, differentiated by 0.5% hydrochloric acid alcohol, and dehydrated with sequential ethanol washes of 1 min each starting with 95% ethanol, followed by 100% ethanol and finishing with a dimethylbenzene wash. Finally, the slides were sealed.
Analysis of Islet β cell Mass
Rat pancreases were sliced systematically through the head-to-tail axis in a thickness of 2 μm each section, and every 200 μm, one section was selected. A total of five sections were collected from each pancreas. Rat pancreatic slices were stained with an anti-insulin antibody. Images of the slices were captured by a Leica APERIO AT TURRO. All β cell clusters (islets) were defined as positive pixel, and the rest of the pancreas area was identified as negative pixel. Analyses of β cell percentage were performed using the Image Scope Version 12.3.3.5048 software (Leica Microsystems Inc., Germany). Β cell mass was calculated by number positive/total number (positive + negative) multiplied by pancreas weight.
Statistical Analysis
Data analysis was performed using SPSS 22.0 (IBM Corporation, Armonk, NY, USA). The results were presented as mean ± SEM. Areas under curves (AUC) of OGTT excursions were calculated by trapezoidal integration. Statistical analysis was performed using two-way or one-way analysis of variance with Bonferroni or Sidak test for multiple comparisons. P < 0.05 was regarded as statistically significant.
Results
Food Intake and Weight
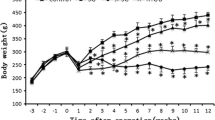
Food intake and weight of each rat were measured 1 week before and after STZ administration, 1, 4, and 8 weeks after operation. After STZ administration, animal with non-fasting plasma glucose level above 16.7 mmol/L was considered diabetic and selected for further studies. Meanwhile, body weight of selected animals dropped sharply. These animals were randomized into three groups, all of which had a comparable baseline in food intake and weight. Then, the SADI-S, DJB, or sham procedure was performed, and significant decrease in food intake for SADI-S and RYGB groups was observed, as compared with the S group (Fig. 3a).
Food intake and body weight 1 week before and after STZ administration, 1, 4, and 8 weeks after surgery. a Food intake did not show significant difference in the three groups before operation. Although food intake decreased obviously in the surgery group than that in the S group, there was no significant difference between the SADI-S and RYGB groups postoperatively. b The change of body weight in the three groups showed a similar pattern. Even animals in the SADI-S and RYGB groups consumed less food than those in the S group after operation, but they did not loss excessive weight at 8th week postoperatively. All values are shown as means ± SEM.(*P < 0.05, **P < 0.01, ***P < 0.001, comparing with the S group)
Before surgical procedure, weight in all three groups was comparable. As mentioned, after surgery, rats in the SADI-S and RYGB groups exhibited decreased food intake; however, they did not lose more weight as compared with the S group at the endpoint of the study, implying both kinds of bariatric surgery would not cause excessive weight loss in mildly obese diabetic rat models (Fig. 3b).
FPG and OGTT
To gain insight into the mechanism underlying how SADI-S and RYGB procedures affect glucose metabolism, the FPG levels and glucose tolerance of all the rats were measured 1 week before and after STZ administration, 1 (only FPG), 4, and 8 weeks after operation. We found that rats had a comparably impaired glucose tolerance after 8 weeks HFD feeding (Fig. 4c). Then, after administration of STZ, FPG increased and glucose tolerance deteriorated obviously in all three groups (Fig. 4a, b, d).
Fasting plasma glucose and OGTT measurements. a Fasting plasma glucose increased significantly in all three groups 1 week after STZ injection, which decreased obviously in the surgery group compared to the S group, as showed at 1, 4, and 8th week measuring point postoperatively. Meanwhile, the SADI-S and RYGB procedures have comparable effect in fasting plasma glucose control. b Area under curve (AUC) calculated from OGTT which were done 1 week before and after STZ administration, 4 and 8 weeks after surgery. Similar to fasting glucose, AUC of the three groups increased sharply after STZ injection, which declined vigorously in the SADI-S and RYGB groups postoperatively, compared with the S group. What is more, there was no significantly different effect on AUC between the SADI-S and RYGB groups. c, d, e, and f show OGTT excursions 1 week before and after STZ administration, 4 and 8 weeks after surgery, respectively. All values are showed as means ± SEM (*P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.001, comparing with the S group)
However, the FPG and OGTT excursions of the SADI-S and RYGB groups decreased significantly and promptly as compared with the S group at 1, 4, and 8th week post-operation. In addition, there was no statistically significant difference in FPG and OGTT between the SADI-S and RYGB groups at any time point.
Hormone Measurements
In the STZ-induced rat models with β cell deficiency, plasma insulin level is a quantitative biomarker of pancreatic β cell quantity and activity. As a result, RYGB does not conserve and SADI-S decreases the proportion of the stomach fundus. Meanwhile, since both of them belong to a intestinal rearrangement procedure, hormones like ghrelin and GLP-1 levels are supposed to change after surgery.
Plasma insulin, GLP-1, and ghrelin levels response to hypertonic glucose fluctuation correspondingly, whereas OGTT results were comparable in the three groups and without time dependent fluctuation preoperatively (Fig. 5a, c, e). Eight weeks after surgery, insulin, GLP-1, and ghrelin secretion in the S group maintained a similar pattern; however, rats in the bariatric surgery group displayed a time-dependent insulin secretion increase at 15 (S versus SADI-S: P < 0.05) and 30 (S versus RYGB, P < 0.05) min in the OGTT (Fig. 4b). Compared with the S and SADI-S groups, GLP-1 secretion (Fig. 5d) increased at 30 min (P< 0.001) in the RYGB group. In addition, RYGB decreased ghrelin level obviously (Fig. 4f) at 0 (S versus RYGB: P< 0.01; SADI-S versus RYGB: P< 0.05), 15 (S versus RYGB: P< 0.05), and 30 (S versus RYGB: P< 0.01) min.
Insulin (a and b), GLP-1 (c and d), and ghrelin (e and f) response after hypertonic glucose challenge during OGTT. a, c, and e All three groups showed a similar insulin, GLP-1, and ghrelin secrete pattern (without time-dependent fluctuation) after glucose challenge during OGTT preoperatively. b, d, and f Compared with the S group, insulin secrete exhibited a time-dependent increase pattern in the SADI-S and RYGB groups postoperatively, but for GLP-1 and ghrelin, only the RYGB procedure induced this pattern of increase (GLP-1) or decrease (ghrelin) and no significant difference was found between the S and SADI-S groups. All values are showed as means ± SEM (*P < 0.05, **P < 0.01, and ***P < 0.001, RYGB versus S; #P < 0.05 and ###P < 0.001, RYGB versus SADI-S)
Insulin Sensitivity and β cell Function
Effect of SADI-S and RYGB on insulin sensitivity and β cell function was assessed during OGTT. Hepatic and peripheral insulin sensitivities were evaluated by HOMA-IR and ISI M, respectively. HOMA-IR decreased and ISI M increased to a similar extent in both the SADI-S and RYGB groups after surgery, and this improvement was not observed in the S group after surgery (Fig. 6a, b). Measurements of β cell function included HOMA-B, DI 0, DI 15, and DI 30. HOMA-B in the SADI-S and RYGB groups both increased to a similar extent after surgery (Fig. 6c), but DI 0 after insulin sensitivity correction did not show obvious changes pre- and post-operation in the three groups (Fig. 6d). However, DI 15 and DI 30 in the SADI-S and RYGB groups both significantly increased to a similar extent after surgery, while no similar result in the S group was found (Fig. 6e, f). This phenomenon implies SADI-S and RYGB have a comparable effectivity in improving insulin secretion ability and rhythm.
Effect of SADI-S and RYGB on insulin sensitivity and β cell function during OGTT. a, b, and c Insulin resistance (HOMA-IR) decreased, peripheral insulin sensitivity (ISI M) and basal insulin secretion (HOMA-B) increased to a similar extent in both the SADI-S and RYGB groups after surgery. There were no significant changes for all of the above parameters in the S group. d, e, and f No obvious difference of DI 0 was found between pre- and post-operation for all three groups. However, DI 15 and DI 30 in the SADI-S and RYGB groups both significantly increased to a similar extent after surgery, while no increase in the S group was observed. All values are showed as means ± SEM (*P < 0.05, **P < 0.01, and ***P < 0.001, pre-operation versus post-operation)
Pancreas Islet β cell Mass
To determine whether SADI-S or RYGB has a beneficial effect on β cell preservation, we quantified the pancreatic β cell mass at the end of this experiment. Unfortunately, we did not find that the amount of retained β cells in the three groups was significantly different (Fig. 7a–d).
Pancreatic islet immunohistochemistry staining and analysis of β cell mass after execution of animals. a, b, and c Representative images of the S, SADI-S, and RGB groups, respectively showed insulin immunohistochemical staining (brown) from rats 8 weeks after surgery. Scale bars, 300 μm. d The results of planimetric analyses using the Image Scope Version 12.3.3.5048 software for β cell mass are shown as means ± SE. There was no obviously difference about β cell mass among the three groups. All values are showed as means ± SEM
Vitamin and Mineral Measurements
Postoperative vitamin and mineral deficiencies are one of the long-term complications of bariatric surgery. In order to compare the risk of vitamin and mineral deficiencies caused by SADI-S and RYGB procedures, vitamin (A, B12, folic acid, B1, B6, D, and E) and mineral (Ca, Fe, Se, Zn, Cu, Mg, and P) were evaluated at 8th week post-operation. Most of the vitamins and minerals levels did not differ significantly in the three groups. Although the levels of vitamin B6 and mineral Cu in the RYGB group were lower than those in the SADI-S group, their levels were not significantly different between the RYGB and S groups (Fig. 8a, d). Only vitamin E level in the RYGB group was significantly lower than those in the SADI-S and S groups (Fig. 8b).
Vitamin and mineral at the end of the 8th week after surgery. a and b No obvious difference was found for vitamins A, B1, B12, D, and folic acid in the S, SADI-S, and RYGB groups. Although vitamin B6 and E in the RYGB group were relatively deficient when compared with the SADI-S group, only vitamin E level in the RYGB group was lower than that in the S group. c and d There was no remarkable difference about Fe, Zn, Mg, P, Ca, and Se levels among all the groups after surgery, except for Cu level, which dropped after the RYGB procedure when compared with the SADI-S group. All values are showed as means ± SEM (*P < 0.05, RYGB versus S; #P < 0.05 and ##P < 0.001, RYGB versus SADI-S)
Discussion
This study compared the efficacy of SADI-S and RYGB in the treatment of mildly obese diabetes and the risk of postoperative micronutrient deficiency in a HFD and STZ-induced mildly obese diabetic rat model, using the sham group as a control. The study found that compared with sham, the SADI-S and RYGB procedures achieved almost equivalent efficacy in reduced food intake, but without significant weight loss as shown at the last check point. What is more, SADI-S and RYGB also provided comparable effectivity in decreased FBG, as well as improvement of glycemic excursion, insulin sensitivity, and β cell function during OGTT. Unexpectedly, only rats in the RYGB group showed significant changes in gut hormones, which was not found in the SADI-S and S groups during OGTT. In addition, we found most of the vitamin and mineral levels did not differ significantly in the three groups. Only vitamin E level in the RYGB group was significantly lower than those in the SADI-S and S groups. These results imply that in mildly obese diabetic rat, SADI-S and RYGB procedures have the comparable efficacy in diabetes remission and risk of micronutrient deficiency, and the mechanisms of diabetes improvement underlying the two procedures should be overlapping and distinct.
Weight loss and metabolic syndrome improvement, especially diabetes, are the main efficacy of bariatric surgery. RYGB has been widely accepted as a classic bariatric surgery, while SADI-S is an emerging procedure. Although the current report has shown that SADI-S not only has comparable mid-term weight loss effect to RYGB but also has better type 2 diabetes resolution rate [9], long-term large-sample follow-up data is still needed to support SADI-S as a standard bariatric procedure [6, 15]. In addition, excessive weight loss after bariatric surgery is often one of the concerns of patients who are considering surgery, especially for people with BMI ≤ 28 kg/m2 or even normal weight metabolic syndrome. Several studies have reported that people with low BMI did not experience underweight after receiving RYGB [16,17,18,19], and so far, there is no report of SADI-S for this population. In this study, SADI-S and RYGB were compared for the first time in a mildly obese rat model. Both of them significantly reduced the food intake of rats when compared with sham surgery; however, the rats in the bariatric surgery groups just weighed slightly lower than those in the S group, which meant the two procedures did not cause excessive weight loss. This result has positive implications for the promotion of these bariatric surgeries in mildly obese (BMI ≤ 30 kg/m2) and even nonobese people with metabolic syndrome.
Except fear of excessive postoperative weight loss, micronutrient deficiencies are also often patients’ concerns, which have largely hindered the promotion of surgical treatment of obesity and metabolic syndrome. Several scholars have studied the micronutrient deficiencies after RYGB, and the postoperative vitamins A, D, B12, and zinc deficiency have been reported [19,20,21,22]. When comparing RYGB with SADI-S, there were only statistical differences in nutritional outcomes with calcium at 1 and 3 years and vitamin D at 1 year [9]. In our study, seven vitamins (A, B12, folic acid, B1, B6, D, and E) and seven minerals (Ca, Fe, Se, Zn, Cu, Mg, and P) were tested at 8 weeks after surgery. Although the levels of vitamins B6, E, and mineral Cu in the RYGB group were lower than those in the SADI-S group, only the vitamin E level in the RYGB group was lower than that in the S group. The type of micronutrient deficiencies found in this animal experiment and clinical study was different, which might be related to different species; however, the results still indicated that in this rat model, postoperative risk of SADI-S and RYGB is not high and comparable.
Gastrointestinal hormone change induced by bariatric surgery is always a hot topic. Because SADI-S and RYGB surgeries both involve gastric volume reduction and intestinal diversion, we tested ghrelin (mainly secreted by the X/A-like cells in the oxyntic glands of the stomach fundus) and GLP-1(released from the L-cells mainly distributing in the terminal ileum). Ghrelin is an orexigenic hormone and GLP-1 participates in the “ileal break” by slowing gastric emptying and intestinal motility. Ghrelin decreased and GLP-1 increased after RYGB, which was related to the success or failure of weight loss and long-term weight maintenance [23,24,25]. Our study yielded similar results, serum GLP-1 levels increased in rats and ghrelin levels decreased after RYGB during OGTT, but not in the SADI-S and S groups. This may be related to different modes of gastrointestinal reconstruction (Roux-en Y bypass and loop bypass), the pylorus preserved or not, and degree of reduction in gastric volume. Further research is needed to confirm this hypothesis, as well as investigate changes of gastric emptying and intestinal motility after surgery.
Although the exact mechanisms by which bariatric surgery drives clinical remission of T2D are not well understood, attenuation of insulin resistance and improvement of β cell function following surgery are considered to play an important role [26, 27]. Reduced caloric intake and weight loss after surgery increase insulin sensitivity, and early exposure of the distal intestinal to nutrients results in a modification of gut hormones, specifically incretins, leading to boosting of β cell function (“hindgut” hypothesis) [28]. In present study, we provided similar evidence that both SADI-S and RYGB improved insulin resistance, insulin secretion rhythms, and β cell function under OGTT. Moreover, this effect might benefit from elevated postoperative GLP-1 in the RYGB group. Previous research reported that GLP-1 evidently also increases β cell mass by promoting proliferation and neogenesis while inhibiting apoptosis [9, 29], but we did not find that the RYGB group retained more β cells by immunohistochemistry. This may be related to the dose of STZ, the rodent model, and the time of detection, so the phenomenon needs further investigation. Dutia et al. [30] showed increased insulin secretion postoperatively was only linked to the oral glucose test and not the isoglycemic intravenous glucose clamp, which implied there were no major changes in intrinsic β cell function after RYGB. We did not detect β cell mass in RYGB more than the other two groups, which might explain this phenomenon to some extent. In addition, we did not find increased GLP-1 expression in the SADI-S group either, which seems to be inconsistent with the hindgut hypothesis, so changes in gut-derived hormone after SADI-S warrant future investigation.
Our study does have a few limitations. First, dumping syndrome is a more dangerous complication after RYGB. In theory, SADI-S retains the pyloric and will effectively prevent this complication. Second, gastrointestinal hormones changed after bariatric surgery involve appetite, gastric emptying, and bowel motility. However, due to the limitations of animal models and experimental equipment, comparisons of the above items were not reflected in this study. Further research is needed in the future to address these issues. At last, because of the different species, animal experimental research does not equal clinical research, so more clinical controlled studies are needed to identify the total effects and safety of the SADI-S.
In conclusion, we have found in the present study that, without excessive weight loss, SADI-S and RYGB achieved almost equal efficacy at caloric restriction and diabetes control in mildly obese diabetic rats through overlapping and distinct mechanisms. Future research needs to address related mechanisms, such as how to prevent excessive weight loss and the effects of different digestive tract reconstruction methods on β cell function after bariatric surgery in mildly obese diabetic rats.
References
Ma RC, Lin X, Jia W. Causes of type 2 diabetes in China. Lancet Diabetes Endocrinol. 2014;2(12):980–91.
Yang W, Lu J, Weng J, et al. Prevalence of diabetes among men and women in China. N Engl J Med. 2010;362(12):1090–101.
Zhang L, Qiao Q, Tuomilehto J, et al. Blood lipid levels in relation to glucose status in seven populations of Asian origin without a prior history of diabetes: the DECODA study. Diabetes Metab Res Rev. 2009;25(6):549–57.
Rubino F, Nathan DM, Eckel RH, et al. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Surg Obes Relat Dis. 2016;12(6):1144–62.
Sánchez-Pernaute A, Rubio Herrera MA, Pérez-Aguirre E, et al. Proximal duodenal-ileal end-to-side bypass with sleeve gastrectomy: proposed technique. Obes Surg. 2007;17(12):1614–8.
Brown WA, Ooi G, Higa K, et al. Single anastomosis duodenal-ileal bypass with sleeve gastrectomy/one anastomosis duodenal switch (SADI-S/OADS) IFSO position statement. Obes Surg. 2018;28:1207–16.
Zaveri H, Surve A, Cottam D, et al. Mid-term 4-year outcomes with single anastomosis duodenal-ileal bypass with sleeve gastrectomy surgery at a single US center. Obes Surg. 2018. https://doi.org/10.1007/s11695-018-3358-x.
Mitzman B, Cottam D, Goriparthi R, et al. Stomach intestinal pylorus sparing (SIPS) surgery for morbid obesity: retrospective analyses of our preliminary experience. Obes Surg. 2016;26(9):2098–2104.9.
Surve A, Cottam D, Sanchez-Pernaute A, et al. The incidence of complications associated with loop duodeno-ileostomy after single-anastomosis duodenal switch procedures among 1328 patients: a multicenter experience. Surg Obes Relat Dis. 2018;14(5):594–601.
Cottam A, Cottam D, Zaveri H, et al. An analysis of mid-term complications, weight loss, and type 2 diabetes resolution of stomach intestinal pylorus-sparing surgery (SIPS) versus Roux-En-Y gastric bypass (RYGB) with three-year follow-up. Obes Surg. 2018;28:2894–902.
Hu C, Zhang G, Sun D, et al. Duodenal-jejunal bypass improves glucose metabolism and adipokine expression independently of weight loss in a diabetic rat model. Obes Surg. 2013;23(9):1436–44.
Wang T, Zhang P, Zhang X, et al. Duodenal-jejunal bypass attenuates progressive failure of pancreatic islets in streptozotocin-induced diabetic rats. Surg Obes Relat Dis. 2017;13(2):250–60.
Xu B, Yan X, Shao Y, et al. A comparative study of the effect of gastric bypass, sleeve gastrectomy, and duodenal–jejunal bypass on type-2 diabetes in non-obese rats. Obes Surg. 2015;25(10):1966–75.
Zhang X, Yu B, Yang D, et al. Gastric volume reduction is essential for the remission of type 2 diabetes mellitus after bariatric surgery in nonobese rats. Surg Obes Relat Dis. 2016;12(8):1569–76.
Kim J. American Society for Metabolic and Bariatric Surgery Clinical Issues Committee. American Society for Metabolic and Bariatric Surgery statement on single-anastomosis duodenal switch. Surg Obes Relat Dis. 2016;12(5):944–5.
Di J, Zhang H, Yu H, et al. Effect of Roux-en-Y gastric bypass on the remission of type 2 diabetes: a 3-year study in Chinese patients with a BMI <30 kg/m2. Surg Obes Relat Dis. 2016;12(7):1357–63.
Liang H, Guan W, Yang Y, et al. Roux-en-Y gastric bypass for Chinese type 2 diabetes mellitus patients with a BMI < 28 kg/m(2): a multi-institutional study. J Biomed Res. 2015;29(2):112–7.
Zhao L, Li W, Su Z, et al. Preoperative fasting C-peptide predicts type 2 diabetes mellitus remission in low-BMI Chinese patients after Roux-en-Y gastric bypass. J Gastrointest Surg. 2018;22:1672–8.
da Cruz SP, Matos A, Pereira S, et al. Roux-en-Y gastric bypass aggravates vitamin A deficiency in the Mother-Child Group. Obes Surg. 2018;28(1):114–21.
Kornerup LS, Hvas CL, Abild CB, Richelsen B, Nexo E. Early changes in vitamin B12 uptake and biomarker status following Roux-en-Y gastric bypass and sleeve gastrectomy. Clin Nutr. 2018;S0261–5614(18):30070-0. https://doi.org/10.1016/j.clnu.2018.02.007.
Chakhtoura M, Rahme M, El-Hajj Fuleihan G, et al. Metabolism in bariatric surgery. Endocrinol Metab Clin N Am. 2017;46(4):947–82.
Mahawar KK, Bhasker AG, Bindal V, et al. Zinc deficiency after gastric bypass for morbid obesity: a systematic review. Obes Surg. 2017;27(2):522–9.
van den Broek M, de Heide LJM, Emous M, et al. Satiety and gastrointestinal hormones during a mixed meal tolerance test after gastric bypass surgery: association with plasma amino acid concentrations. Surg Obes Relat Dis. 2018;14(8):1106–17.
de Hollanda A, Casals G, Delgado S, et al. Gastrointestinal hormones and weight loss maintenance following Roux-en-Y gastric bypass. J Clin Endocrinol Metab. 2015;100(12):4677–84.
de Hollanda A, Jiménez A, Corcelles R, et al. Gastrointestinal hormones and weight loss response after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2014;10(5):814–9.
Jørgensen NB, Dirksen C, Bojsen-Møller KN, et al. Exaggerated glucagon-like peptide 1 response is important for improved beta-cell function and glucose tolerance after Roux-en-Y gastric bypass in patients with type 2 diabetes. Diabetes. 2013;62(9):3044–52.
Bojsen-Møller KN. Mechanisms of improved glycaemic control after Roux-en-Y gastric bypass. Dan Med J. 2015;62(4):B5057.
Mingrone G, Castagneto M. Bariatric surgery: unstressing or boosting the beta-cell? Diabetes Obes Metab. 2009;11(Suppl. 4):130–42.
Fujitani Y. How does glucagon-like peptide 1 stimulate human beta-cell proliferation? A lesson from islet graft experiments. J Diabetes Investig. 2018;9:1255–7.
Dutia R, Brakoniecki K, Bunker P, et al. Limited recovery of beta-cell function after gastric bypass despite clinical diabetes remission. Diabetes. 2014;63(4):1214–23.
Acknowledgments
The authors would like to thank Dr. Tao Yang for his excellent language editing support and valuable suggestions for manuscript revision. And thanks are also due to Ting Cao, Wen Wu, and Xiong Zhang for assistance with the experiments. This study was supported by the Science and Technology Development Fund of Shanghai Pudong New Area (Grant No. PKJ2015-Y35).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The study protocol was reviewed and approved by the Institutional Animal Care and Utilization Committee (IACUC) of Fudan University Pudong Medical Center.
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
The research followed all applicable institutional and/or national guidelines for the care and use of animals, as well as was performed according to the recommendations of the Guide for the Care and Use of Laboratory Animals of the Ministry of Health, China.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, T., Shen, Y., Qiao, Z. et al. Comparison of Diabetes Remission and Micronutrient Deficiency in a Mildly Obese Diabetic Rat Model Undergoing SADI-S Versus RYGB. OBES SURG 29, 1174–1184 (2019). https://doi.org/10.1007/s11695-018-03630-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-018-03630-5