Abstract
Background
Bariatric surgery leads to remission of several obesity-related comorbidities, including hypertension. Although antihypertensive medication use is decreased after bariatric surgery, the exact time course of decrease in blood pressure after surgery is not known.
Methods
A database of patients undergoing bariatric surgery at our institute was used to study the effect of surgery on time course of blood pressure changes. Data from surgeries performed between January 2010 and December 2012 were used.
Results
Maximum blood pressure and body weight decreases were observed at 2 weeks and 1 year after surgery, respectively. Average decrease in the mean arterial pressure (MAP) was 4.46 mmHg (61.5 ± 17.1% of maximal decrease) and 7.17 mmHg (maximum decrease) at 1 and 2 weeks after surgery, when the decrease in body weight is 22.8 ± 1.6 and 28 ± 1.4% of maximal weight loss, respectively. In hypertensive patients, MAP decreased from 98.5 ± 0.78 to 92.3 ± 1.76 and 93.1 ± 0.92 mmHg at 1 and 2 weeks post-surgery, respectively. In normotensive patients, the MAP decreased from 96.2 ± 0.79 to 88.7 ± 1.25, 90.0 ± 0.94, 86.5 ± 1.35, 88.0 ± 1.13, and 86.4 ± 2.13 mmHg at 2 weeks, 3 and 6 months, and 1 and 3 years after surgery, respectively.
Conclusions
These data demonstrate that significant decrease in MAP occurs within 2 weeks after bariatric surgery in hypertensive as well as normotensive patients. Future studies are required to investigate the weight-independent mechanisms of blood pressure decreases after bariatric surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bariatric surgery is an effective treatment option to fight the increasing obesity epidemic. Roux-en-Y gastric bypass, laparoscopic sleeve gastrectomy, biliopancreatic diversion, and adjustable gastric band can have significant effects on long-term weight loss [1,2,3]. In addition, these procedures have been shown to decrease the incidence of several obesity-related comorbidities such as diabetes, hypertension, dyslipidemia, and sleep apnea [4,5,6]. However, the underlying pathophysiologic mechanisms are not well understood.
Differential effects of surgery on time course of diabetic remission and weight loss provided a rationale for studying the underlying mechanisms. Several mechanisms such as decreased hepatic glucose production [7], increased glucose utilization in intestines [8], changes in eating behavior [9], and altered entero-endocrine responses to meal ingestion [10] have been proposed. Of these different mechanisms, the majority of published studies have focused on the entero-endocrine changes and increased incretin response after surgery is widely accepted as at least partly being responsible for diabetes remission.
Although strong evidence exists for remission of other obesity comorbidities [2, 6], investigation of their underlying mechanisms has received much less attention. For example, hypertension remission is consistently reported after bariatric surgery [11, 12], but very limited information is available on the underlying pathophysiology. However, it is common practice to decrease antihypertensive medications after surgery, along with anti-diabetic medication. The underlying assumption is that hypertension remission also follows a similar time course of diabetes remission. Although a few preliminary reports suggested early remission of hypertension after bariatric surgery [13,14,15], this hypothesis has not been formally evaluated. The goal of this study is to compare the time course of blood pressure changes after bariatric surgery with that of body weight.
Methods
Data
A retrospective chart review was conducted using 249 patients who underwent laparoscopic sleeve gastrectomy (LSG), and laparoscopic Roux-en-Y gastric bypass (RYGB), from January 2010 to December 2012 at our institute. A preoperative average blood pressure was obtained by using the mean blood pressure of the most recent four blood pressures taken within 3 months of the patient undergoing bariatric surgery. Postoperative blood pressures and mean arterial pressures were then recorded at routine postoperative intervals: 1 to 2 weeks, 3 months, 6 months, 1 year, 3 years, and 5 years. Preoperative antihypertensive medications as well as postoperative trends in medication management were recorded at the same time intervals.
Patient Selection
All adult patients (> 18 years) who underwent bariatric surgery were included. All patients that would undergo bariatric surgery at our institute meet the 1991 NIH criteria for bariatric surgery, which includes BMI greater than or equal to 40 kg/m2 or BMI 35–39.9 kg/m2 with any obesity-related comorbid conditions. Psychiatric evaluation was used to identify any patients that are likely to be unsuccessful with bariatric surgery due to coexisting behavioral or personality disorders such as binge eating disorder. Patients also underwent preoperative esophagogastroduodenoscopy to identify patients with possible hiatal hernia, Helicobacter pylori infection, or other esophageal, gastric, or duodenal pathology. Patients who are noted to have a hiatal hernia are offered concomitant hernia repair at the time of surgery. Patients who are identified to have H. pylori are treated with standard triple therapy regimens (e.g., amoxicillin, clarithromycin, and a proton pump inhibitor). Anyone who has undergone previous bariatric surgery and subsequently underwent second bariatric procedure was excluded from study. Patients are classified to be hypertensive if they are on antihypertensive medication or if their pre-surgery systolic blood pressure is 140 mmHg. Medication for each patient was managed according to the patient history, the clinical presentation, and the class of antihypertensive. The primary goal of medication management after surgery is to maintain the systolic blood pressure below 140 mmHg.
Outcomes
Body weight and mean arterial pressure at different time points were analyzed as primary outcome measures. All blood pressure measurements were recorded by trained nurses using Welch Allyn automatic machines. Blood pressures were measured on the arm of a seated patient at rest. In addition, the number of medications used and the dosage of different classes of antihypertensive medicines were also analyzed across different time points. The antihypertensive medication classes which were overwhelmingly utilized and monitored were beta blockers, calcium channel blockers, ACE inhibitors, angiotensin receptor blockers (ARB’s), and thiazide diuretics.
Statistical Analyses
A within-subject statistical analysis was used to compare mean arterial pressure (MAP) values, body weight, number of medications, and dose of medications. This design eliminates the inter-individual variability associated with baseline (pre-surgery) values in each parameter. However, the variation in individual responses in body weight and MAP to surgical intervention was analyzed by using paired statistical tests. Body weight and MAP data had Gaussian distribution, and thus parametric statistical analyses (paired t tests) were used to compare changes in body weight and MAP at different time points with those of pre-surgery values. The number of antihypertensive medications used is a discontinuous variable and does not follow normal distribution. Wilcoxon paired test was used to compare the changes in number of medications at different intervals after surgery with that of pre-surgery. Antihypertensive medications were classified into seven different categories. All dosage data within each medication class were normalized to pre-surgery values for each patient. Fractional data of each medication class at different post-surgery time points were analyzed to test a significant deviation from the normalized value of 1, using a Student’s t test.
Results
Baseline characteristics of patient population are described in Table 1. Of the total 249 patients included in this study, 135 met the criteria for hypertension while the rest are classified as normotensive. Majority of patients (91%) were female, reflecting the demographics of bariatric patients at military and civilian hospitals. Most of the surgeries performed (85.5%) were LSG, as this is the preferred surgical intervention at our institute. Baseline pre-surgical data on dosage of seven different classes of antihypertensive medications shown in Table 2 provide reference points for interpreting normalized data analyses presented below. In addition, the frequency distribution of baseline medication usage shows that ACE inhibitor/ARB, thiazide, beta adrenergic blockers, and calcium channel blockers are the most used drugs in that order (Table 2).
Time Course of MAP and Body Weight Changes
As expected, bariatric surgery led to a progressive decrease in body weight from 1 week to 1 year after surgery (Fig. 1a). This trend reversed after 1 year with an increased body weight at 3- and 5-year post-surgical intervals compared to that of 1 year. A statistically significant decrease in MAP was observed at all the time points after surgery, except at 5 years. Similarly, a decrease in systolic blood pressure was observed at all the time points after surgery (Supplemental Figure 1). The magnitude of MAP decrease was greatest at 2 weeks post-surgery with comparable values at 1-week, 3-month, 6-month, 1-year, and 3-year time points. This discrepancy in time course of the magnitude of changes in body weight and MAP is readily apparent when the same data are visualized as percent of maximal change. Data were normalized to the values observed at 2 weeks (MAP) or 1 year (body weight) within each subject and expressed as percent maximal change. Figure 1b shows that 61.5 and 100% of maximal MAP changes were observed at 1 and 2 weeks after surgery when the body weight decreases were only 22.8 and 28% of maximal changes observed, respectively. These data illustrate that significant MAP decreases occur within the first 2 weeks after surgery and are not related to the magnitude of body weight changes.
Effect of bariatric surgery on body weight and MAP. a Body weight (kg) and MAP (mmHg) before and different time points after bariatric surgery are shown as mean ± SEM. Asterisk symbol represents significant difference from pre-surgery values (P < 0.05). N values are 249, 88, 205, 160, 114, 157, 137, and 52 at pre-surgery, 1 week, 2 weeks, 3 months, 6 months, 1 year, 3 years, and 5 years, respectively. Percent of excess BMI lost at different post-surgery time points was 44.81 ± 2.01, 46.06 ± 1.93, 62.69 ± 1.63, 71.62 ± 1.70, 80.44 ± 1.91, 75.70 ± 2.04, and 71.91 ± 4.23, respectively. b Body weight and MAP are shown as percent of maximal change after surgery (1 year for body weight and 2 weeks for MAP). MAP mean arterial pressure
Time Course of Changes in Antihypertensive Medication Use
Analyses of changes in number and dosage of antihypertensive medications are shown in Fig. 2a, b, respectively. A statistically significant decrease in the number of medications was observed at all the post-surgical time points with the exception of 5-year data. Analyses of changes in the dosage of four of the most commonly used medications show that a statistically significant decrease in dosage is observed at all the time points for thiazides, beta adrenergic blockers, and calcium channel blockers. ACE inhibitor/ARB usage was decreased at 1-week to 6-month period, while the changes at 1–5 years were statistically not significant. Maximum fractional decrease was observed at 1 week for thiazides, while the other three classes showed comparable decrease at this time point. A progressive trend towards higher doses after 1 week was observed for thiazides and ACE inhibitor/ARB, but not beta adrenergic blockers and calcium channel blockers.
Effect of bariatric surgery on use of hypertension medication. a Average number (± SEM) of antihypertensive medication used before and different time points after surgery is shown. Asterisk symbol represents statistically significant difference from pre-surgery values (P < 0.05). b Fraction of antihypertensive medication used at different time points after surgery is shown as mean ± SEM. Pre-surgery dosage in each drug category was defined as 1. All post-surgery values except those indicated by asterisk symbols are statistically different from corresponding pre-surgery values (P < 0.05). Only the top four categories based on pre-surgery usage are shown
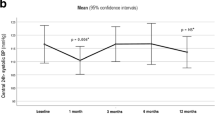
Comparison of MAP Changes in Normotensive and Hypertensive Subjects
The above data show that the changes in MAP observed in Fig. 1 occur in presence of decreased medication use. Thus, it is difficult to assess the true effect of bariatric surgery on MAP changes. Furthermore, to our knowledge, the effect of bariatric surgery on blood pressure in normotensive subjects has not been reported. We next analyzed the time course of changes in MAP in normotensive and hypertensive patients. The average pre-surgery MAP in normotensive patients (104.2 ± 0.80 mmHg) was lower than that in hypertensive patients (107.7 ± 1.72 mmHg) (P < 0.01). Figure 3 shows that a statistically significant decrease in MAP was observed at 1 and 2 weeks post-surgery in hypertensive patients. In normotensive subjects, a statistically significant decrease in MAP was observed at 2 weeks, 3 months, 6 months, 1 year, and 3 years after surgery. The magnitude of this decrease was 7.6, 6.9, 10.8, 9.7, and 8.7 mmHg, respectively. Similar decreases in systolic blood pressure were also observed in these two cohorts after surgery (Supplemental Figure 2). These data demonstrate that the decrease in blood pressure after bariatric surgery occurs as early as 2 weeks in both normotensive and hypertensive patients.
Discussion
In a comprehensive analysis of a temporal discord between hypertension remission and weight loss after bariatric surgery, we found that decreases in blood pressure occur within 2 weeks in both hypertensive and normotensive patients. To the best of our knowledge, this is the first evidence of blood pressure decreases in clinically normotensive human subjects after bariatric surgery. It is difficult to assess the exact effect of bariatric surgery on blood pressure because of simultaneous changes in medication in hypertensive patients in the post-surgical period. Thus, the magnitude of blood pressure changes in normotensive patients reflects the true effect of surgery. As mentioned in the “Introduction,” three other studies reported decreases in blood pressure within weeks after bariatric surgery. However, a few methodological limitations of the earlier studies preclude a firm conclusion about the true effect of bariatric surgery on the blood pressure. A recent study reported decreases in blood pressure in hypertensive patients but not in normotensive patients after laparoscopic Roux-en-Y gastric bypass [15]. This study had small set of study subjects (n=12), and the earliest time point of post-surgical blood pressure measurement was 6 weeks. An earlier study reported decreases in blood pressure as early as 1 week after laparoscopic Roux-en-Y gastric bypass [13]. However, the majority of the hypertensive subjects and half of the subjects in normotensive group in this study were on antihypertensive medication. The changes in postoperative medication make it difficult to assess the true effect of surgery on blood pressure, similar to the limitation in the hypertensive group of our study. The third study is a preliminary report from our institute including a majority of sleeve gastrectomy patients [14]. This study also has the same limitation of simultaneous medication changes along with the changes in blood pressure. Thus, the present study provides the first conclusive evidence of blood pressure changes in normotensive bariatric surgery patients and offers an incentive to study the possible underlying mechanisms of weight loss-independent decreases in blood pressure.
Data on changes in blood pressure medication are presented in order to present a context in which the blood pressure changes in hypertension group were observed. It would be difficult to read into these changes as they were not designed to meet any criteria, but rather follow a standard of care protocol at our institution. Another limitation of our study is that it is retrospective in nature and suffers from all the limitations of such an experimental design. For example, there are missing observations that are responsible for some of the variability seen in blood pressure. However, our statistical analysis of within-subjects design was able to overcome this limitation and provide meaningful information on blood pressure changes after surgery. Another limitation of our study is that it is conducted at a single institution and we pooled different surgery types. The majority of the subjects underwent LSG, and when data were analyzed in LSG group alone, the conclusions were not changed.
Phenotype of obese individual is one of the risk factors for hypertension where abdominal fat rather than subcutaneous fat is associated with hypertension [16, 17]. Multiple mechanisms are involved in pathophysiology of obesity-associated hypertension [18]. Increased activity of the sympathetic nervous system is one of the primary causes for obesity-associated increase in humans as well as animal models [19, 20]. In addition, hyperinsulinemia, activation of the renin-angiotensin-aldosterone system, abnormal levels adipokines, and altered spectrum of cytokines acting at the vascular endothelial level have all been suggested to play a role in obesity-associated hypertension [18, 21, 22]. While some of these mechanisms might be responsible for improvement in blood pressure during later phases (beyond 3–6 months) after bariatric surgery, they are unlikely to account for early improvements after bariatric surgery. The correlation of body weight and blood pressure changes observed at later phases, but not at 1–2 weeks after surgery provides evidence for differences in the respective mechanisms of blood pressure improvement.
Augmented entero-endocrine responses are at least partly responsible for early diabetic remission after bariatric surgery. For example, increased glucagon-like peptide 1 (GLP-1) release can cause increased insulin production and better glycemic control within a week of surgery [23]. There are several plausible mechanisms by which GLP-1 can have a hypotensive effect. Renin-angiotensin-aldosterone system, immune cell activation, vasorelaxation, and natriuresis have all been hypothesized to play a role in incretin-mediated improvements in blood pressure [24]. Indeed, long-term administration of a GLP1 analog (exenatide) can decrease blood pressure in humans [25, 26]. While some of these systems, such as renin-angiotensin-aldosterone system, are involved in bodyweight-related changes in blood pressure, the exact mode of their involvement after augmented incretin response is likely to be different. Other hormones, such as atrial natriuretic peptide and inflammatory mediators, have also been proposed to mediate hypotensive effects of bariatric surgery [15, 27]. Future studies are required to analyze the precise role of different endocrine mediators and their downstream effects on blood pressure regulation after bariatric surgery.
These future studies in humans and animal models can be designed with the benefit of the data presented in this manuscript. For example, we show that pre-existing hypertension is not necessarily required for studying the changes in blood pressure after bariatric surgery. Indeed, the interpretation of blood pressure changes in this cohort, in absence of any medication changes after surgery, is relatively simpler and provides additional support to the effectiveness of surgery in blood pressure regulation. Precise animal models will enable us to dissect the role of different mechanisms outlined above. Similarly, non-invasive prospective studies in humans such as monitoring natriuresis after surgery can provide useful leads.
References
Eldar S, Heneghan HM, Brethauer SA, et al. Bariatric surgery for treatment of obesity. Int J Obes. 2011;35(Suppl 3):S16–21. https://doi.org/10.1038/ijo.2011.142.
Puzziferri N, Roshek 3rd TB, Mayo HG, et al. Long-term follow-up after bariatric surgery: a systematic review. JAMA. 2014;312(9):934–42. https://doi.org/10.1001/jama.2014.10706.
Payne JH, DeWind LT. Surgical treatment of obesity. Am J Surg. 1969;118(2):141–7. https://doi.org/10.1016/0002-9610(69)90113-5.
Benaiges D, Mas-Lorenzo A, Goday A, et al. Laparoscopic sleeve gastrectomy: more than a restrictive bariatric surgery procedure? World J Gastroenterol. 2015;21(41):11804–14. https://doi.org/10.3748/wjg.v21.i41.11804.
Courcoulas AP, Yanovski SZ, Bonds D, et al. Long-term outcomes of bariatric surgery: a National Institutes of Health symposium. JAMA Surg. 2014;149(12):1323–9. https://doi.org/10.1001/jamasurg.2014.2440.
Muller-Stich BP, Senft JD, Warschkow R, et al. Surgical versus medical treatment of type 2 diabetes mellitus in nonseverely obese patients: a systematic review and meta-analysis. Ann Surg. 2015;261(3):421–9. https://doi.org/10.1097/SLA.0000000000001014.
Breen DM, Rasmussen BA, Kokorovic A, et al. Jejunal nutrient sensing is required for duodenal-jejunal bypass surgery to rapidly lower glucose concentrations in uncontrolled diabetes. Nat Med. 2012;18(6):950–5. https://doi.org/10.1038/nm.2745.
Saeidi N, Meoli L, Nestoridi E, et al. Reprogramming of intestinal glucose metabolism and glycemic control in rats after gastric bypass. Science. 2013;341(6144):406–10. https://doi.org/10.1126/science.1235103.
Munzberg H, Laque A, Yu S, et al. Appetite and body weight regulation after bariatric surgery. Obes Rev. 2015;16(Suppl 1):77–90. https://doi.org/10.1111/obr.12258.
Mingrone G. Role of the incretin system in the remission of type 2 diabetes following bariatric surgery. Nutr Metab Cardiovasc Dis. 2008;18(8):574–9. https://doi.org/10.1016/j.numecd.2008.07.004.
Hatoum IJ, Blackstone R, Hunter TD, et al. Clinical factors associated with remission of obesity-related comorbidities after bariatric surgery. JAMA Surg. 2016;151(2):130–7. https://doi.org/10.1001/jamasurg.2015.3231.
Neff KJ, Baud G, Raverdy V, et al. Renal function and remission of hypertension after bariatric surgery: a 5-year prospective cohort study. Obes Surg. 2017;27(3):613–9. https://doi.org/10.1007/s11695-016-2333-7.
Ahmed AR, Rickards G, Coniglio D, et al. Laparoscopic Roux-en-Y gastric bypass and its early effect on blood pressure. Obes Surg. 2009;19(7):845–9. https://doi.org/10.1007/s11695-008-9671-z.
Bland CM, Tritsch AM, Bookstaver DA, et al. Hypertension and diabetes mellitus medication management in sleeve gastrectomy patients. Am J Health Syst Pharm. 2013;70(12):1018–20. https://doi.org/10.2146/ajhp120607.
Bonfils PK, Taskiran M, Damgaard M, et al. Roux-en-Y gastric bypass alleviates hypertension and is associated with an increase in mid-regional pro-atrial natriuretic peptide in morbid obese patients. J Hypertens. 2015;33(6):1215–25. https://doi.org/10.1097/HJH.0000000000000526.
Hamdy O, Porramatikul S, Al-Ozairi E. Metabolic obesity: the paradox between visceral and subcutaneous fat. Curr Diabetes Rev. 2006;2(4):367–73.
Matsuzawa Y, Funahashi T, Nakamura T. The concept of metabolic syndrome: contribution of visceral fat accumulation and its molecular mechanism. J Atheroscler Thromb. 2011;18(8):629–39. https://doi.org/10.5551/jat.7922.
Vaneckova I, Maletinska L, Behuliak M, et al. Obesity-related hypertension: possible pathophysiological mechanisms. J Endocrinol. 2014;223(3):R63–78. https://doi.org/10.1530/JOE-14-0368.
Prior LJ, Davern PJ, Burke SL, et al. Exposure to a high-fat diet during development alters leptin and ghrelin sensitivity and elevates renal sympathetic nerve activity and arterial pressure in rabbits. Hypertension. 2014;63(2):338–45. https://doi.org/10.1161/HYPERTENSIONAHA.113.02498.
Wofford MR, Anderson Jr DC, Brown CA, et al. Antihypertensive effect of alpha- and beta-adrenergic blockade in obese and lean hypertensive subjects. Am J Hypertens. 2001;14(7 Pt 1):694–8. https://doi.org/10.1016/S0895-7061(01)01293-6.
Hall JE, do Carmo JM, da Silva AA, et al. Obesity-induced hypertension: interaction of neurohumoral and renal mechanisms. Circ Res. 2015;116(6):991–1006. https://doi.org/10.1161/CIRCRESAHA.116.305697.
Seven E, Husemoen LL, Wachtell K, et al. Overweight, adipocytokines and hypertension: a prospective population-based study. J Hypertens. 2014;32(7):1488–1494; discussion 94. https://doi.org/10.1097/HJH.0000000000000207.
Jorgensen NB, Dirksen C, Bojsen-Moller KN, et al. Exaggerated glucagon-like peptide 1 response is important for improved beta-cell function and glucose tolerance after Roux-en-Y gastric bypass in patients with type 2 diabetes. Diabetes. 2013;62(9):3044–52. https://doi.org/10.2337/db13-0022.
Ryan D, Acosta A. GLP-1 receptor agonists: nonglycemic clinical effects in weight loss and beyond. Obesity (Silver Spring). 2015;23(6):1119–29. https://doi.org/10.1002/oby.21107.
Blonde L, Klein EJ, Han J, et al. Interim analysis of the effects of exenatide treatment on A1C, weight and cardiovascular risk factors over 82 weeks in 314 overweight patients with type 2 diabetes. Diabetes Obes Metab. 2006;8(4):436–47. https://doi.org/10.1111/j.1463-1326.2006.00602.x.
Buse JB, Drucker DJ, Taylor KL, et al. DURATION-1: exenatide once weekly produces sustained glycemic control and weight loss over 52 weeks. Diabetes Care. 2010;33(6):1255–61. https://doi.org/10.2337/dc09-1914.
Aghamohammadzadeh R, Greenstein AS, Yadav R, et al. Effects of bariatric surgery on human small artery function: evidence for reduction in perivascular adipocyte inflammation, and the restoration of normal anticontractile activity despite persistent obesity. J Am Coll Cardiol. 2013;62(2):128–35. https://doi.org/10.1016/j.jacc.2013.04.027.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent Statement
For this type of study, formal consent is not required.
Electronic Supplementary Material
ESM 1
(DOCX 128 KB)
Rights and permissions
About this article
Cite this article
Hawkins, D.N., Faler, B.J., Choi, Y.U. et al. Time Course of Blood Pressure Decrease After Bariatric Surgery in Normotensive and Hypertensive Patients. OBES SURG 28, 1845–1851 (2018). https://doi.org/10.1007/s11695-017-3091-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-017-3091-x