Abstract
Background
Leaks are considered one of the major complications of laparoscopic sleeve gastrectomy (LSG) with a reported rate up to 7 %. Drainage of the collection coupled with SEMS deployment is the most frequent treatment. Its success is variable and burdened by high morbidity and not irrelevant mortality. The aim of this paper is to suggest and establish a new approach by endoscopic internal drainage (EID) for the management of leaks.
Methods
Since March 2013, 67 patients presenting leak following LSG were treated with deployment of double pigtail plastic stents across orifice leak, positioning one end inside the collection and the other end in remnant stomach. The aim of EID is to internally drain the collection and at the same time promote leak healing.
Results
Double pigtails stent were successfully delivered in 66 out of 67 patients (98.5 %). Fifty patients were cured by EID after a mean time of 57.5 days and an average of 3.14 endoscopic sessions. Two died for event not related to EID. Nine are still under treatment; five failure had been registered. Six patients developed late stenosis treated endoscopically.
Conclusions
EID proved to be a valid, curative, and safe mini-invasive approach for treatment of leaks following SG. EID achieves complete drainage of perigastric collections and stimulates mucosal growth over the stent. EID is well tolerated, allows early re-alimentation, and it is burdened by fewer complications than others technique. Long-term follow-up confirms good outcomes with no motility or feeding alterations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Laparoscopic sleeve gastrectomy (LSG) is a stand-alone procedure in the treatment of morbid obesity. Surgical complications (bleeding, leaks, and stenosis) occur in 10 to 13.2 % of cases [1]. Leaks are considered a major complication with a reported rate up to 7 % after primary LSG [2]. Surgical/percutaneous drainage coupled with self expandable metal stent (SEMS) is nowadays the most frequent treatment. Success rate depends greatly on delay of intervention [3]; moreover, the use of SEMS is burdened with high migration rate [4], occlusion by ingrowth tissue [5], esophageal stenosis [6], and rupture [7].
Biodegradable plugs [8], glue [9], over-the-scope clip [10], endoluminal vacuum therapy [11], and trans-orificeal plastic stent [12] have been proposed as alternative endoscopic techniques with limited success rate.
Up to now, well-established guidelines concerning management of leaks after bariatric surgery are missing.
Hereby, according to our previous experience [13] with trans-oral endoscopic internal drainage coupled with enteral nutrition (EDEN), we suggest a new treatment protocol for management of leak following LSG.
Patients and Methods
From March 2013 to December 2014, a total of 67 patients (57 females) with a median age of 43 years (23–70) were treated with endoscopic internal drainage (EID) technique for leaks following SG. Informed consent was obtained from all patients. Institutional Review Boards approved the study for human research.
Data were collected in prospectively maintained database and retrospectively analyzed.
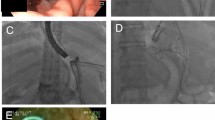
The technical peculiarities of EID technique are as follows: firstly evaluation of leak area and perigastric collection cavity if present and secondly deployment of double pigtails stent (Advanix®, Boston Scientific®, MA, USA). Stents were delivered across the orifice placing one end inside the cavity to be drained and the other end in digestive lumen in order to avoid migration (Fig. 1a–c). Stents were changed every 4–6 weeks until complete fistula healing. According to collection extent, shape and to leak size, from 1 to 3, 7, or 10 Fr stent were inserted. Nasojejunal feeding tube (NJT) (Corflo®, Corpak® MedSystems, IL, USA) was left in place in the third part of the duodenum if necessary. After the first 15 cases, CO2 was used in order to reduce risk of pneumoperitoneum related to air insufflation.
Leak location was as follows: 56 at cardia level while 11 located in the middle portion of staple line. Eight patients presented complete dehiscence of one staple fire, and one patient had a concomitant gastric-bronchial fistula (Fig. 2).
Leaks were diagnosed at an average time interval of 52.2 days (1–1450) from surgery, and EID procedure was carried out 60.5 days (4–1460) after LSG. According to Rosenthal’s classification [14], leaks in this series were classified as acute in 26 cases, early in 32, late in 3, and chronic in 6 patients in which leaks were diagnosed after 12 weeks.
Of 66 patients, 42 had a drainage positioned close to the leak after 52.12 days (0–1430) from primary surgery for diffuse or localized peritonitis while the remaining 25 patients did not needed any surgical/radiological drainage. NJT was inserted in 41/66 patients, 6/66 patients presented surgical performed jejunostomy while 8/66 patients had a peripherally inserted central catheter (PICC) and refused feeding tube. All these patients were kept nil by mouth with enteral nutrition (Impact® Enteral, Nestlé Health Science, Lausanne, Switzerland) at least till the first endoscopic control.
The remaining 11/66 patients were allowed normal diet due to complete blockage of small leak by means of stent insertion. Demographics data and leak’s characteristics are listed in Tables 1 and 2.
Results
Technical success, defined as successful deployment of pigtail stent across the leak, was achieved in 66 out of 67 patients (98.5 %).
In one patient, we failed to perform EID due to an intra-procedural perforation related to erroneous guidewire manipulation (Fig. 3a). Patient required emergency surgery fully recovering after 3 months of enteral nutrition.
a Extravasation of medium contrast in the peritoneal cavity due to erroneous guidewire manipulation during selective catheterism of perigastric collection. b Extravasation of medium contrast in peritoneal cavity, underneath diaphragms (red arrow) due to a detachment of perigastric collection from the stomach (yellow arrow) (color figure online)
Three patients were not considered in the results: other than one technical failure, two patients died at days 1 and 13 respectively for pulmonary embolism.
Therefore, we report the long-term results of EID protocol in 64 patients.
Clinical success was defined as absence of free contrast medium extravasation in the peritoneal cavity neither around the stomach nor through fistula orifice. Pseudodiverticula comunicating with gastric tube with assured internal empting was considered as a clinical success..
All patients underwent first check endoscopy after an average of 31.3 days (10–54). Twenty-six out of 50 had a good outcome showing leak closure after removal of the stent while 24 patients required deployment of new ones. During the second endoscopic session, one patient developed a septic shock due to intra-abdominal abscess requiring surgical drainage; nonetheless, the patient continued EID treatment, and she fully recovered after 88 days of treatment.
At second check, endoscopy leak closure was achieved in 16 out of 24 patients after an overall average time of 62.3 days (48–88) of stenting. The remaining eight patients healed respectively after 3 (3 pts), 5 (2 pts), 6 (1 pt), 7 (1 pt), and 8 (1 pt) endoscopic sessions.
Clinical success was achieved in 50 of 64 (78.2 %) patients after a mean time of 57.5 days (10–206) with an average of 3.14 (2–16) endoscopic sessions per patient while 9 patients (14 %) are still under treatment after an average of 36 days (2–100).
We registered five clinical failure (7.8 %) fistula. Two patients with chronic fistula were successfully cured by n-butyl-2-cyanoacrylate glue (Glubran® 2, GEM, Viareggio, Italy) after failure of EID (average of 368 days of treatment and 12 endoscopic sessions) due to recurrent pigtail stent migration. The other three patients (two late and one chronic fistula) were definitively treated by total gastrectomy for chronic sepsis after an average of three endoscopic sessions for a total of 90 days of EID treatment.
During the first endoscopic drainage, two patients developed pneumoperitoneum. In the first case, medium contrast extravasation was detected intra-procedurally (Fig. 3b) while in the second case, insertion of a 10 Fr stent in a small cavity induced its rupture with consequent air leak. These two cases brought us to deploy only 7 Fr stents in case of small cavity. Nonetheless, both EID were successful and neither patients required surgical procedure.
After an average of 29.5 days (7–80) from EID 5 out of 50 patients were re-admitted to the hospital due to CRP elevation and/or fever. All subjects underwent CT scan and upper endoscopy showing a gastrocutaneous fistula in two patients successfully treated endoscopically by deployment of pigtail stent in order to promote fistula healing and to drain the collection (Fig. 5a–c). One patient with chronic fistula and perispleen abscess far from sleeve was considered a clinical failure and underwent total gastrectomy. Two patients did not present any medium extravasation and fully recovered after few days of fasting. Results are summarized in Tables 3 and 4.
Six of 50 patients developed a stenosis after an average of 36 days (15–45) from the end of EID. All patients underwent an average of three dilations with achalasia balloon (Rigiflex®, Boston Scientific, MA, USA) up to a diameter of 40 mm. In one case, after balloon dilation failure, FCSEMS was deployed for 5 weeks. All stenosis were successfully treated (Fig. 4a–d). Four out of six of these patients had more than 2-cm-long complete dehiscence of staple line. Granulation tissue induced by the pigtails and the subsequent scar retraction were most probably responsible for stenosis formation. Mean follow-up for the 50 patients treated by means of EID is of 316 days (20–600), and all patients are on full diet, symptom free, and showed no weight regain.
Discussion
Gastric leaks (GL) after bariatric surgery (BS) represent one of most dreaded complications due to its associated high morbidity and mortality. No standard protocol for management of GL exists. Surgical revision, due to surrounding inflammation and ischemic edges, is often unsuccessful and burdened with high post-operative complications [15, 16]. Surgical treatment should be reserved to patients presenting severe sepsis or multiorgan failure.
Mainstay of nonsurgical treatment consists of complete drainage of any fluid collection, enteral hyper-alimentation, and antibiotics therapy [17].
Up to now, deployment of SEMS is the most popular endoscopic approach. In literature, several studies are present; however, the success rate is very variable and study populations are often limited. Moreover, use of SEMS is limited by poor tolerance due to nausea, vomiting, retrosternal discomfort and by significant morbidity and mortality. Migration is still an open issue occurring with a frequency variable from 33 [18] to 83 % [19] of cases; widening of leak due to excessive SEMS radial force and refilling of fistula due to occlusion of distal end of metallic stent by overgrowth has been also observed in our experience in particular with the new developed stents that in order to reduce migration were designed longer and with a larger diameter.
Pequignot et al. firstly reported the use of pigtail drain in post-SG leaks. This approach showed to be efficacious, better tolerated, requiring fewer procedures, and shorter healing time compared to SEMS [9].
We think that the indications for SEMS in the managementof leak following BS should be carefully re-evaluated considering the abovementioned shortcomings. We believe that rather than by-passing (span) the leak with SEMS, the key to success is to accomplish complete internal drainage of any collection and to induce orifice traumatism to promote healing. Supported by this theory since March 2013, we abandoned the use of SEMS in favor of EID technique.
According to our previous experience [13], we suggest an algorithm for the use of endoscopic internal drainage with or without enteral nutrition as first-line management of GL following SG. Our study, amounting to 67 patients, is the largest to date. We believe that pigtail stent acting as a foreigner body promotes re-epithelialization while guarantying internal drainage of infected fluid collection. It allows early removal of surgical drainage (if present) avoiding fistula tract to become chronic. Moreover, stent do not interfere with early oral re-alimentation after a short period of enteral nutrition.
Paramount importance has to be given to leak site and perigastric collection evaluation in order to correctly assess its extension and anatomical relations. We performed not only intra-procedural contrast study but also, whenever feasible, direct view inspection by means of endoscopic cavity exploration (NOTES procedure) [20] (video 1).
Systematic evaluation after 4–6 weeks was performed in order to avoid pigtail obstruction and more importantly to induce fistula’s edge traumatism to help granulation tissue formation and watertight closure of the fistula (video 2). Another key point is C02 insufflation. It reduces risk of pneumoperitoneum, air embolization, and post-procedural patient discomfort [21]. For small cavity, it is important to deploy only small caliber pigtail stent. They are softer and easier to insert reducing the risk of perforating the cavity. After the first 21 patients, we realized that persistence of small orifice or pseudodiverticula (Fig. 6) does not have any pathological impact on re-alimentation or on motility and stomach empting and thus does not require any further treatment.
In our series, average interval between primary surgery and EID was of 60.3 days (4–1460), classifying our leaks as “late” fistula [14]. EID proved efficient even for leaks generally associated with longer healing time and lower response to SEMS deployment [22].
We recommend EID approach even for acute and early fistula (within 6 weeks). EID guarantees a fast fistula resolution and avoidance of surgical cumbersome procedure.
Draining the cavity from inside proved efficient, easier, and more physiological than surgical or radiological drainage allowing avoidance of potentially long term external fistula.
In almost one third of the cases, EID resulted in an all-in-one procedure allowing simultaneously closure of leak and drainage of infected cavity. Differently from SEMS, EID reduced necessity for external drainage by means of surgical re-intervention or radiological percutaneous procedure, thus reducing interval time between diagnosis and treatment, complications related to different procedures and costs. We suggest an “algorithm” that allows us to choose the correct therapeutic plan according to patient’s general status and leak’s characteristics (Fig. 7). Double pigtail stent expulsion occurred in six patients due to granulation tissue pushing the stent outside the cavity, but no re-intervention was required.
No patient required treatment interruption not even in case of complications related to stent deployment. EID was continued until healing, allowing oral diet after the first endoscopic controls, with no external drainage.
Conclusion
EID proved to be a valid, curative, and safe mini-invasive approach for treatment of leaks following SG. According to our experience, EID protocol should be considered as primary management for both early and late leaks if no diffuse peritonitis or multiorgan failure is present. Although multiple endoscopic sessions are required, EID achieves complete drainage of perigastric collections, simultaneously stimulating mucosal growth over the stent. EID is well tolerated, less expensive than SEMS, and burdened by fewer complications. Long-term follow-up confirms good outcomes with no motility or feeding alterations.
References
Trastulli S, Desiderio J, Guarino S, et al. Laparoscopic sleeve gastrectomy compared with other bariatric surgical procedures: a systematicreview of randomized trials. Surg ObesRelat Dis. 2013;9(5):816–29.
Fuks D, Verhaeghe P, Brehant O, et al. Results of laparoscopic sleeve gastrectomy: a prospective study in 135 patients with morbidobesity. Surgery. 2009;145:106–13.
Donatelli G, Dhumane P, Perretta S, et al. Endoscopic placement offully covered self expanding metal stents for management of postoperative foregut leaks. J Minim Access Surg. 2012;8(4):118–24.
Nguyen NT, Nguyen XM, Dholakia C. The use of endoscopic stent in management of leaks after sleeve gastrectomy. Obes Surg. 2010;20:1289–92.
VanBoeckel PG, Dua KS, Weusten BL, Schmits RJ, Surapaneni N, Timmer R, et al. Fully coveredself-expandable metal stents (SEMS), partially coveredSEMS and self-expandable plastic stents for the treatmentof benign esophageal ruptures andanastomoticleaks. BMC Gastroenterol. 2012;12:19.
Iossa A, Fiocca F, Cereatti F, Silecchia G. Severe events related to use of stent in case of bariatric complications. In press MN JSLS.2014.00223R1
Donatelli G, Dhumane P, Vergeau BM et al. Succesfullremoval from the esophagus of a self-expandble metal stenthadshriveled up into a tangledball. Endoscopy. 2013;45Suppl 2 UCTN:E410-1
Toussaint E, Eisendrath P, Kwan V, et al. Endoscopic treatment of postoperative enterocutaneous fistulas after bariatric surgery with theuse of a fistula plug: report of five cases. Endoscopy. 2009;41(6):560–3.
Campos JM, Pereira EF, Evangelista LF, et al. Gastrobronchial fistula after sleeve gastrectomy and gastric bypass: endoscopicmanagementand prevention. Obes Surg. 2011;21(10):1520–9.
Donatelli G, Leblanc S, Vienne A, et al. Is Over-the-Scope Clip a permanently implanted device? Outcome and follow up of clipdelivery for fistulas, perforations and bleeding. Gastroint Endosc. 2013;77(5):AB 207–8.
Seyfried F, Reimer S, Miras AD, et al. Successful treatment of a gastric leak after bariatric surgery using endoluminal vacuum therapy. Endoscopy. 2013;45:E267–68.
Pequignot A, Fuks D, Verhaeghe P, et al. Is there a place for pigtail drains in the management of gastric leaks after laparoscopic sleevegastrectomy? Obes Surg. 2012;22:712–20.
Donatelli G, Ferretti S, Vergeau BM, et al. Endoscopic internal drainage with enteral nutrition (EDEN) for treatment of leaks following sleeve gastrectomy. Obes Surg. 2014;24:1400–07.
Rosenthal RJ. International Sleeve Gastrectomy Expert Panel Consensus statement: best practice guidelines based on experience of >12000 cases. SOARD. 2012;8:8–19.
Dakwar A, Assalia A, Khamaysi I, et al. Late complication of laparoscopic sleeve gastrectomy. Case Rep Gastrointest Med. 2013;2013:136153. doi:10.1155/2013/136153.
Roller JE, Provost DA. Revision of failed gastric restrictive operations to Roux-en-Y gastric bypass: impact of multiple prior bariatric operations on outcome. Obes Surg. 2006;16:865–9.
Sakran N, Goitein D, et al. Gastric leaks after sleeve gastrectomy: a multicenter experience with 2,834 patients. Surg Endosc. 2013;27:240–5.
Merrifield BF, Lautz D, Thompson CC. Endoscopic repair of gastric leaks after Roux-en-Y gastric bypass: a less invasive approach. Gastrointest Endosc. 2006;63:710–4.
Edwards CA, Bui TP, Astudillo JA, et al. Management of anastomotic leaks after Roux-en-Y bypass using self-expanding polyester stents. Surg Obes Relat Dis. 2008;4:594–9.
Begè T, Emungania O, Vitton V, et al. An endoscopic strategy for managment of anastomotic complications from bariatric surgery: a prospective study. Gastrointestendosc. 2011;73:238–44.
Inoue H, Minami H, Kobayashi Y. Peroral endoscopic myotomy (POEM) for esophageal achalasia. Endoscopy. 2010;42:265–71.
Simon F, Siciliano I, Gillet A, et al. Gastric leak after laparoscopic sleeve gastrectomy: early covered self-expandable stent reduces healing time. Obes Surg. 2013;23(5):687–92.
Compliance with Ethical Standards
We declare that this article does not contain any studies with human participants or animals performed by any of the authors. Informed consent was obtained from all individual participants included in the study.
Conflict of Interest
Gianfranco DONATELLI: no conflict of interest
Jean-Loup DUMONT: no conflict of interest
Fabrizio CEREATTI: no conflict of interest
Stefano FERRETTI: no conflict of interest
Bertrand Marie Vergeau: no conflict of interest
Thierry TUSZYNSKI: no conflict of interest
Guillaume POURCHER: no conflict of interest
Hadrien TRANCHART: no conflict of interest
Paola MARIANI: no conflict of interest
Alexandre MEDURI:no conflict of interest
Jean-Marc CATHELINE: no conflict of interest
Ibrahim DAGHER: no conflict of interest
Fausto FIOCCA: no conflict of interest
Jean-Pierre MARMUSE: no conflict of interest
Bruno MEDURI: no conflict of interest
The authors have no financial arrangements or commercial associations that might be a conflict of interest in relation to this manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Videos 1
Direct endoscopic exploration of peritoneal cavity (NOTES procedure) through a large fistula defect followed sleeve gastrectomy. (MOV 12545 kb)
Videos 2
Granulation tissue formation and watertight closure of large fistula defect at the level of medium gastric line. (MOV 9326 kb)
Rights and permissions
About this article
Cite this article
Donatelli, G., Dumont, JL., Cereatti, F. et al. Treatment of Leaks Following Sleeve Gastrectomy by Endoscopic Internal Drainage (EID). OBES SURG 25, 1293–1301 (2015). https://doi.org/10.1007/s11695-015-1675-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-015-1675-x