Abstract
Background
Visfatin is an adipokine linked to obesity and inflammation, and it has insulin-mimetic properties. Apelin is an adipokine with positive cardiac inotropic effects, and it may be related to inflammatory molecules. Variations in plasma visfatin and apelin levels following bariatric surgery remain controversial.
Methods
In this study, patients who underwent a biliopancreatic diversion with duodenal switch (BPD-DS) were compared to a severely obese group (control group). Anthropometric measures and blood samples were taken before surgery, on days 1 and 5, as well as at 6 and 12 months after surgery in the BDP-DS group. For the control group, the tests were performed at baseline and at 6 and 12 months.
Results
Seventy subjects in the BPD-DS group and 28 in the control group were included. The expected reduction in body weight at 1 year after a BPD-DS was observed (85.9 ± 18.5 vs. 136.6 ± 27.7 kg at baseline; p < 0.001). Plasma visfatin levels decreased at day 1 (16.13 ± 5.56 vs. 18.82 ± 7.36 ng/mL at baseline; p = 0.001), while plasma apelin levels decreased at day 5 (0.50 ± 0.28 vs. 0.55 ± 0.33 ng/mL at baseline; p = 0.040) after surgery. There were no changes at 6 and 12 months compared to baseline, and no changes were observed in the control group.
Conclusions
Our data show that 1-year weight loss induced by BPD-DS did not influence the overall plasma visfatin and apelin levels in severely obese patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity is a chronic disease of pandemic proportions. The increased prevalence of obesity, initially seen in industrialized countries, has reached the rest of the world and now touches upon developing nations [1, 2]. In Canada, in 2004, the percentage of overweight adults [body mass index (BMI) between 25.0 and 29.9 kg/m2] and obese adults (BMI ≥30.0 kg/m2) was 59.1 and 23.1 %, respectively. It is important to emphasize that the most rapidly expanding group is the severely obese population [3, 4]. In the USA, between 1986 and 2000, people with BMI >30, 40, and 50 kg/m2 have doubled, quadrupled, and quintupled, respectively [5]. Although minimal weight loss can improve the comorbidities associated with obesity [6], only a small proportion of obese individuals are able to maintain long-term weight loss [7]. At the moment, bariatric surgery is the only established treatment that allows for significant and durable long-term weight loss, along with associated remission or sustained improvements in weight-related comorbidities [8–11].
Adipose tissue has long been viewed as mainly a site of energy storage. It is now seen as an endocrine organ [12]. Molecules that are secreted from the adipose tissue (i.e., adipokines) have multiple roles: (1) appetite and energy balance, (2) immunity, (3) insulin sensitivity, (4) angiogenesis, (5) inflammation and acute-phase response, (6) blood pressure, (7) lipid metabolism, and (8) hemostasis [13]. Visfatin is expressed mainly in visceral adipose tissue, has insulin-mimetic effects in cultured cells, and lowers plasma glucose levels in mice [14]. The link between visfatin, obesity, or type 2 diabetes has been reinforced in some studies [15, 16], but not all [17–19]. Visfatin has also been proposed as a pro-inflammatory molecule and an immunomodulator as it has been reported to be independently correlated with C-reactive protein (CRP), interleukin 6 (IL-6) [20], and tumor necrosis factor (TNF-α) [21]. Apelin is an endogenous ligand for the orphan G-protein-coupled receptor, APJ (putative receptor protein related to AT1) [22], and is secreted by adipocytes [23]. Apelin is one of the most potent endogenous positive inotropic substances yet identified [24] and is emerging as a novel mediator of cardiovascular control [25]. Apelin activates nitric oxide synthase, exerts a hypotensive effect, and plays a counter-regulatory role against the pressor action of angiotensin II [26]. Studies have reported a direct link between plasma apelin levels, obesity, and type 2 diabetes [23, 27].
Plasma visfatin and apelin level responses to bariatric surgery are controversial [16, 28–31]. Both adipokines are associated with comorbidities, like type 2 diabetes, hypertension, or chronic state of inflammation as often found in obesity. One may speculate that biliopancreatic diversion with duodenal switch (BPD-DS) surgery-induced weight loss will positively influence these adipokines. Controversial data from previous study in the field of bariatric surgery could have been the results of unmeasured parameters that impact apelin and visfatin regulation. The main objective of this study is to evaluate the acute and chronic (6 and 12 months) changes in serum levels of visfatin and apelin after BPD-DS surgery in severely obese patients. We gathered a comprehensive set of data in order to try to evaluate acutely and chronically the impact of BPD-DS on apelin and visfatin levels. The main hypothesis of this study is that baseline plasma visfatin and apelin levels will be higher in patients with higher BMI and with higher fasting glucose and insulin levels. Considering that both adipokines may be pro-inflammatory mediators, our second hypothesis was that the level of both adipokines will increase shortly after bariatric surgery due to acute stress. Finally, our last hypothesis was in regards to the long-term effects of BPD-DS. We hypothesized that weight loss and improvements/remission in comorbidities will be associated with decreased levels of visfatin and apelin.
Materials and Methods
Subjects and Surgical Procedure
We included 70 randomly selected men and women, 18 years of age or older, with an indication for bariatric surgery (BMI ≥ 40 or ≥ 35 kg/m2 with associated comorbidities). Subjects who had previously undergone bariatric surgery or those bearing a pacemaker were excluded: as per the manufacturer's safety indication, a patient with a pacemaker cannot undergo electrical bioimpedance assessment. Subjects undergoing BPD-DS were recruited through the bariatric surgery clinic at the Institut universitaire de cardiologie et de pneumologie de Québec (IUCPQ). Twenty-eight severely obese control subjects (control group) were randomly selected on the waiting list to match subjects in the BPD-DS group for age and gender. The experimental protocol was approved by the ethics committee of the IUCPQ, and all patients gave their written informed consent. Blood samples were taken before surgery (baseline), at days 1 and 5, at 6 and 12 months after surgery in the BDP-DS group. For the control group, the tests were performed at baseline and at 6 and 12 months.
Anthropometric Measurements and Clinical Variables
Height was measured using a stadiometer (SECA, 216 1814009, Brooklyn, NY, USA). Total body mass, BMI, and lean and fat mass were evaluated with an electrical bioimpedance balance (Tanita TBF-310, Tokyo, Japan) following a 12-h fast. Blood pressure and resting heart rate were measured in a seated position, following a 45-min resting period, while subjects were lying on their side during an echocardiogram study [32]. Blood pressure was measured using an adapted size blood pressure cuff and an electronic sphygmomanometer (Welch-Allyn, 5200 series, Arden, NC, USA). Medical history was collected for diabetes, hypertension, coronary artery disease, and dyslipidemia as well as for pharmacological therapy.
Blood Sampling
Following a 12-h fast, blood was collected into 6-mL tubes containing K2EDTA. Samples were rapidly placed on ice until centrifugation. One tube was sent to the hospital biochemistry laboratory for glycated hemoglobin (HbA1C) analysis, while other samples were centrifuged within 15 min following collection at 3,500 rpm, 4 °C, for 15 min. Plasma samples were collected and frozen in 2-mL aliquots at −80 °C until further analysis.
Plasma Analysis
HbA1c was measured with a turbidimetric inhibition immunoassay, and high sensitive C-reactive protein (hs-CRP) and apolipoprotein B (apoB) levels were measured with an immunoturbidimetric method, all using a Roche Diagnostics Integra 800 System. LDL-cholesterol concentrations were calculated using Friedewald formula [33]. Homeostasis model assessment of insulin resistance was calculated with the following formula: fasting glucose (mmol/L) × fasting insulin (μU/mL)/22.5 [34]. We converted insulin (ρmol/L) to insulin (μU/mL) by dividing the former by 6.945. Visfatin was assayed in duplicate using a commercial enzyme immunoassay (EIA) kit (Phoenix Pharmaceuticals, Inc., Belmont, CA, USA) following the manufacturer's instructions. The sensitivity was 1.85 ng/mL, with a 12 % inter-assay coefficient of variation (CV) and a 5 % intra-assay CV. Apelin-36 was analyzed in duplicate also by an EIA kit, of the same company, following the manufacturer's instructions (Phoenix Pharmaceuticals, Inc., Belmont, CA, USA). The sensitivity of this technique was 0.08 ng/mL and the intra- and inter-assay errors CV% reported by the manufacturer were <5 and <14 %, respectively. The EIA has 100 % cross-reactivity with human apelin-12, -13, and −23.
Statistical Methods
Results are reported as the mean ± standard deviation unless specified otherwise. Comparisons of continuous variables between groups at baseline were performed using unpaired t-test or Mann–Whitney U-test as needed. Measured characteristics were compared between time points using the chi-square statistic for categorical variables and repeated-measures ANOVA for continuous variables. Spearman correlation was used for qualitative data and Pearson correlation for quantitative data. All statistical analyses were performed with statistical software SPSS, version 19.0 (SPSS Inc., Chicago, IL, USA). A P value of 0.05 was considered as statistically significant.
Results
Baseline Characteristics
Seventy severely obese patients were included in the BPD-DS group and were age- and sex-matched with 28 severely obese waiting for bariatric surgery (control group). Baseline anthropometric and biochemical characteristics of both groups are summarized in Table 1. BPD-DS patients were heavier with higher BMI (50.0 ± 7.2 vs. 45.7 ± 8.1 kg/m2; p = 0.012), which was mostly explained by a greater fat mass (70.8 ± 17.9 vs. 60.5 ± 19.3 kg; p = 0.014) rather than fat-free mass (66.3 ± 14.0 vs. 66.2 ± 17.1 kg; p = ns). In the BPD-DS group, there were more patients with a diagnosis of type 2 diabetes (49 vs. 25 %; p = 0.017) and sleep apnea (66 vs. 36 %; p = 0.006).
Characteristics After Bariatric Surgery
Anthropometric measurements are presented in Table 2 at baseline, 6 months, and 1 year. As expected, at 6 months and 1 year after surgery, there was a reduction in body weight and BMI in the BPD-DS group. The percentage of the excess weight loss after BPD-DS was 55.7 ± 14.4 and 75.9 ± 24.0 % at 6 and 12 months, respectively (Table 2). Except for diastolic blood pressure and IL-6, both of which significantly improved between 6 and 12 months postoperatively, all other parameters significantly improved within the first 6 months; most of them also continued to improve beyond 6 months. Baseline values for the control group are shown in Table 1; there were no significant changes in the control group measurements during the study period (data not shown). There was an improvement in diabetes after BPD-DS. At baseline, 49 % of the BPD-DS group were diabetic; this dropped to 25 % at 6 months and 7 % at 12 months, a remission of 49 and 86 %, respectively. Also, there was a remission rate of 51 % in hypertension after 1 year.
Table 3 shows the acute effects of BPD-DS on anthropometric parameters, comorbidities, and biochemical analysis. At 24 h, no anthropometric measures were performed due to the recent surgery, and there were no changes in the medications taken for comorbidities. As shown in Table 3, except for TNF-α, there is an activation of inflammatory processes, which was expressed by a marked increase in CRP and IL-6 levels. At 5 days after surgery, the opposite was observed—TNF-α increases while CRP and IL-6 decreases, both relative to 24 h and to baseline values. In addition, all biochemical measures in our BPD-DS group improved by the fifth postoperative day, except for triglyceride level which returns to initial value after 5 days.
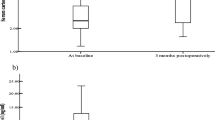
Before BPD-DS surgery, baseline plasma visfatin level in the BPD-DS group was 18.82 ± 7.36 ng/mL (Fig. 1a). Visfatin correlated positively with fat mass (r = 0.247; p = 0.04). Visfatin levels decreased significantly at 24 h after BPD-DS (16.13 ± 5.56 ng/mL; p = 0.003); these 24-h levels were also significantly lower than the concentrations measured at 1 year after surgery (19.00 ± 8.29 ng/mL; p = 0.002). The observed changes were not sex dependent. The control group did not show any changes in visfatin levels (Fig. 1a). Plasma apelin concentrations are shown in Fig. 1b. Plasma apelin levels were significantly lower at day 5 after BPD-DS (p < 0.05). Day 5 concentrations were also significantly lower (p = 0.002) than those measured at 1 year. There was also a difference between the concentrations at 6 months and 1 year (p = 0.001). The changes were not sex dependent. The control group did not show any changes in plasma apelin concentrations over this time period (Fig. 1b).
a Plasma visfatin levels in the BPD-DS group before and at different points after BPD-DS surgery and for the control group at 0, 6, and 12 months. Measures were obtained after an overnight fast. Bars represent mean ± standard error. Means are indicated on the top of each bar. Baseline and 24 h, p = 0.002; single asterisk = between baseline and 24 h, double asterisks = between 24 h and 1 year. b Plasma apelin levels in the BPD-DS group before and at different points after BPD-DS surgery and for the control group at 0, 6, and 12 months. Measures were obtained after an overnight fast. Bars represent mean ± standard error. Means are indicated on the top of each bar. *p < 0.05, **p < 0.05, ***p = 0.001; single asterisk = baseline and 5 days, double asterisks = 5 days and 1 year, triple asterisks = 6 months and 1 year
Correlations Regarding Variation of Both Adipokines over Time
The decrease from baseline in visfatin levels observed at 24 h and from baseline in apelin seen at 5 days post-BPD-DS was not associated with any changes in other parameters over the same time frame. The increase in apelin levels seen between 6 and 12 months was associated positively with changes in fasting insulin (r = 0.255; p = 0.035) and negatively with changes in TNF-α levels (r = −0.284; p = −0.021) during the same time period. Although there was no statistical difference overall between baseline and 1-year plasma visfatin and apelin levels, changes in visfatin levels were negatively associated with both changes in fat-free mass (r = −0.335; p = 0.005) and changes in CRP levels (r = −0.332; p = 0.005) and positively associated with changes in HDL levels (r = 0.284; p = 0.017).
Discussion
The effects of weight loss on plasma visfatin and apelin levels are still debated. At the moment, only a few studies have been designed to explore changes in the levels of these adipokines after weight loss. As with the relation to body weight per se [15, 17, 18, 35], the impact of weight loss on plasma visfatin and apelin levels is controversial. Studies have demonstrated decreases [16, 30, 31], increases [28, 36], or no change [29, 37] with weight loss. Our findings demonstrate that, even though visfatin and apelin are recognized adipokines, there was no relationship with body composition measures at baseline other than a weak correlation between visfatin and fat mass. Also, we did not observe any overall change in visfatin and apelin concentrations following weight loss induced by BDP-DS. Our findings do support an acute modulation of visfatin and apelin levels after bariatric surgery. To our knowledge, no studies have looked at the short-term impact of bariatric surgery on these two adipokines. Considering that both adipokines may be pro-inflammatory mediators, our hypothesis was that the levels of both adipokines might increase shortly after bariatric surgery. Surprisingly, both visfatin and apelin decreased acutely following BPD-DS. This process may be considered as an acute phase reactant. However, this decrease was not associated with any changes in other parameters, including inflammatory markers such as CRP, TNF-α, and IL-6.
Comparing our long-term results with those of other studies is limited due to differences in study design. We identified six studies that evaluated the impact of weight loss on visfatin levels. Two studies [37, 38] used diet and/or exercise. Subjects were less obese than the ones in our study and the follow-up time was shorter (3 months). Two other studies [16, 28] used gastric banding and results were reported at 6 and 7 months after surgery. After banding, patients lost less weight than those in our study after BPD-DS. The last two studies [29, 36] were more comparable to ours: a mixed surgical procedure was used and subjects were followed for at least 12 months. In Garcia-Fuentes et al. [36], visfatin levels increased, while de Luis et al. [29] reported no change. Regarding apelin, only three studies were identified, but only one study evaluated the impact of a surgical procedure [31]. The others evaluated the impact of diet on apelin levels [30, 39]. In Soriguer et al. [31], subjects underwent a mixed surgical procedure and post-surgical follow-up was at 7 months. The authors observed a decrease in apelin levels, but only in severely obese patients with type 2 diabetes.
Patient characteristics and approaches used to induce weight loss might play a role in explaining differences between studies. Also, depending on associated comorbidities, concentrations and variations in plasma adipokine levels could be different. Indeed Haider et al. [16] found that the release of visfatin by adipocytes was dependent on the duration and magnitude of glucose elevation. We did not observe any association between diabetes duration and visfatin levels (data not shown). It is unlikely that differing results are due to methods used to measure visfatin and apelin since all studies referenced here used visfatin and apelin assays similar to ours.
We found correlations only for visfatin levels. Changes in visfatin between baseline and at 1 year were negatively associated with changes in fat-free mass and CRP levels and positively associated with changes in HDL levels. HDL is a good marker of overall metabolic state. After surgery, decrease in body weight is principally explained by decreased fat mass. Unfortunately, there is also a small decrease in fat-free mass. HDL levels also decreased. HDL has anti-inflammatory properties, which could respond to inflammatory molecules like CRP. This might explain why changes in these three parameters are related with changes in visfatin levels, but the clinical relevance of these findings is unknown. Also, little is known about the clearance or the bioavailability of both adipokines as both have a short half-life [14, 40, 41]. Another important point to consider when measuring plasma visfatin or apelin levels is that adipose tissue is not the only organ that secretes these adipokines. Visfatin is also known to be secreted by skeletal muscles, bone marrow, and liver [42], while apelin can be secreted from the gastrointestinal, nervous, and cardiac systems [22, 43]. The exact contribution of each organ is still unknown.
Our study has limitations that need to be discussed. Subjects in the control group were matched using age and gender but not based on a subject's comorbidities. In their preparation for surgery, BPD-DS subjects underwent several pre-operative tests. Consequently, new comorbidities were unmasked following these tests. We used bioimpedance to assess body composition in our patients because dual-energy X-ray absorptiometry was not possible due to equipment limitations. Even though bioimpedance may not be recognized as a fully valid tool, it is often used in clinics and many investigators have used this approach to report their data. Our balance could assess weight up to 600 pounds (270 kg). Nevertheless, we think that our results are relevant to the field of bariatric surgery.
Conclusion
In conclusion, our data show that 1-year weight loss induced by BPD-DS did not influence overall plasma visfatin and apelin levels in severely obese patients. However, we observed an acute decrement (within days) in plasma visfatin and apelin levels days after the surgery. Thus, more studies are needed to determine the impact of bariatric surgery on plasma visfatin and apelin levels and assess the exact roles of these two adipokines in the short- and long-term improvements of comorbidities induced by bariatric surgery.
References
Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser 2000; 894:i-253
Deitel M. Overweight and obesity worldwide now estimated to involve 1.7 billion people. Obes Surg. 2003;13:329–30.
Tjepkema M. Adult obesity. Health Rep. 2006;17:9–25.
Renquist K. Obesity classification. Obes Surg. 1998;8:480.
Sturm R. Increases in clinically severe obesity in the United States, 1986–2000. Arch Intern Med. 2003;163:2146–8.
Goldstein DJ. Beneficial health effects of modest weight loss. Int J Obes Relat Metab Disord. 1992;16:397–415.
Franz MJ, VanWormer JJ, Crain AL, et al. Weight-loss outcomes: a systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. J Am Diet Assoc. 2007;107:1755–67.
Fobi MA. Surgical treatment of obesity: a review. J Natl Med Assoc. 2004;96:61–75.
Marceau P, Biron S, Hould FS, et al. Duodenal switch: long-term results. Obes Surg. 2007;17:1421–30.
Biron S, Hould FS, Lebel S, et al. Twenty years of biliopancreatic diversion: what is the goal of the surgery? Obes Surg. 2004;14:160–4.
Sjostrom L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683–93.
Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–56.
Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr. 2004;92:347–55.
Fukuhara A, Matsuda M, Nishizawa M, et al. Visfatin: a protein secreted by visceral fat that mimics the effects of insulin. Science. 2005;307:426–30.
Berndt J, Kloting N, Kralisch S, et al. Plasma visfatin concentrations and fat depot-specific mRNA expression in humans. Diabetes. 2005;54:2911–6.
Haider DG, Schindler K, Schaller G, et al. Increased plasma visfatin concentrations in morbidly obese subjects are reduced after gastric banding. J Clin Endocrinol Metab. 2006;91:1578–81.
Pagano C, Pilon C, Olivieri M, et al. Reduced plasma visfatin/pre-B cell colony-enhancing factor in obesity is not related to insulin resistance in humans. J Clin Endocrinol Metab. 2006;91:3165–70.
Tan BK, Chen J, Digby JE, et al. Increased visfatin messenger ribonucleic acid and protein levels in adipose tissue and adipocytes in women with polycystic ovary syndrome: parallel increase in plasma visfatin. J Clin Endocrinol Metab. 2006;91:5022–8.
Varma V, Yao-Borengasser A, Rasouli N, et al. Human visfatin expression: relationship to insulin sensitivity, intramyocellular lipids, and inflammation. J Clin Endocrinol Metab. 2007;92:666–72.
Oki K, Yamane K, Kamei N, et al. Circulating visfatin level is correlated with inflammation, but not with insulin resistance. Clin Endocrinol (Oxf). 2007;67:796–800.
Moschen AR, Kaser A, Enrich B, et al. Visfatin, an adipocytokine with proinflammatory and immunomodulating properties. J Immunol. 2007;178:1748–58.
Tatemoto K, Hosoya M, Habata Y, et al. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem Biophys Res Commun. 1998;251:471–6.
Boucher J, Masri B, Daviaud D, et al. Apelin, a newly identified adipokine up-regulated by insulin and obesity. Endocrinology. 2005;146:1764–71.
Szokodi I, Tavi P, Foldes G, et al. Apelin, the novel endogenous ligand of the orphan receptor APJ, regulates cardiac contractility. Circ Res. 2002;91:434–40.
Chen MM, Ashley EA, Deng DX, et al. Novel role for the potent endogenous inotrope apelin in human cardiac dysfunction. Circulation. 2003;108:1432–9.
Ishida J, Hashimoto T, Hashimoto Y, et al. Regulatory roles for APJ, a seven-transmembrane receptor related to angiotensin-type 1 receptor in blood pressure in vivo. J Biol Chem. 2004;279:26274–9.
Heinonen MV, Purhonen AK, Miettinen P, et al. Apelin, orexin-A and leptin plasma levels in morbid obesity and effect of gastric banding. Regul Pept. 2005;130:7–13.
Krzyzanowska K, Mittermayer F, Krugluger W, et al. Increase in visfatin after weight loss induced by gastroplastic surgery. Obesity (Silver Spring). 2006;14:1886–9.
de Luis DA, Izaola O, Conde R, et al. Visfatin levels in female, morbid, nondiabetic obese patients after biliopancreatic diversion surgery. Surg Obes Relat Dis. 2011;7:195–8.
Castan-Laurell I, Vitkova M, Daviaud D, et al. Effect of hypocaloric diet-induced weight loss in obese women on plasma apelin and adipose tissue expression of apelin and APJ. Eur J Endocrinol. 2008;158:905–10.
Soriguer F, Garrido-Sanchez L, Garcia-Serrano S, et al. Apelin levels are increased in morbidly obese subjects with type 2 diabetes mellitus. Obes Surg. 2009;19:1574–80.
Martin J, Bergeron S, Pibarot P, et al. Impact of bariatric surgery on N-terminal fragment of the prohormone brain natriuretic peptide and left ventricular diastolic function. Can J Cardiol 2013. doi:10.1016/j.cjca.2012.11.010.
Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502.
Muniyappa R, Lee S, Chen H, et al. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab. 2008;294:E15–26.
Telejko B, Kuzmicki M, Wawrusiewicz-Kurylonek N, et al. Plasma apelin levels and apelin/APJ mRNA expression in patients with gestational diabetes mellitus. Diabetes Res Clin Pract. 2010;87:176–83.
Garcia-Fuentes E, Garcia-Almeida JM, Garcia-Arnes J, et al. Plasma visfatin concentrations in severely obese subjects are increased after intestinal bypass. Obesity (Silver Spring). 2007;15:2391–5.
Bo S, Ciccone G, Baldi I, et al. Plasma visfatin concentrations after a lifestyle intervention were directly associated with inflammatory markers. Nutr Metab Cardiovasc Dis. 2009;19:423–30.
de Luis DA, Gonzalez SM, Conde R, et al. Effect of a hypocaloric diet on serum visfatin in obese non-diabetic patients. Nutrition. 2008;24:517–21.
Heinonen MV, Laaksonen DE, Karhu T, et al. Effect of diet-induced weight loss on plasma apelin and cytokine levels in individuals with the metabolic syndrome. Nutr Metab Cardiovasc Dis. 2009;19:626–33.
Japp AG, Cruden NL, Amer DA, et al. Vascular effects of apelin in vivo in man. J Am Coll Cardiol. 2008;52:908–13.
Castan-Laurell I, Dray C, Attane C, et al. Apelin, diabetes, and obesity. Endocrine. 2011;40:1–9.
Samal B, Sun Y, Stearns G, et al. Cloning and characterization of the cDNA encoding a novel human pre-B-cell colony-enhancing factor. Mol Cell Biol. 1994;14:1431–7.
Lee DK, Cheng R, Nguyen T, et al. Characterization of apelin, the ligand for the APJ receptor. J Neurochem. 2000;74:34–41.
Acknowledgments
P Poirier holds a FRSQ chercheur-boursier clinicien senior. K Cianflone holds a Canada Research Chair in adipose tissue. SM Caron-Cantin was supported by the Fonds d'enseignement et de recherche of University of Laval, Faculty of Pharmacy.
Disclosure
The authors declare that there is no competing financial interest in relation to the present work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Caron-Cantin, SM., Martin, J., Bastien, M. et al. Acute and Chronic Effects of Biliopancreatic Diversion with Duodenal Switch Surgery on Plasma Visfatin and Apelin Levels in Patients with Severe Obesity. OBES SURG 23, 1806–1814 (2013). https://doi.org/10.1007/s11695-013-0952-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-013-0952-9