Abstract
Background
The physiological role of apelin in obesity and diabetes remains unclear. Although apelin has been studied in persons with different conditions, no studies have yet examined the joint influence of obesity and diabetes on apelin levels. We measured the changes in apelin levels in morbidly obese subjects, with and without diabetes, and in the inverse situation of improvement in carbohydrate metabolism as a result of bariatric surgery.
Methods
The study was undertaken in 54 morbidly obese persons, 16 of whom had type 2 diabetes mellitus, before and 7 months after undergoing bariatric surgery, and in 12 healthy, nonobese persons. Measurements were made of apelin levels and insulin sensitivity by an intravenous glucose tolerance test.
Results
The apelin levels in the morbidly obese patients prior to surgery were significantly higher than those of the controls only when the morbidly obese subjects were diabetic (P < 0.005). Apelin levels correlated significantly in the morbidly obese patients with serum triglycerides (r = 0.292, P = 0.032) and glucose (r = 0.337, P = 0.039). Bariatric surgery resulted in a significant decrease in apelin levels only in the morbidly obese subjects with impaired fasting glucose or diabetes. The change in apelin levels correlated significantly in the morbidly obese patients with the changes in serum glucose (r = 0.338, P = 0.038) and insulin sensitivity (r = −0.417, P = 0.043).
Conclusions
This study demonstrates that obesity is not the main determinant of the rise in apelin levels. The association between apelin levels and glucose concentrations and insulin sensitivity provides evidence that apelin may play a role in the pathogenesis of diabetes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The number of overweight and obese subjects is taking on alarming proportions worldwide. Obesity is a multifactorial disease that involves an alteration of energy balance. Different studies have shown the important role of adipose-tissue-derived hormones in the complications associated with obesity. A certain number of adipose-tissue-derived hormones, such as leptin, adiponectin, resistin, and visfatin, are involved in carbohydrate metabolism control [1]. The adipose tissue has recently been reported to synthesize a new peptide, apelin. This peptide, first isolated from bovine stomach [2], is derived from a 77-amino-acid precursor, which is processed to several active molecular forms, such as apelin-36 or apelin-13 and apelin-12 in different tissues [3]. In humans, apelin has been shown to be widely expressed [4]. This protein is an endogenous ligand of APJ, a G-protein-coupled receptor [2, 5].
The role of apelin is currently unknown. The receptor and the ligand are colocalized in many tissues. Apelin may have different effects, depending on the tissue in question [6]. Apelin appears to exert its effect on the cardiovascular system [7–9] and the nervous system [10], among others. Apelin also increases drinking behavior and reduces food intake in model experiments in rodents [11]. Recently, apelin expression has been reported to be regulated by insulin [12], and it has been suggested to be involved in regulation of glucose homeostasis and in obesity [12–15]. However, there are certain contradictory findings. While some studies have found apelin to be increased in diabetic persons [15], another study showed its serum levels to be reduced in newly diagnosed type 2 diabetes mellitus as compared with a group of control subjects [16]. Apelin has also been associated with inhibition of glucose-stimulated insulin secretion in mice [14].
One of the main treatments for morbid obesity and its accompanying disorders, such as diabetes, is bariatric surgery. Although calorie restriction is the main reason for improvement, it is nevertheless not sufficient to explain all the resulting changes. The regulation of energy balance is a complex phenomenon involving numerous factors [17]. The changes undergone by different hormones can have repercussions on these processes. Very few studies have examined the evolution of apelin over time in different disorders [13, 18]. In fact, as far as we are aware, no study has yet been undertaken in morbidly obese persons undergoing bariatric surgery with restrictive malabsorption techniques. Following surgery, an important change is produced in the gastrointestinal physiology that can affect plasma levels of apelin and its effects. Although the association between apelin and obesity and diabetes is now beginning to be studied, the physiological role of apelin is still unknown. Thus, the aim of this study was to investigate the levels of apelin in morbidly obese persons with and without type 2 diabetes mellitus, as well as the changes in apelin levels after the improvement in carbohydrate metabolism as a result of bariatric surgery.
Materials and Methods
Subjects
Measurements were taken in 54 morbidly obese persons (19 men and 35 women) before and 7 months after bariatric surgery and in 12 healthy, nonobese persons with normal fasting glucose (four men and eight women) as controls. The morbidly obese persons were classified into three groups, according to their fasting glucose levels prior to bariatric surgery: morbidly obese with normal fasting glucose (MO-NFG; glucose <5.6 mmol/l; n = 15), morbidly obese with impaired fasting glucose (MO-IFG; glucose ≥5.6 and <7.0 mmol/l; n = 23), and morbidly obese with diabetes mellitus (MO-DM; glucose ≥7.0 mmol/l; n = 16). None of the morbidly obese persons with type 2 diabetes mellitus were receiving insulin therapy. Each patient was weighed, their height measured, and their body mass index (BMI) calculated (kg/m2). All the patients underwent bariatric surgery with mixed techniques, combining gastric reduction with an intestinal bypass: open biliopancreatic diversion of Scopinaro (n = 39) or laparoscopic Roux-en-Y gastric bypass (n = 15) [19]. All participants gave their informed consent, and the study was reviewed and approved by the Ethics and Research Committee.
Intravenous Glucose Tolerance Test
An intravenous glucose tolerance test (IVGTT) [20] was performed in the healthy persons and in the morbidly obese persons prior to surgery and 7 months after bariatric surgery. The study protocol commenced at 08:30 a.m. after a 10–12 h fast. Baseline blood samples were obtained at 15, 10, and 5 min before glucose administration. At point 0, glucose was administered (50% dextrose; 11.4-g/m2 body surface area) in less than 1 min. Blood samples were taken after 2, 3, 4, 5, 6, 8, 10, 12, 14, 16, 19, 22, 25, 30, 40, 50, 60, 70, 80, 100, 120, 140, 160, and 180 min for the measurement of concentrations of glucose and insulin. The serum was separated, aliquoted within 30 min of extraction, and immediately frozen at −80°C. The insulin sensitivity (SI) and the acute insulin response (AIRG) were calculated after introduction of the results for glucose and insulin obtained during the IVGTT into the MINMOD program [21].
Laboratory Measurements
Blood samples were collected after a 12-h fast. All the samples were analyzed simultaneously by a common laboratory. Serum biochemical parameters were measured in duplicate. Serum glucose, cholesterol, high-density lipoprotein cholesterol, triglycerides (RANDOX Laboratories, Antrim, UK), and free fatty acids (WAKO Chemicals, Richmond, VI) were determined by standard enzymatic methods. Low-density lipoprotein cholesterol was calculated by the Friedewald formula. The insulin was analyzed by an immunoradiometric assay (BioSource International, Camarillo, CA, USA), showing a 0.3% cross-reaction with proinsulin. The intra-assay and interassay coefficients of variation (CV) were 1.9% and 6.3%, respectively. Apelin-12 was analyzed by enzyme immunoassay (enzyme-linked immunosorbent assay, ELISA) kits (Phoenix Pharmaceutical, Inc. Belmont, CA, USA). The sensitivity of the technique was 0.04 ng/ml and the intra-assay and interassay CV were <5% and <14%, respectively. Leptin was analyzed by enzyme immunoassay (ELISA) kits (DSL, Webster, TX, USA). The sensitivity of the technique was 0.05 ng/ml and the intra-assay and interassay CV were 3.8% and 4.4%, respectively. Adiponectin was analyzed by enzyme immunoassay (ELISA) kits (DRG Diagnostics GmbH, Germany). The intra-assay and interassay CV were 3.4% and 5.7%, respectively.
Statistical Analysis
The results are given as the mean ± SD. Logarithmic adjustments are made when the variables are not normally distributed. Comparison between the results of the different groups was made with the Student t test or with the one-way analysis of variance, and the post hoc analysis was done with Duncan’s multiple-range test. The differences in the variables within the same group before and after bariatric surgery were compared with the Student t test for paired samples. The Pearson correlation coefficient was calculated to estimate the linear correlations between variables. Values were considered to be statistically significant when P ≤ 0.05. The statistical analysis was done with SPSS (version 11.5 for Windows; SPSS, Chicago, IL, USA).
Results
Clinical Characteristics of the Study Group
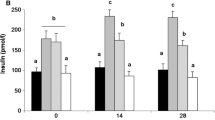
Table 1 summarizes the anthropometric and biochemical characteristics of the control subjects and the morbidly obese subjects, before and after bariatric surgery. The group of morbidly obese patients was extremely obese, with a mean presurgery BMI of 54.0 ± 6.8 kg/m2. This study was carried out at the same time at two different centers, and although the assignment of the morbidly obese patients to one or other of the techniques was not random, no significant differences were found prior to bariatric surgery for the different study variables according to the type of surgery later undergone (biliopancreatic diversion or Roux-en-Y gastric bypass; data not shown). When the morbidly obese patients were grouped according to their serum glucose concentrations, significant differences were only found in the serum glucose (Fig. 1a), triglycerides (Fig. 1b), SI (Fig. 1c), and AIRG.
Serum glucose (a), triglycerides (b), insulin sensitivity (SI; c), and apelin (d) in control subjects (n = 12), morbidly obese patients with NFG (n = 15), IFG (n = 23), or type 2 diabetes mellitus (n = 16), before (black bars) and after (white bars) bariatric surgery, according to their presurgery fasting glucose levels. Data are presented as means ± SD. Different letters indicate significant differences between the means of the different groups of preoperative morbidly obese patients and the controls (P < 0.05). *P < 0.05; † P < 0.01; ‡ P < 0.001: significant differences within the same group of morbidly obese subjects before and after bariatric surgery
Serum apelin concentrations
The apelin levels in the morbidly obese patients prior to surgery were significantly higher than those of the controls (1.68 ± 1.25 vs. 1.12 ± 0.51 ng/ml, P = 0.017). However, this was solely at the expense of the morbidly obese patients who were diabetic (Fig. 1d). No significant differences were noted according to whether the morbidly obese patients were or were not being treated with oral antidiabetic agents (OAA; MO-DM with OAA 1.62 ± 1.31 ng/ml, MO-DM no OAA 1.99 ± 1.63 ng/ml, P = 0.626). No significant differences were noted according to the sex of the patient or the hospital involved (data not shown).
Baseline serum apelin correlated significantly in all subjects with triglycerides, glucose, and insulin (Table 2). In the group of morbidly obese patients, apelin correlated significantly with serum triglycerides (r = 0.292, P = 0.032) and glucose (r = 0.337, P = 0.039).
Bariatric surgery resulted in a significant decrease in apelin levels (1.68 ± 1.25 vs. 0.88 ± 0.48 ng/ml, P = 0.003) in the morbidly obese patients. Again, this was due to the significant reduction in the morbidly obese patients with DM and IFG (Fig. 1d). No significant differences were found in apelin levels according to the type of bariatric surgery undergone (data not shown).
After surgery, apelin did not correlate significantly in the morbidly obese patients with any of the variables studied. However, the change in its levels (∆apelin) correlated significantly in the morbidly obese patients with the changes in serum glucose (∆glucose) and SI (ΔSI; Table 3).
Discussion
The main results of this study are that (a) serum apelin levels were only significantly increased in morbidly obese patients with type 2 diabetes mellitus, and (b) bariatric surgery only led to a significant reduction in apelin levels in those who had IFG or type 2 diabetes mellitus before surgery. The change in serum apelin levels after surgery was greater as the change in serum glucose levels increased.
Studies over recent years have shown that more and more adipose-tissue-derived hormones are involved in the regulation of the fat store, metabolism, and obesity-associated disorders, including type 2 diabetes mellitus [1, 15, 17]. Adipose tissue has also recently been shown to secrete an adipokine called apelin, which seems to be involved in obesity and carbohydrate metabolism [22–24]. However, very few studies have examined apelin in humans. Our results, like those of others [12, 13, 24], show that serum apelin levels in obese persons are significantly raised and may be involved in the pathophysiology of obesity. Even so, while some studies found a positive correlation of circulating apelin with BMI [15, 24], others (including ours) failed to detect this association [16, 25, 26].
To date, the studies on apelin in obesity have been done in persons with hyperinsulinemia and glucose levels within normal ranges [12, 13] or with IFG [24]. Although these studies showed the increased levels of apelin [12, 13, 24], studies in experimental models of obesity have shown that apelin is significantly elevated only in those states associated with hyperinsulinemia [12]. Our group of morbidly obese persons had significantly higher levels of insulin, glucose, and triglycerides than the control subjects. Only the glucose and the triglycerides, though, correlated positively with the apelin levels. Clarification of the role played by hyperinsulinemia and obesity in apelin regulation is difficult as obesity is often accompanied by hyperinsulinemia. Nevertheless, this study shows that serum apelin concentrations are significantly raised in morbidly obese persons with type 2 diabetes mellitus, even though all the morbidly obese patients (NFG, IFG, and DM) had raised serum insulin levels and no significant differences between groups. Our results could suggest that obesity is not the determining factor of circulating levels of apelin, as occurs with other adipokines [1].
The data reported here are in agreement with studies undertaken in nonobese persons with varying degrees of carbohydrate metabolism disorders [15]. Serum apelin levels were significantly increased in patients with type 2 diabetes mellitus compared with NFG or control subjects. Others have found reduced apelin levels in newly diagnosed and untreated patients with type 2 diabetes mellitus [16]. We, however, found no significant differences in apelin levels between the morbidly obese patients with type 2 diabetes mellitus depending on whether they were or were not taking OAA, though this may be due to the low number of patients who were taking OAA.
Our results suggest an association between apelin and carbohydrate metabolism. As with other adipokines [1], we are unable to determine from this study whether there is first an increase in apelin levels followed by hyperinsulinemia and/or hyperglycemia or whether the hyperinsulinemia and/or hyperglycemia is responsible for the greater apelin levels. Boucher et al. found upregulation of apelin in adipocytes by insulin [12]. Other studies have shown that apelin-36 inhibits glucose-stimulated insulin secretion both in vivo and in vitro in mice [14]. Apelin also inhibited the insulin response to intravenous glucose in high-fat-fed mice [14]. The authors suggested that the inhibition of glucose-stimulated insulin secretion by apelin would fit activation of the sympathetic nervous system. In our case, no association was detected between plasma levels of apelin and insulin secretion after an intravenous glucose overload (AIRG).
In this study, we show clearly that, following bariatric surgery, the levels of apelin are decreased, especially and significantly so in morbidly obese patients with IFG or DM, whereas no significant change was found in the group of morbidly obese patients with NFG. These findings suggest that weight loss is not the sole reason for the reduction in apelin levels. If weight loss was associated with apelin reduction, the reduction would have been seen in all three groups of morbidly obese patients, not just the groups with IFG or DM. We are only aware of one study examining the effect of weight loss on apelin levels [13]. In that study, a hypocaloric diet associated with weight reduction resulted in a reduction of plasma apelin levels in obese individuals, approaching the range found in lean controls. The study concluded that weight loss and reduction in insulin resistance promote a reduction in the high levels of apelin found in obesity.
Our results clearly show that the changes in serum apelin concentrations following bariatric surgery are correlated with the changes in serum glucose and insulin sensitivity. As we saw, those groups of morbidly obese patients who experienced a greater reduction in glucose and a greater increase in insulin sensitivity were those that also experienced a greater drop in apelin levels. Furthermore, the original differences between the various morbidly obese groups disappeared after bariatric surgery. These results confirm that the improvement in glucose metabolism plays an important role in the reduction in apelin levels. Given that the regulation of energy balance is a complex phenomenon involving numerous factors, we cannot rule out the possibility that the change undergone by different gastrointestinal hormones after bariatric surgery can have repercussions on the apelin levels. Unfortunately, we have taken samples only once after the operation. It would be of interest to observe the effect of the operation per se, before any significant weight loss had been achieved.
In conclusion, the present study demonstrates that obesity is not the main determinant of the rise in apelin levels. Apelin levels were significantly increased in morbidly obese subjects with type 2 diabetes mellitus but not those with NFG. The improvement seen in glucose levels and insulin sensitivity resulting from bariatric surgery was associated with a reduction in apelin levels. However, the effects of improved glycemic control by measures other than significant weight reduction should be explored. These data provide evidence that apelin may play a role in the pathogenesis of insulin resistance and type 2 diabetes mellitus. More studies are necessary to clarify the role of apelin in obesity and diabetes. A better definition of the link between apelin and the pathogenesis of insulin resistance is therefore warranted.
References
García-Fuentes E, García-Almeida JM, García-Arnés J, et al. Plasma visfatin concentrations in severely obese subjects are increased after intestinal bypass. Obesity (Silver Spring). 2007;15:2391–5.
Tatemoto K, Hosoya M, Habata Y, et al. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem Biophys Res Commun. 1998;251:471–6.
Masri B, Knibiehler B, Audigier Y. Apelin signalling: a promising pathway from cloning to pharmacology. Cell Signal. 2005;17:415–26.
De Falco M, De Luca L, Onori N, et al. Apelin expression in normal human tissues. In Vivo. 2002;16:333–6.
Falcao-Pires I, Leite-Moreira AF. Apelin: a novel neurohumoral modulator of the cardiovascular system. Pathophysiologic importance and potential use as a therapeutic target. Rev Port Cardiol. 2005;24:1263–76.
Carpéné C, Dray C, Attané C, et al. Expanding role for the apelin/APJ system in physiopathology. J Physiol Biochem. 2007;63:359–73.
Tatemoto K, Takayama K, Zou MX, et al. The novel peptide apelin lowers blood pressure via a nitric oxide-dependent mechanism. Regul Pept. 2001;99:87–92.
Cheng X, Cheng XS, Pang CC. Venous dilator effect of apelin, an endogenous peptide ligand for the orphan APJ receptor, in conscious rats. Eur J Pharmacol. 2003;470:171–5.
Kleinz MJ, Skepper JN, Davenport AP. Immunocytochemical localisation of the apelin receptor, APJ, to human cardiomyocytes, vascular smooth muscle and endothelial cells. Regul Pept. 2005;126:233–40.
Lee DK, Cheng R, Nguyen T, et al. Characterization of apelin, the ligand for the APJ receptor. J Neurochem. 2000;74:34–41.
Sunter D, Hewson AK, Dickson SL. Intracerebroventricular injection of apelin-13 reduces food intake in the rat. Neurosci Lett. 2003;353:1–4.
Boucher J, Masri B, Daviaud D, et al. Apelin, a newly identified adipokine up-regulated by insulin and obesity. Endocrinology. 2005;146:1764–71.
Castan-Laurell I, Vítkova M, Daviaud D, et al. Effect of hypocaloric diet-induced weight loss in obese women on plasma apelin and adipose tissue expression of apelin and APJ. Eur J Endocrinol. 2008;158:905–10.
Sorhede Winzell M, Magnusson C, Ahren B. The apj receptor is expressed in pancreatic islets and its ligand, apelin, inhibits insulin secretion in mice. Regul Pept. 2005;131:12–7.
Li L, Yang G, Li Q, et al. Changes and relations of circulating visfatin, apelin, and resistin levels in normal, impaired glucose tolerance, and type 2 diabetic subjects. Exp Clin Endocrinol Diabetes. 2006;114:544–8.
Erdem G, Dogru T, Tasci I, et al. Low plasma apelin levels in newly diagnosed type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes. 2008;116:289–92.
Fetner R, McGinty J, Russell C, et al. Incretins, diabetes, and bariatric surgery: a review. Surg Obes Relat Dis. 2005;1:589–97.
Tasci I, Erdem G, Ozgur G, et al. LDL-cholesterol lowering increases plasma apelin in isolated hypercholesterolemia. Atherosclerosis. 2008;204:222–8.
Garcia-Fuentes E, Garrido-Sanchez L, Garcia-Almeida JM, et al. Different effect of laparoscopic Roux-en-Y gastric bypass and open biliopancreatic diversion of Scopinaro on serum PYY and ghrelin levels. Obes Surg. 2008;18:1424–9.
García-Fuentes E, García-Almeida JM, García-Arnés J, et al. Morbidly obese individuals with impaired fasting glucose have a specific pattern of insulin secretion and sensitivity: effect of weight loss after bariatric surgery. Obes Surg. 2006;16:1179–88.
Bergman RN. Lilly lecture. Toward physiological understanding of glucose tolerance. Minimal-model approach. Diabetes. 1989;38:1512–27.
Wei L, Hou X, Tatemoto K. Regulation of apelin mRNA expression by insulin and glucocorticoids in mouse 3 T3-L1 adipocytes. Regul Pept. 2005;132:27–32.
Rayalam S, Della-Fera MA, Krieg PA, et al. A putative role for apelin in the etiology of obesity. Biochem Biophys Res Commun. 2008;368:815–9.
Heinonen MV, Purhonen AK, Miettinen P, et al. Apelin, orexin-A and leptin plasma levels in morbid obesity and effect of gastric banding. Regul Pept. 2005;130:7–13.
Tasci I, Dogru T, Naharci I, et al. Plasma apelin is lower in patients with elevated LDL-cholesterol. Exp Clin Endocrinol Diabetes. 2007;115:428–32.
Chong KS, Gardner RS, Morton JJ, et al. Plasma concentrations of the novel peptide apelin are decreased in patients with chronic heart failure. Eur J Heart Fail. 2006;8:355–60.
Acknowledgments
This work was supported in part by a grant from the Instituto de Salud Carlos III (CP04/00133) and Servicio Andaluz de Salud (0438/2006, 0255/2007). CIBER Fisiopatología de la Obesidad y Nutrición (CB06/03) and CIBER de Diabetes y Enfermedades Metabólicas Asociadas (CIBERDEM) are ISCIII projects. We also thank Ian Johnstone for help with the English language version of the text.
Conflict of interest
The authors declare that there was no conflict of interest associated with this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Soriguer, F., Garrido-Sanchez, L., Garcia-Serrano, S. et al. Apelin Levels Are Increased in Morbidly Obese Subjects with Type 2 Diabetes Mellitus. OBES SURG 19, 1574–1580 (2009). https://doi.org/10.1007/s11695-009-9955-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-009-9955-y