Abstract
Background
Restrictive bariatric surgery procedures currently used include adjustable gastric banding, sleeve gastrectomy (SG), and gastric plication (GP), of which the last two techniques still lack sufficient data and long-term studies on weight loss, surgical complications, resolution of comorbidities, and mechanisms of weight loss. Therefore, gastric plication and sleeve gastrectomy as a standalone procedure are still considered experimental. Our aim was to analyze the effects of SG and GP on body weight, food intake, and endocrine profile.
Methods
Forty-four male Wistar rats were randomized into six weight-matched groups and submitted either to SG, GP, or sham-operated. Sham-operated rats were divided into pair-fed and fed ad libitum controls, one for each procedure. Animals were followed up for 21 days after surgery, while body weight and food intake were recorded daily, when fasting ghrelin, leptin, insulin and glucose plasma levels, and ghrelin expression in the stomach were measured.
Results
Rats submitted to SG and GP showed a significant decrease in body weight gain to the same extent as rats pair-fed to the surgical groups when compared to sham-operated fed ad libitum controls. After surgery, SG rats showed no difference in body composition, ghrelin, leptin, insulin, or glucose levels, while GP rats displayed lower body fat content and leptin levels compared to controls. Ghrelin was also lower in GP rats compared to sham-operated pair-fed rats. Ghrelin expression displayed a pattern similar to circulating ghrelin.
Conclusions
SG and GP result in weight loss, although with differences in body composition and metabolic and endocrine profiles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The obesity epidemic has grown in severity over the past several decades and is now a worldwide public health priority [1–3]. Bariatric surgical procedures are currently the most effective approach to achieve long-term weight loss treating individuals who have clinically severe obesity [4, 5] and are at the highest risk for obesity-related mortality and comorbidity [6]. Besides weight loss, bariatric surgery also provides the possibility of resolving or improving several comorbidities associated with obesity, such as type 2 diabetes [7]. The increasing demand for effective bariatric procedures that are associated with lower complication rates has led to the development of new surgical techniques.
Currently, restrictive bariatric surgery procedures include adjustable gastric banding, sleeve gastrectomy (SG), and gastric plication (GP) or imbrication. Adjustable gastric banding provides an excess body weight loss of 46.2 %, and the remission rate of diabetes after gastric banding is 56.7 % in overall studies that include patients with less than 2 and more than 2 years of follow-up [7]. Despite its widespread use, adjustable gastric banding involves surgical reoperations in 25 % of patients, either by secondary failure with weight regain, or due to complication inherent to the placement of a long duration prosthetic device, such as migration or slippage of the gastric band, as well as the risks of obstruction, erosion, and herniation [8].
SG provides reduction of gastric volume through the resection of the stomach along the greater gastric curvature and construction of a tubular gastric pouch [9]. The effectiveness of this procedure, which is associated with a variable excess body weight loss ranging from 33 to 90 % that appeared to be sustained up to 3 years [9], has been attributed to the restriction of the stomach capacity as well as to the decrease of ghrelin levels, a gastric hormone that stimulates appetite [10]. However, the creation of a long stapled line during SG can lead to complications, such as leaks and bleeding, and the irreversibility of this operation has been a detraction for some surgeons and patients [11]. SG is thought to have an improved safety profile as compared to the Roux-en-Y gastric bypass but has nevertheless demonstrated comparable efficacy in inducing the resolution of type 2 diabetes [12, 13].
Complication rates and morbidity associated with gastric banding and SG led to the search of new restrictive surgical procedures, namely GP or imbrication. GP can be performed by two different techniques, by invagination of the greater gastric curvature or by invagination of the anterior gastric wall [11]. GP of the greater curvature has been the most widely used procedure [11, 14]. The surgery resembles to SG without gastric resection, as it creates a gastric tube and eliminates the greater curvature; the reported excess body weight loss 1 year after GP was between 53.4 and 69.6 %, in small series studies [11, 14]. In the largest series of patients submitted to GP, nausea and vomiting were the most common complications; the reoperation rate was 2.6 % due to gastric perforation secondary to tear of the suture line or thermal injury of the stomach [15].
Because SG as a standalone procedure and GP are recently described techniques, there is still lack of sufficient studies on weight loss and on resolution of associated complications and comorbidities. Therefore, animal models of these bariatric procedures may provide valuable insight into the endocrine and metabolic mechanisms associated with body weight reduction after SG and GP. Although data arising from animal models may not be suitable for direct extrapolation to humans, they offer the advantage of postmortem analysis and also the investigation of factors that are impossible to be evaluated in patients due to ethical reasons. Our aim was to analyze the effect of the bariatric surgical models of SG and GP in the Wistar rat with regards to body weight, food intake, and endocrine and metabolic profiles, by measuring fasting ghrelin, leptin, insulin, and glucose plasma levels and ghrelin expression in the stomach.
Material and Methods
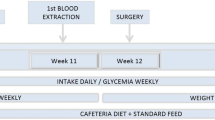
Forty-four male Wistar rats, purchased from a local breeder (Charles River, Barcelona, Spain), were maintained in individual cages under controlled temperature (21–23 °C), humidity, and light (12 h light, 12 h dark, lights on at 7.00 hours) with free access to standard rat chow (4RF21, Mucedola, MI, Italy) and tap water.
Animals were acclimatized to the local facilities for 7 days before surgery, and only healthy growing animals were used in the experiments. Wistar rats were randomized into weight-matched groups to be submitted either to SG (n = 10; 420.1 ± 50.6 g), GP (n = 10; 340.5 ± 2.31 g), sham-operated pair-fed to the amount eaten by rats with SG (n = 6; 428.2 ± 46.8 g), sham-operated pair-fed to the amount eaten by rats with GP (n = 6; 350.2 ± 17.3 g), and sham-operated fed ad libitum that were used as controls (n = 6/surgical group; 422.8 ± 43.0 and 346.3 ± 14.5 g for SG and GP, respectively). The reason for including two separate groups of sham-operated fed ad libitum rats as controls was derived from the fact that it was technically impossible for the same surgeon to perform all surgeries on the same day, and since the animals were a few days older and there is always some genetic variability in growth and weight gain, this has been compensated by using a control group for each and animals were randomized according to body weight in each procedure group. Therefore to increase the statistical strength of the study, all rats in each study group, SG and GP, were submitted to surgery on the same day and then later sacrificed also on the same day. The daily ration in the pair-fed animals was given as a single portion before dark. As rats normally start eating at the beginning of the dark phase and eat most of their food during that period, the animals were fed just before dark to comply with their natural habits. Pair-fed animals were food-restricted, but only to the same extent as rats submitted to the restrictive surgeries, in order to allow the dissociation between the specific effects of surgery from the ones derived from food restriction per se. In spite that pair-fed were always food-restricted and given the same amount of food as eaten by the surgical groups, actual food intake was measured and displayed in the graphs. In the instances when the animals consumed less food than provided, it was left in the hopper for the following days. All procedures were approved by the local Ethics Board for Animal Research and followed the European Union laws on animal protection (86/609/EC).
Surgical Procedures
After an overnight 12 h fast, rats were sedated with a subcutaneous injection of acepromazine (2 mg/kg), butorfanol (2.5 mg/kg), and diazepam (2 mg/kg). Anesthesia was initiated with isoflurane 3 % and then reduced to isoflurane 1.5 % administered through a mask for rodents. The animals were kept in spontaneous ventilation.
Prophylactic antibiotherapy consisting of cefazolin (100 mg/kg) was administered intraperitoneally immediately before surgery, and all surgical procedures were performed under sterile conditions. In order to perform the SG, after a 1.5-cm upper midline incision was made, the stomach was externalized and the gastroesplenic ligament was divided using electrocautery; a vascular clamp was placed along the greater curvature from the antrum to the fundus across the stomach calibrated by a nasogastric tube of the same size for all the animals and scissors were used to divide the greater curvature along the clamp, removing approximately 90 % of the forestomach and 80 % of the glandular stomach. The divided stomach was then closed with 5-0 adsorbable polyglyconate suture (Maxon, Surgical US, USA) in two layers in a continuous fashion creating the gastric sleeve (Fig. 1a). To perform the GP, after performing a 1.5-cm upper midline incision, the stomach was externalized and the gastroesplenic ligament was divided using electrocautery; GP was created by imbrication of the greater gastric curvature over a nasogastric tube applying a first row of extramucosal interrupted stitches of 4-0 nonadsorbable polyester suture (Ethibond, Ethicon, NJ, USA), and this row guided two subsequent rows created with running suture lines of 4-0 nonadsorbable polypropylene suture (Prolene, Ethicon) (Fig. 1b). For the sham-operated groups, after performing a 1.5-cm upper midline incision, the stomach was externalized, manipulated, and then returned to the abdomen, which was then closed using 4-0 adsorbable coated polyglactin (Vicryl, Ethicon) followed by 4-0 adsorbable coated polyglactin (Vicryl Rapid, Ethicon) for the skin.
All animals were given 5 ml sterile warmed saline subcutaneously to avoid dehydration and allowed to recover spontaneously from anesthesia and surgery. Rats were returned to their home cages and, on the first postoperative day, were restricted to water-only diet and placed on regular solid rat chow afterwards until the end of the experiment.
Feeding Studies Protocols
Body weight was measured daily at 9.00 hours using a scale (Monobloc, Metterr, Toledo, USA) recording to the nearest 1 g and the remaining food in the hopper was reweighed at the same time using a scale (Kern, KB 5000-1) recording to the nearest 0.1 g, which allowed daily food intake to be calculated. All animals had ad libitum access to standard rat chow, except for the pair-fed groups which were fed daily with the same amount eaten by animals submitted to SG or GP.
Hormone Measurements
Twenty-one days after the surgeries, 12-h fasted rats were deeply anesthetized with CO2 and the whole blood was collected by cardiac puncture into chilled lithium heparin tubes containing a protease inhibitor (0.02 ml/10 ml; Trasylol, Bayer, Portugal). The tubes were kept on ice and centrifuged at 3,000 rpm for 8 min at 4 °C. Plasma was separated and stored at −20 °C until the assays were performed. Plasma levels of total ghrelin (EZRGRT91, Linco Research, St. Charles, MO, USA), leptin (EZRL-83 K, Linco Research), and insulin (EZRMI-13 K, Linco Research) were determined by ELISA using specific commercial kits according to the manufacturer’s instructions. Blood glucose levels were analyzed by the glucose oxidase method using a glucometer (One Touch Ultra, Lifescan, Johnson and Johnson, Milipitas, CA, USA).
RNA Extraction and Real-Time PCR
Total RNA was isolated from stomach fundus using RNeasy Mini Kit (Qiagen, Germany) according to the manufacturer’s instructions and 500 ng of RNA was retrotranscribed into cDNA using High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA). The RNA expression of ghrelin in the stomach was studied by using TaqMan real-time PCR in Step One Plus system (Applied Biosystems) using specific primers and probes obtained from inventoried TaqMan Gene Expression Assays (Applied Biosystems). All reactions were carried out using the following cycling parameters: 50 °C for 2 min, 95 °C for 10 min followed by 40 cycles of 95 °C for 15 s, 60 °C for 1 min. For the analysis of the data, the RNA level of the gene of interest was normalized using β-actin for values according to the 2 − ΔΔCt method.
Epididymal White Adipose Tissue Weight
After sacrifice, epididymal white adipose tissue pads were removed and weighed using a scale recording to the nearest 0.001 g (Kern 440, Version 3.2).
Statistical Analysis
Results are shown as means ± SEM, unless otherwise specified. One-way analysis of variance ANOVA was used for comparison of the means between the groups with post hoc Bonferroni correction when appropriate. p < 0.05 was considered to be statistically significant.
Results
Sleeve Gastrectomy
Rats submitted to SG displayed a significant decrease in body weight gain when compared to sham-operated fed ad libitum controls, while there was no difference in body weight when compared to pair-fed rats (−46.5 ± 7.69 g SG, −51.17 ± 2.40 g PF-SG, p = NS) (Fig. 2a). After SG, rats displayed a significant decrease in cumulative food intake (229.73 ± 24.44 g SG, 396.58 ± 11.65 g controls, p < 0.001) (Table 1) and daily until 14 days after surgery (12.45 ± 2.26 g SG, 20.85 ± 1.13 controls, p < 0.05) (Fig. 2b). There was no difference in relative body fat content, assessed by the percentage of epididymal white adipose tissue between the three groups of rats (2.22 ± 0.29 SG, 2.47 ± 0.25 PF-SG, 2.40 ± 0.32 controls, p = NS). Rats submitted to SG showed no differences in fasting glucose, total ghrelin, leptin, or insulin plasma levels when compared to sham-operated pair-fed or sham-operated fed ad libitum controls (Table 1). After SG, there was no significant difference in ghrelin expression in the gastric fundus between the three experimental groups of rats (0.32 ± 0.17 SG, 0.66 ± 0.13 PF-SG, 1.00 controls, p = NS) (Table 1).
Graphs showing body weight gain of rats submitted to SG and sham operation pair-fed and fed ad libitum. There was a significant decrease in body weight gain of rats submitted to SG and rats pair-fed when compared to sham-operated fed ad libitum controls (***p < 0.001) (a). There was a significant decrease in daily food intake of rats submitted to SG and rats pair-fed when compared to sham-operated and fed ad libitum controls for the first 14 days after surgery (***p < 0.001) (b)
Gastric Plicature
Rats submitted to GP and sham-operated pair-fed rats displayed a significant decrease in body weight gain when compared to sham-operated rats fed ad libitum controls (−40 ± 15.36 g GP, 22.83 ± 4.64 g controls, p < 0.01) (Fig. 3). Rats submitted to GP also displayed a significant decrease in cumulative food intake when compared to sham-operated fed ad libitum controls (416.72 ± 41.63 g GP, 541.95 ± 12.48 g controls, p < 0.01) (Table 2) and daily food intake until 14 days after surgery (19.81 ± 2.61 g GP, 28.93 ± 1.55 controls, p < 0.05) (Fig. 3b). Body fat content, assessed by the percentage of epididymal fat of rats submitted to GP, was also significantly lower when compared to sham-operated fed ad libitum controls (1.38 ± 0.25 GP, 1.87 ± 0.12 PF-GP, controls, p < 0.05).
Graphs showing body weight gain of rats submitted to GP and sham operation pair-fed and fed ad libitum. There was a significant decrease in body weight gain of rats submitted to GP and sham-operated pair-fed when compared to fed ad libitum controls (**p < 0.01) (a). There was a significant decrease in daily food intake of rats submitted to GP and sham-operated when compared to fed ad libitum controls for the first 14 days after surgery (**p < 0.01) (b)
Total ghrelin plasma levels of rats submitted to GP were significantly lower when compared to sham-operated pair fed rats and similar to the levels displayed by sham-operated rats fed ad libitum (p < 0.01) (Table 2). Also, leptin plasma levels of rats submitted to GP were significantly lower than those presented by sham-operated rats pair-fed and sham-operated fed ad libitum controls (p < 0.05 and p < 0.01, respectively) (Table 2). There were no differences in fasting glucose or insulin plasma levels between the three groups of rats (Table 2). After GP, there was no significant difference in ghrelin expression in the gastric fundus between the three experimental groups of rats (1.35 ± 0.76 GP, 4.10 ± 1.51 PF-SG, 1.00 controls, p = NS) (Table 2).
The mortality rate of rats submitted to SG was 10 % (n = 1), of rats submitted to GP 60 % (n = 6), and for sham-operated rats 0 %. Mortality in the group of animals subjected to SG and GP occurred during anesthesia induction and within the first hours after surgery, respectively. The mortality has been attributed to respiratory arrest as a result of anesthesia complications, since the procedure for GP lasted significantly longer than the procedure for SG or sham surgeries (43.5 ± 1.93 min for GP vs 30.4 ± 2.09 min for SG vs 11.1 ± 0.64 for sham operation, p < 0.001), and at the autopsy, there were no signs of hemorrhage or intra-abdominal sepsis. No late complications were reported for any of the animals in this study. These rats have been excluded from the statistical analysis.
Discussion
SG is a restrictive bariatric procedure that was initially proposed as a first step operation to be followed by biliopancreatic diversion in high-risk super obese patients (BMI > 60 kg/m2) [16]. More recently, it has also been indicated to be effective as a single procedure in patients with lower BMI [16, 17]. The mechanisms of weight loss from SG and its effectiveness as a standalone procedure in improving comorbidities have not yet been extensively characterized, and thus, it is still considered experimental. To help overcome this lack of information, animal models of SG may be useful to define the metabolic and endocrine changes taking place after the surgical procedure.
Our current investigation used the Wistar rat to reproduce the SG and GP procedures that are used in the human setting, as previously described [18, 19], in order to investigate the metabolic and endocrine changes attending weight loss induced by surgery. Sham-operated pair-fed rats and rats fed ad libitum where used as controls; the comparison of SG with sham-operated rats allowed the evaluation of the effects due to surgical trauma. The analysis of the data of the pair-fed group reflected the impact of the decrease in food intake.
Similar to previous reports, rats submitted to SG showed a significant decrease in body weight gain when compared with sham-operated control animals, and this difference was similar to food-restricted rats (pair-fed), suggesting that the weight loss was due to food restriction since there were no differences between SG rats and pair-fed rats [18, 20–24]. Rats submitted to SG showed a significant decrease in the cumulative food intake for the study time span and of daily food intake for the first 14 days after surgery, followed by a gradual increase in food intake until reaching baseline food intake. This could be explained by the loss of the restrictive capacity due to the restrictive enlargement or pouch dilatation, as other possible explanations such as vomiting as observed in humans do not occur in rats since the species is devoid of vomiting reflex. To support this hypothesis, a calibration nasogastric tube has been used for holding the sleeve and a gastric dilation of the tube has been found post mortem in all animals. Ghrelin is a gastrointestinal peptide hormone, produced mainly in the stomach that stimulates appetite [25]. Plasma ghrelin levels correlate inversely with body mass index; thus, ghrelin is reduced in most obese individuals when compared to normal body weight controls [26]. Ghrelin replacement has been shown to reverse the reduction in weight gain and body fat in gastrectomized mice [27]. SG has been shown to decrease fasting ghrelin levels, both in rodents and humans [10, 28–30], since this surgery involves resection of the majority of the gastric fundus which is the main location of ghrelin production [10, 31]. However, variable changes in plasma ghrelin levels were reported following SG procedures [32]. Ghrelin levels have been shown to be decreased only in the first six postoperative months after SG and not to increase significantly thereafter [10], and also to be unchanged 14 days after SG in Zucker rats, suggesting that the effects in weight loss after SG might be independent of changes in ghrelin levels [33]. The type of surgical technique, whether or not involving sectioning of the vagus nerve, may also contribute to changes in ghrelin secretion after SG [34]. In the present investigation, similar to previous studies [33], ghrelin levels were not significantly different 21 days after surgery, a period of time that equals 2 years in human lifespan [35]. This may be explained by the putative compensatory increase in ghrelin secretion by the remainder gastric tissue or by extra gastric ghrelin sources, as ghrelin expression displayed a similar pattern as found for circulating ghrelin [25]. In addition, the fact that ghrelin levels were determined in a single time point precludes the knowledge of the kinetics of ghrelin secretion over the study time span. Also, it is pertinent to take into account that the anatomy of the rat and human stomach is different; therefore, the deployment of the esophagus at the lesser curvature compels the surgeon when creating the gastric tube to leave part of the fundus intact, leaving in place part of the organ responsible for ghrelin production. Rats submitted to SG did not show any difference in body fat composition or leptin levels, opposite to what was previously described [36].
GP is a restrictive bariatric surgical procedure developed to overcome the inconvenience of gastric partitioning or the use of prosthetic devices with their risks of obstruction, erosion, and herniation. After GP, rats displayed a significant decrease in body weight gain when compared with the sham-operated control group, similar to what was previously reported by other research groups [19, 37]. This decrease was even more marked in the group of rats subjected to GP when compared with the group of controls fed ad libitum. Relative body fat content, evaluated by the epididymal fat weight, was also lower in rats submitted to GP when compared with controls. After the GP and despite the weight loss, total plasma ghrelin levels and ghrelin expression in the stomach were not significantly different from controls, suggesting that weight loss induced by GP is not a stimulus to ghrelin secretion as is usually observed after food restriction only. Because ghrelin levels were not increased, as what occurred in pair-fed rats, it is plausible to consider that the lack of contact of food with gastric mucosa might be responsible for preventing the rise in ghrelin eventually due to over-ride inhibition. These data support the hypothesis that weight loss observed after GP might not depend solely on food restriction.
In the present study, no significant differences were found with regards to values of fasting glucose and insulin levels in both SG and GP surgical groups. However, one should bear in mind that the surgery was performed in healthy rats carrying no glucose metabolic disturbance.
Conclusion
Reduction in gastric capacity can be achieved using SG and GP, and this reduction is accompanied by a decrease in food intake and body weight gain, but the two surgical approaches lead to differences in body composition and metabolic and endocrine profiles of the surgically treated rats.
References
Ashrafian H, Bueter M, Ahmed K, et al. Metabolic surgery: an evolution through bariatric animal models. Obes Rev. 2010;11(12):907–20.
Prentice AM. The emerging epidemic of obesity in developing countries. Int J Epidemiol. 2006;35(1):93–9.
Puska P, Nishida C, Porter D. World Health Organization, Obesity and overweight. Available at http://www.who.int/dietphysicalactivity/publications/facts/obesity/en/print.html. Acessed 3 July 2009 2006.
Brolin RE. Bariatric surgery and long-term control of morbid obesity. JAMA. 2002;288(22):2793–6.
Mun EC, Blackburn GL, Matthews JB. Current status of medical and surgical therapy for obesity. Gastroenterology. 2001;120(3):669–81.
Buchwald H. The future of bariatric surgery. Obes Surg. 2005;15(5):598–605.
Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122(3):248–56. e5.
Chevallier JM, Zinzindohoué F, Douard R, et al. Complications after laparoscopic adjustable gastric banding for morbid obesity: experience with 1,000 patients over 7 years. Obes Surg. 2004;14(3):407–14.
Shi X, Karmali S, Sharma AM, et al. A review of laparoscopic sleeve gastrectomy for morbid obesity. Obes Surg. 2010;20(8):1171–7.
Langer FB, Reza Hoda MA, Bohdjalian A, et al. Sleeve gastrectomy and gastric banding: effects on plasma ghrelin levels. Obes Surg. 2005;15(7):1024–9.
Brethauer SA, Harris JL, Kroh M, et al. Laparoscopic gastric plication for treatment of severe obesity. Surg Obes Relat Dis. 2011;7(1):15–22.
Jacobs M, Bisland W, Gomez E, et al. Laparoscopic sleeve gastrectomy: a retrospective review of 1- and 2-year results. Surg Endosc. 2010;24(4):781–5.
Rosenthal R, Li X, Samuel S, et al. Effect of sleeve gastrectomy on patients with diabetes mellitus. Surg Obes Relat Dis. 2009;5(4):429–34.
Ramos A, Galvao Neto M, Galvao M, et al. Laparoscopic greater curvature plication: initial results of an alternative restrictive bariatric procedure. Obes Surg. 2010;20(7):913–8.
Talebpour M, Amoli BS. Laparoscopic total gastric vertical plication in morbid obesity. J Laparoendosc Adv Surg Tech A. 2007;17(6):793–8.
Basso N, Casella G, Rizzello M, et al. Laparoscopic sleeve gastrectomy as first stage or definitive intent in 300 consecutive cases. Surg Endosc. 2011;25(2):444–9.
Cottam D, Qureshi FG, Mattar SG, et al. Laparoscopic sleeve gastrectomy as an initial weight-loss procedure for high-risk patients with morbid obesity. Surg Endosc. 2006;20(6):859–63.
de Bona CJ, Bettiol J, d'Acampora AJ, et al. Sleeve gastrectomy model in Wistar rats. Obes Surg. 2007;17(7):957–61.
Fusco PE, Poggetti RS, Younes RN, et al. Evaluation of gastric greater curvature invagination for weight loss in rats. Obes Surg. 2006;16(2):172–7.
Patrikakos P, Toutouzas KG, Perrea D, et al. A surgical rat model of sleeve gastrectomy with staple technique: long-term weight loss results. Obes Surg. 2009;19(11):1586–90.
Valenti V, Martin M, Ramirez B, et al. Sleeve gastrectomy induces weight loss in diet-induced obese rats even if high-fat feeding is continued. Obes Surg. 2011;21(9):1438.
Kodama Y, Zhao CM, Kulseng B, et al. Eating behavior in rats subjected to vagotomy, sleeve gastrectomy, and duodenal switch. J Gastrointest Surg. 2010;14(10):1502–10.
Wang Y, Liu J. Sleeve gastrectomy relieves steatohepatitis in high-fat-diet-induced obese rats. Obes Surg. 2009;19(7):921–5.
Schlager A, Khalaileh A, Mintz Y, et al. A mouse model for sleeve gastrectomy: applications for diabetes research. Microsurgery. 2011;31(1):66–71.
Kojima M, Kangawa K. Ghrelin: structure and function. Physiol Rev. 2005;85(2):495–522.
Tschop M, Weyer C, Tataranni PA, et al. Circulating ghrelin levels are decreased in human obesity. Diabetes. 2001;50(4):707–9.
Dornonville de la Cour C, Lindqvist A, Egecioglu E, et al. Ghrelin treatment reverses the reduction in weight gain and body fat in gastrectomised mice. Gut. 2005;54(7):907–13.
Wang Y, Liu J. Plasma ghrelin modulation in gastric band operation and sleeve gastrectomy. Obes Surg. 2009;19(3):357–62.
Li F, Zhang G, Liang J, et al. Sleeve gastrectomy provides a better control of diabetes by decreasing ghrelin in the diabetic Goto-Kakizaki rats. J Gastrointest Surg. 2009;13(12):2302–8.
Wang Y, Yan L, Jin Z, et al. Effects of sleeve gastrectomy in neonatally streptozotocin-induced diabetic rats. PLoS One. 2011;6(1):e16383.
Cohen R, Uzzan B, Bihan H, et al. Ghrelin levels and sleeve gastrectomy in super-super-obesity. Obes Surg. 2005;15(10):1501–2.
Pereferrer FS, Gonzàlez MH, Rovira AF, et al. Influence of sleeve gastrectomy on several experimental models of obesity: metabolic and hormonal implications. Obes Surg. 2008;18(1):97–108.
Lopez PP, Nicholson SE, Burkhardt GE, et al. Development of a sleeve gastrectomy weight loss model in obese Zucker rats. J Surg Res. 2009;157(2):243–50.
Williams DL, Grill HJ, Cummings DE, et al. Vagotomy dissociates short- and long-term controls of circulating ghrelin. Endocrinology. 2003;144(12):5184–7.
Quinn R. Comparing rat's to human's age: how old is my rat in people years? Nutrition. 2005;21(6):775–7.
Stefater MA, Pérez-Tilve D, Chambers AP, et al. Sleeve gastrectomy induces loss of weight and fat mass in obese rats, but does not affect leptin sensitivity. Gastroenterology. 2010;138(7):2426–36. 2436 e1-3.
Fusco PE, Poggetti RS, Younes RN, et al. Comparison of anterior gastric wall and greater gastric curvature invaginations for weight loss in rats. Obes Surg. 2007;17(10):1340–5.
Acknowledgments
The authors thank Joana Matos and Patrícia Carvalho, DVM, CCEA for support with animal work. UMIB is funded by grants from FCT (POCTI/FEDER), Portugal. M.C.C. is funded by the Xunta de Galicia through a research-staff contract “Angeles Alvarinño.”
Conflict of Interest
All contributing authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guimarães, M., Nora, M., Ferreira, T. et al. Sleeve Gastrectomy and Gastric Plication in the Rat Result in Weight Loss with Different Endocrine Profiles. OBES SURG 23, 710–717 (2013). https://doi.org/10.1007/s11695-013-0886-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-013-0886-2