Abstract
Backround
Diabetes surgery in nonobese or moderately obese patients is an emerging topic. The identification of preoperative factors predicting diabetes outcome following bariatric surgery, especially for metabolic nonresponders, is imperative.
Methods
Between 2005 and 2011, 235 patients underwent bariatric surgery for morbid obesity. Eighty-two of 235 patients had type 2 diabetes mellitus (T2DM). Data from this subgroup were investigated with univariate and multivariate analyses to identify predictors for metabolic nonresponse after surgery.
Results
Diabetes did not improve in 17/82 patients within 3 months after surgery. No correlation between excess body weight loss and metabolic response was detected. In univariate analysis, preoperative duration of diabetes was significantly longer in the nonresponder group (9.146 vs. 6.270 years; *p = 0.016), preoperative HbA1c levels were significantly higher among the nonresponders than among the responders (8.341 vs. 7.781 %; *p = 0.033), and more patients in the nonresponder group were reliant on a multi-drug approach preoperatively (*p = 0.045). In multivariate analysis, age, preoperative doses of insulin, and preoperative oral antidiabetics showed positive correlation to metabolic nonresponse after surgery (*p = 0.04; *p = 0.021; *p = 0.021). Metabolic failure rate was lower after Roux-en-Y gastric bypass compared to other bariatric procedures (**p = 0.008).
Conclusions
A long history of preoperative T2DM, high preoperative HbA1c levels, and a preoperative therapy consisting of diverse approaches to diabetes treatment may be factors predicting failure of diabetes improvement in the early postoperative course after bariatric surgery. Age, preoperative insulin, and oral antidiabetic medication can be regarded as independent, significant predictors for metabolic outcome after bariatric surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bariatric surgery and especially Roux-en-Y gastric bypass (RYGB) have been shown to have an impressive impact on metabolic response and diabetes control: almost immediately postoperatively and usually long before a relevant weight loss [1]. These effects have led to the assumption that a diabetic condition is “curable by metabolic surgery” [2]. There is evidence [3] that long-term remission of type 2 diabetes mellitus (T2DM) after RYGB can be expected in about 84 % of patients. However, there is still an ongoing discussion on how these positive effects should be defined as several studies used different definitions of diabetes “improvement,” “resolution,” “remission,” or “cure.” The problem of metabolic nonresponse is important, since 15–20 % of diabetic obese patients undergoing bariatric surgery do not show diabetes remission in the long term [4].
Due to the remarkable improvement of glycemic control in obese patients after bariatric surgery, the question was raised if bariatric/metabolic surgery is also applicable to moderately obese and nonobese patients with metabolic disorders [4]. Data from pilot studies focusing on metabolic outcome rather than weight loss effectiveness are promising [4] and randomized controlled trails have already been conducted to gain more evidence on diabetes control in patients with a body mass index (BMI) between 30 and 35 kg/m² [4]. Therefore, the identification of independent predictors on diabetes outcome after bariatric/metabolic surgery is increasingly important, especially for moderately obese and nonobese patients.
Even when surgery has no metabolic effect, obese patients might benefit from a clinically relevant weight loss. Patients hoping to improve their metabolic situation need to know about metabolic nonresponse after surgery, since these patients otherwise undergo surgical risks without achieving the desired health benefits.
In order to identify nonresponders, we analyzed the data with regards to metabolic and weight loss status preoperatively and within the first 3 months after bariatric surgery. Furthermore, we performed univariate and multivariate analyses in order to detect and isolate factors predicting metabolic outcome.
Patients and Methods
Patient Population
Between 2005 and 2011, all patients (n = 235) who underwent bariatric surgery were documented in a prospective database. Bariatric procedures were either sleeve gastrectomy (SG), gastric bypass (RYGB), or laparoscopic adjustable gastric banding (LAGB). Patients were diagnosed with T2DM based on WHO criteria: fasting glucose levels >126 mg/dl or a high pathological 2-h value in the oral glucose tolerance test (>200 mg/dl) [5].
Definitions
Diabetes Improvement Failures
According to the criteria for a possible success of a nonoperative, purely medication-based antidiabetic treatment [6], diabetes improvement failures were defined as:
-
(a)
Nonresponse (metabolic failure): postoperative insulin dose ≥1/4 of preoperative dose; example: preoperative—90IE insulin/day–postoperative—85IE = no diabetes remission (improvement failure)
-
(b)
Nonresponse (metabolic failure): postoperative dose of medication ≥1/2 of preoperative dose; example: preoperative—2,000 mg metformin/day–postoperative—1,500 mg metformin/day = no diabetes remission (improvement failure)
-
(c)
No effective reduction of HbA1c postoperative (less than 0.5 % HbA1c reduction within 3 months or not reaching HbA1c <6.5 %)
Definitions were made prior to reporting process.
Successful Weight Loss
Excess body weight is defined as preoperative weight−ideal weight (based on a BMI of 25 kg/m2). As there is no clear-cut definition for “successful weight loss after 3 months,” limits were calculated as the mean value of excess body weight loss (EBWL) after 3 months minus 1 standard deviation (SD). “Successful weight loss after 3 months” was therefore defined as an EBWL of 0.23 after sleeve gastrectomy, 0.26 after gastric bypass, and 0.09 after laparoscopic adjustable gastric banding.
Example:
-
Mean excess body weight loss for RYGB patients at 3 months postoperatively—0.389 (±0.133 SD)
-
Patient no. 82: preoperative excess weight 108–94 kg (3 months postoperative excess weight) = EBWL 0.13 (<0.26 = weight loss failure)
Definitions were made prior to reporting process.
Statistical Analysis
Statistical analysis was done using univariate analysis of the following: duration of preoperative diabetes, evolution of HbA1c levels, preoperative antidiabetic therapy (insulin and oral antidiabetic medication), weight loss failure (as defined above), preoperative BMI, age, and type of surgery. Furthermore, a multiple regression analysis was performed regarding age, BMI, operative procedure, diabetes duration, HbA1c, and antidiabetic treatment. All data were expressed as means ± SD and 95 % confidence interval (CI). Comparisons between groups were performed using chi-squared and parametric or nonparametric tests (Student’s t test, Mann–Whitney U, Kendall and Spearman rank correlation, Kruskal–Wallis one-way analysis of variance) when appropriate. Statistical significance was set at a p value < 0.05. Statistics were performed using the Statistical Package for Social Sciences (SPSS™), version 19 (IBM SPSS Inc, Chicago, IL, USA).
Results
Patient Data
The study population comprised 235 morbidly obese patients who underwent SG (n = 68, 28.9 %), RYGB (n = 126, 53.6 %), or LAGB (n = 41, 17.5 %) from 2005 to 2011 at the Center for Bariatric and Metabolic Surgery, University Hospital of Würzburg. Among the 235 patients, 80 (34 %) were males and 155 (66 %) were females; mean age was 44.1 ± 11.0 years and mean BMI 50.98 ± 8.28 kg/m2.
In 217 (92.3 %) of these cases, the operation was carried out laparoscopically. Conversion of a laparoscopic procedure into open surgery was necessary in 7 (4.7 %) cases; 11 (3 %) patients underwent primary open surgery. T2DM was diagnosed in 82 (34.9 %) of the 235 patients preoperatively. The diagnosis was based on WHO criteria mentioned above [5].
Using the criteria for “diabetes improvement failure,” 17 of these 82 patients (20.7 %) were defined as initial metabolic nonresponders 3 months after surgery (see Table 1).
Univariate Analysis
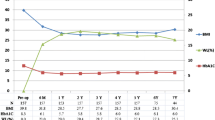
Metabolic nonresponders showed a mean preoperative diabetes mellitus duration of 9.1 years in contrast to 6.3 years in the group of patients with metabolic response (*p = 0.016). Preoperative HbA1c levels were 8.3 % in the nonresponder group and 7.8 % in the responder group (*p = 0.033). No data concerning preoperative duration of T2DM or preoperative HbA1c level were available for six and two patients, respectively. The univariate analysis identified preoperative diabetes duration and preoperative HbA1c levels as significant prognostic parameters for metabolic nonresponse (Table 2).
Eleven of 17 patients showing no metabolic improvement postoperatively and 26/65 responders were dependent on insulin therapy preoperatively (p = 0.068). Nine of 11 insulin-dependent nonresponders and 17/26 insulin-dependent responders received insulin within the scope of a multi-approach therapy with antidiabetic medication (*p = 0.045) (Table 3).
Insufficient weight loss according to the definitions mentioned above was seen in 28 of the 235 morbidly obese patients (12.07 %). There was no follow-up for three patients. Within the group of the 82 diabetic patients, there were only three patients showing both weight loss failure and metabolic nonresponse (3.61 %; p = 0.57). Therefore, there was no correlation between insufficient weight loss and metabolic failure (Table 4).
Mean age of all nonresponding patients was 52.8 years with a mean preop BMI of 51.8 kg/m2. The mean age of metabolic responders was 48.5 years with a mean preop BMI of 50.9 kg/m2. Therefore, no relationship between a patient’s age or preop BMI and metabolic failure could be observed (Tables 5 and 6).
Within the group of the 17 nonresponders, 8 patients underwent SG, 7 patients RYGB, and 2 patients LAGB. Within the group of the 65 responders, 17 patients underwent SG, 42 patients RYGB, and 6 patients LAGB. There was no significant difference between the type of surgery and metabolic response (p = 0.20, Mehta and Patel’s exact chi-square test) 3 months after the bariatric procedure (Table 7).
Multiple Regression Analysis
Multiple regression analysis (Table 8) identified four independent, significant predictors for postoperative metabolic failure. Age, preoperative insulin, and preoperative oral antidiabetics showed significant correlation with postoperative initial metabolic nonresponse (*p = 0.04; *p = 0.021; *p = 0.021). RYGB as an operative procedure was significantly correlated with less metabolic failures and was, therefore, an independent predictor for positive outcome in the first 3 months after metabolic surgery (**p = 0.008).
Discussion
According to the latest International Diabetes Federation statement, “the global prevalence of type 2 diabetes is rising dramatically, driven by an ‘obesogenic’ environment” [7]. Currently, about 366 million people worldwide are diagnosed with type 2 diabetes mellitus. Obesity is the primary risk factor [16], since 85 % of diabetic individuals are overweight or obese. For patients with a BMI of 35+ kg/m2 and type 2 diabetes, bariatric surgery is accepted as an appropriate treatment if recommended treatment targets cannot be achieved with medical therapies and other obesity-related comorbidities are present.
In western populations, approximately 15 % of the people with T2DM are not overweight [8]. The role of surgery in diabetes treatment algorithms, especially for these patients, needs to be defined. The aim of obesity surgery is defined as substantial weight loss and control of associated comorbidities as far as possible. The success of metabolic surgery in nonobese or moderately obese diabetic individuals depends mainly on postoperative glycemic control. Patients not showing the expected improvement of T2DM undergo surgical risks without any benefits. Today, even moderately obese patients (e.g., with BMI < 35) are treated with bariatric (metabolic) procedures [9–13]. A recent meta-analysis by Li et al. includes 13 trials involving 357 T2DM patients with a BMI < 35 kg/m2; 80.0 % of the patients achieved adequate glycemic control (HbA1c < 7 %) without antidiabetic medication [14]. Although this is encouraging, it means that approximately 20 % of patients undergoing bariatric/metabolic surgery are at surgical risk without clinically relevant health benefits. This rate of treatment failure would be unacceptable for other surgical therapies, e.g., antireflux surgery. A careful patient selection is, therefore, mandatory. Following these considerations, the identification of preoperative factors accounting for metabolic failure postoperatively is of utmost importance. In our study, 20.7 % of the treated T2DM patients did not show the expected metabolic response in the early postoperative course.
With no clear-cut definition of either “nonresponse” or “diabetes improvement failure” 3 months after surgery, a definition consisting of special limits and values had to be established. A successful modern multi-approach drug therapy for diabetes mellitus can result in a decrease in HbA1c levels of −0.6 % after 12 weeks [6]. This study defined “early metabolic nonresponse” following bariatric surgery as the reduction of less than 0.5 % of the initial preoperative HbA1c level along with unsuccessful reductions of insulin or oral antidiabetic medication. These definitions and limits were established in consensus with experienced diabetologists according to their medical results in long-standing clinical practice.
An additional artificial definition of adequate EBWL (3 months) was defined to correlate metabolic response with weight loss 3 months postoperatively. Weight loss failure according to the definitions mentioned above was seen in 12.07 % of the patients. We were unable to detect a correlation between inadequate weight loss and metabolic failure (see Table 4).
According to the results of the present study, a long history of preoperative T2DM, high preoperative HbA1c levels, and a preoperative therapy consisting of diverse approaches to diabetes treatment (insulin + oral antidiabetic medication) might be the factors predicting failure of diabetes improvement in the early postoperative course after bariatric surgery, as far as the results of univariate analysis are concerned. While there was no relationship between age, BMI, type of surgery, and metabolic nonresponse in univariate analysis, multiple regression analysis revealed age, preoperative insulin, oral AD, and RYGB to be significant predictors for metabolic outcome in the short term. Concerning the type of surgery, these different results might be due to the small number of cases in each operation category. Therefore, the impact of the type of surgery on metabolic response cannot sufficiently be answered by the present analysis. The fact that the duration of T2DM was not a significant predictor in the multiple regression analysis might be due to the significant predictor age that correlates strongly with the duration of T2DM.
Hall et al. [15] examined 110 T2DM patients undergoing RYGB. Patients with a baseline HbA1c > 10 % had a 50 % rate of remission compared to 77.3 % with an HbA1c of 6.5–7.9 %. A preoperative history of T2DM longer than 10 years was shown to significantly reduce the chances of remission (p = 0.005). Their study also supported findings that a shorter duration and a better control of diabetes preoperatively correspond to a higher rate of remission—a possible argument for early surgical intervention in the morbidly obese diabetic patient. In contrast to the present study, the period under consideration was 6, 12, and 24 months postoperatively. Schauer et al. confirmed that the degree of severity of preoperative T2DM might be a predictor of postoperative outcome after metabolic surgery [16]. This observation is confirmed by our own data (Table 2). In the present study, preoperative oral AD and insulin were significantly correlated with metabolic outcome (negative predictors). Interestingly, oral AD medication seemed to be the strongest factor (odds ratio 9.0888) identified by multiple regression analysis.
In conjunction with the results of the univariate analysis, the severity of preoperative T2DM seems to play an important role in postoperative outcome. Again, mean duration of follow-up was 19.7 months (range 6–54 months). Huang et al. [17] examined 22 patients with a BMI of 25–35 and T2DM concerning remission rates at 12 months. Fourteen out of 22 (63.6 %) patients showed T2DM remission, 6 (27. 3 %) showed glycemic control, and 2 (9.1 %) showed improvement of their initial diabetic metabolic status. The remission group had a higher BMI (p = 0.001), younger age (p = 0.002), and shorter history of diabetes (p = 0.001). Though a direct comparison is not possible according to different patient populations, definitions of outcome, and time points of examination, this partially confirms the results of the present study: age was a significant factor for metabolic nonresponse (Table 7, β = 1.600, OR = 4.9515, *p = 0.040). T2DM duration seems to be significantly correlated with metabolic outcome at 3 months in univariate analysis. No correlations were observed for preoperative BMI. Concerning age at the time of surgery, Hamza et al. came to the same conclusions. In their study, a multivariate analysis of 74 T2DM patients among 487 patients undergoing bariatric procedures revealed a younger age to be an independent predictor of postoperative remission of T2DM. This is also in line with the findings of the present study. In contrast, they also found a greater % EBWL (excessive body weight loss) to predict postoperative diabetes remission [18].
Many studies suggest that the choice of surgical procedure is an important determinant of metabolic outcome [19]. RYGB might lead to greater diabetes remission rates than LAGB [20], whereas recent studies suggest a similar positive impact on diabetes control with sleeve gastrectomy and gastric bypass [21, 22]. In the present study, 3 months after surgery, no significant relationship between type of surgery and metabolic nonresponse could be observed in univariate analysis, whereas multivariate analysis revealed RYGB to be an independent predictor for metabolic outcome at 3 months (Table 8). The difference between the results of univariate and multiple regression analyses is, indeed, surprising. RYGB seems to have an impact only if other factors are considered simultaneously during multiple regression analysis. This might be due to small category numbers.
Limitations
The data presented in this study are short-term results. Therefore, the comparability to the available literature representing long-term results is limited. However, our data are congruent with recent findings [20]. Additionally, long-term follow-up of patients identified as “initial metabolic nonresponders” is both interesting and mandatory in order to display the correlation of initial metabolic failure and long-term metabolic outcome.
Our data are collected in a morbidly obese cohort. Whether these finding are applicable to nonobese patients must be further investigated. Due to lack of data, our own investigations did not reflect on preoperative C peptide levels. C peptide levels would have been interesting and useful, since several studies have defined higher preoperative C peptide levels (C peptide > 3 ng/ml) to be important predictors of diabetes resolution, helping to define the best candidates for surgical treatment of diabetes [23].
Conclusion
Following bariatric/metabolic surgery, a minority of patients did not show the expected metabolic health benefits. There is no correlation between inadequate weight loss and metabolic nonresponse. Age and a long history of preoperative diabetes mellitus, high preoperative HbA1c levels, and a preoperative multi-drug diabetes medication can be assumed to be negative predictors for metabolic (diabetes) failure in the early postoperative course.
References
Rubino F, Gagner M. Potential of surgery for curing type 2 diabetes mellitus. Ann Surg. 2002;236(5):554–9.
Rubino F. Is type 2 diabetes an operable intestinal disease? A provocative yet reasonable hypothesis. Diabetes Care. 2008;31 Suppl 2:290–6.
Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292(14):1724–37.
Rubino F, Kaplan LM, Schauer PR, et al. The Diabetes Surgery Summit consensus conference: recommendations for the evaluation and use of gastrointestinal surgery to treat type 2 diabetes mellitus. Ann Surg. 2010;251(3):399–405.
Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15(7):539–53.
Rosenstock J, Banarer S, Fonseca VA, et al. The 11-beta-hydroxysteroid dehydrogenase type 1 inhibitor INCB13739 improves hyperglycemia in patients with type 2 diabetes inadequately controlled by metformin monotherapy. Diabetes Care. 2010;33(7):1516–22.
Dixon JB, Zimmet P, Alberti KG, et al. Bariatric surgery: an IDF statement for obese Type 2 diabetes(1). Diabet Med. 2011;28(6):628–42.
Gregg EW, Cheng YJ, Narayan KM, et al. The relative contributions of different levels of overweight and obesity to the increased prevalence of diabetes in the United States: 1976–2004. Prev Med. 2007;45(5):348–52.
Cohen R, Pinheiro JS, Correa JL, et al. Laparoscopic Roux-en-Y gastric bypass for BMI < 35 kg/m2: a tailored approach. Surg Obes Relat Dis. 2006;2(3):401–4. discussion 04.
Lee WJ, Wang W, Lee YC, et al. Effect of laparoscopic mini-gastric bypass for type 2 diabetes mellitus: comparison of BMI > 35 and <35 kg/m2. J Gastrointest Surg. 2008;12(5):945–52.
Scopinaro N, Adami GF, Papadia FS, et al. Effects of biliopanceratic diversion on type 2 diabetes in patients with BMI 25 to 35. Ann Surg. 2011;253(4):699–703.
de Paula AL, Macedo AL, Prudente AS, et al. Laparoscopic sleeve gastrectomy with ileal interposition (“neuroendocrine brake”)—pilot study of a new operation. Surg Obes Relat Dis. 2006;2(4):464–7.
Gianos M, Abdemur A, Fendrich I, et al. Outcomes of bariatric surgery in patients with body mass index <35 kg/m2. Surg Obes Relat Dis. 2011;8(1):25–30.
Li Q, Chen L, Yang Z, et al. Metabolic effects of bariatric surgery in type 2 diabetic patients with BMI <35 kg/m2. Diabetes Obes Metab. 2011;14(3):262–70.
Hall TC, Pellen MG, Sedman PC, et al. Preoperative factors predicting remission of type 2 diabetes mellitus after Roux-en-Y gastric bypass surgery for obesity. Obes Surg. 2010;20(9):1245–50.
Schauer PR, Burguera B, Ikramuddin S, et al. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg. 2003;238(4):467–84. discussion 84-5.
Huang CK, Shabbir A, Lo CH, et al. Laparoscopic Roux-en-Y gastric bypass for the treatment of type II diabetes mellitus in Chinese patients with body mass index of 25–35. Obes Surg. 2011;21(9):1344–9.
Hamza N, Abbas MH, Darwish A, et al. Predictors of remission of type 2 diabetes mellitus after laparoscopic gastric banding and bypass. Surg Obes Relat Dis. 2011;7(6):691–6.
Shukla AP, Ahn SM, Patel RT, et al. Surgical treatment of type 2 diabetes: the surgeon perspective. Endocrine. 2011;40(2):151–61.
Rubino F, Schauer PR, Kaplan LM, et al. Metabolic surgery to treat type 2 diabetes: clinical outcomes and mechanisms of action. Annu Rev Med. 2010;61:393–411.
Benaiges D, Goday A, Ramon JM, et al. Laparoscopic sleeve gastrectomy and laparoscopic gastric bypass are equally effective for reduction of cardiovascular risk in severely obese patients at one year of follow-up. Surg Obes Relat Dis. 2011;7(5):575–80.
Lakdawala MA, Bhasker A, Mulchandani D, et al. Comparison between the results of laparoscopic sleeve gastrectomy and laparoscopic Roux-en-Y gastric bypass in the Indian population: a retrospective 1 year study. Obes Surg. 2010;20(1):1–6.
Lee WJ, Ser KH, Chong K, et al. Laparoscopic sleeve gastrectomy for diabetes treatment in nonmorbidly obese patients: efficacy and change of insulin secretion. Surgery. 2010;147(5):664–9.
Conflict of interest
All authors declare to have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Christian Jurowich and Andreas Thalheimer contributed equally to this work.
Rights and permissions
About this article
Cite this article
Jurowich, C., Thalheimer, A., Hartmann, D. et al. Improvement of Type 2 Diabetes Mellitus (T2DM) After Bariatric Surgery—Who Fails in the Early Postoperative Course?. OBES SURG 22, 1521–1526 (2012). https://doi.org/10.1007/s11695-012-0676-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-012-0676-2