Abstract
Background
Ghrelin is secreted mainly in the stomach and plays a role in food intake regulation. Morbidly obese (MO) individuals report a decline in appetite after sleeve gastrectomy (SG), presumably due, in part, to ghrelin cell removal. Ghrelin cell distribution and expression were determined in three areas of resected stomach specimens from MO patients subjected to SG.
Methods
Resected stomach specimens from 20 MO patients undergoing SG were analyzed. Real-time polymerase chain reaction of ghrelin mRNA and immunohistostaining for ghrelin cells in three stomach regions (fundus, body, and pre-antral areas) were performed. Body mass index (BMI) and total plasma ghrelin levels were obtained before and 3 months postoperatively.
Results
Ghrelin mRNA was detected throughout the stomach, its expression decreasing from the fundus towards the antrum. The relative quantification for ghrelin mRNA expression was 0.043, 0.026, and 0.015 at the fundus, body, and pre-antral region, respectively (P = 0.05, fundus vs. pre-antral region). Average ghrelin cell counts declined from 60 ± 40 to 45 ± 20 and 39 ± 13 cells/high power fields in the fundus, body, and pre-antral region, respectively. Three months after surgery, total plasma ghrelin levels decreased from 1,676 ± 470 to 1,179 ± 188 pg/ml (P < 0.00001) and BMI dropped from 46 ± 6 to 38 ± 5 kg/m2 (P < 0.00001).
Conclusions
Distribution and expression of ghrelin-secreting cells throughout the stomach were defined, emphasizing the importance of meticulous resection of the fundus during SG for maximal ghrelin cell removal.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ghrelin, a ligand for the growth hormone secretagogue receptor, is an orexigenic hormone produced primarily by cells in the oxyntic glands of the stomach [1–4]. It plays a physiological role in the regulation of food intake with plasma ghrelin levels rising shortly before meals and declining rapidly after food is consumed in humans and rodents [3, 5, 6].
Ghrelin is a 28 amino acid gut peptide derived from the 117 amino acid proghrelin. Following cleavage by proprotein convertase 1, it is stored in secretory vesicles of endocrine cells. Further acylation by ghrelin O-acyltransferase, adding an octanoyl group to a serine residue, has been shown to be crucial for its biological function [2]. Ghrelin is a multifunctional molecule, involved in many biological processes ranging from appetite regulation and growth hormone release to gut motility. Though produced throughout the length of the intestine, ghrelin is mainly secreted by the stomach, followed by the small intestine. Negligible quantities are also expressed by peripheral tissues, such as the pancreas and brain [1, 7, 8].
Plasma ghrelin levels rise during food deprivation and decline after food ingestion; this peptide is therefore referred to as a “meal initiator” [5]. Interestingly, fasting plasma ghrelin concentration is negatively correlated with body mass index (BMI) [6].
Sleeve gastrectomy (SG) is rapidly gaining acceptance both as a first stage in high risk patients or standalone procedure for treatment of morbid obesity. It entails a vertical resection of most of the stomach volume, removing part of the antrum, most of the body, and all of the fundus [9]. The proposed mechanism of action of this procedure is a combination of volume restriction, creation of a high-pressure system, and the induction of a favorable hormonal change [10]. Low plasma ghrelin levels were monitored up to 5 years after SG [11].
To better elucidate the “hormonal” mechanism of SG, we examined the distribution of ghrelin mRNA expression by real-time polymerase chain reaction (RT-PCR) and ghrelin-producing cell distribution by immunohistostaining of resected stomach specimens obtained in the course of SG in morbidly obese subjects. Circulating plasma ghrelin levels before and 3 months after surgery were measured as well.
Materials and Methods
Subjects
Twenty morbidly obese patients (3 males and 17 females) scheduled to undergo SG were enrolled in the study. Institutional review board approval was obtained prior to study initiation and all subjects signed an informed consent form.
All subjects met the standard NIH criteria for bariatric surgery (BMI >40 kg/m2 or BMI >35 kg/m2 with obesity-related comorbidities). Exclusion criteria included previous bariatric surgery, chronic liver or renal disease, and heart failure.
Laparoscopic Sleeve Gastrectomy
Surgical technique was identical to our standard practice. A five-port laparoscopic procedure was performed with two 12-mm working ports at the patient’s left upper and right mid-abdomen, one 10.5-mm port for the camera in the supra-umbilical midline, and two 5-mm ports at the subxyphoid and left costal margin, anterior axillary line, for retraction. The greater curvature of the stomach was freed from the omentum using ultrasonic shears from 5 cm proximal to the pylorus to the angle of His. Extra care was taken in dissecting the posterior aspect of the fundus to expose the lesser curvature caudally, all the way to the left crus of the diaphragm, with meticulous division of all short gastric vessels. The gastric sleeve was constructed over a 42F bougie (Pilling; Maloney Bougie Tungsten, Teleflex Medical, High Wycomb, UK). A linear-cutting stapler (Echelon 60 Endopath®, Ethicon Endosurgery, Cincinnati, OH, USA) was used from 5 cm proximal to the pylorus to the angle of His with varying stapler heights, according to tissue thickness, maintaining uniform sleeve diameter throughout its length, with over-suturing of the entire stapler line. The entire fundus was thus completely resected and removed.
Plasma Ghrelin Determination
The levels of plasma total ghrelin were measured by a commercial radioimmunoassay kit (LINCO Cat# GHRT-89HK). Blood samples were taken from patients before and 3 months after surgery after an overnight fast according to the manufacturer’s kit specifications. Radioactivity was determined by the WALLAC gamma counter (1261). Two samples were excluded due to technical error in sample handling.
Immunohistochemistry
Stomach specimens resected during SG were thoroughly washed in saline immediately following extraction to remove debris (blood, loose fat tissue, and mucus). Stomachs were fixed in buffered formalin 10% for 24 h.
After fixation, three tissue samples were obtained from three areas of each stomach: the fundus, body, and pre-antral area. These samples were paraffinized, sectioned, and mounted on slides. Tissue samples were treated by sequential immersion in xylene and graded concentrations of ethanol for removal of paraffin and rehydration. They were then rinsed in distilled water. Endogenous peroxidase activity was blocked by immersion of the slides in 3% hydrogen peroxide diluted in phosphate-buffered saline (PBS) for 10 min followed by rinsing in PBS. Nuclear Decloaker (Biocare Medical # CB911M) with color-coded pH indicator was used for antigen retrieval from the formalin-fixed tissue. Slides were incubated 1 h with GHRL monoclonal antibody (M01) clone 2F4 Abnova (#H00051738-M01). As control, duplicate slides from the same areas were incubated with nonspecific anti-IgG antibodies. After rinsing, slides were incubated for 30 min with Histofine Simple Stain MAX PO (Nichirei Biosciences Inc.) universal immunoperoxidase polymer, anti-mouse, and anti-rabbit and washed in PBS. Chromogen/substrate reagent was added and slides were rinsed and mounted with DAKO liquid DAB (#k3466). Positive cells were counted in 9 of 20 high (×10) magnification fields randomly chosen for each specimen.
Real-Time Polymerase Chain Reaction
The expression of ghrelin at different regions of the stomach was analyzed. RNA was isolated from the stomach tissue using RecoverAll™ Total Nucleic Acid Isolation (Ambion #1975). RNA was purified using glass fiber filter, with on-filter nuclease treatment, and eluted into water. Single-stranded cDNA from total RNA was synthesized using high capacity cDNA reverse transcription (Applied Biosystems #4374966).
RT-PCR analysis was performed (TaqMan, 7000 sequence detection system, Applied Biosystems). A mixture of 5 μL 1:5 diluted cDNA, 100 nM of primer, 200 nM of probe, and Taqman Universal Master Mix in 20 μL aliquots was prepared. The reaction was initiated by 2 min incubation at 50°C and an initial 10-min denaturation at 94°C. This was followed by 40 cycles consisting of denaturation at 94°C (15 s) and annealing and elongation at 60°C (1 min).
mRNA levels are calculated by comparing the cycle where ghrelin was detected to a control gene. This is the difference in cycle time or ΔCT. The relative quantification (RQ) is the relation between ghrelin and control gene expression and is calculated by the formula RQ = 2−ΔCT. The control gene used was S18, coding for ribosomal RNA, which is avidly transcribed in all tissues.
Statistical Analysis
Data are presented as mean values ± standard deviation. Paired Student’s t test was used for continuous normally distributed variables. Pearson correlation coefficient (r) and the significance for it (P) were calculated between variables.
Analysis of variance using the Duncan multiple comparison option and non-parametric Kruskal–Wallis tests were used to compare measured values from the various regions of the stomach. Statistical significance was considered as a probability of a type 1 error <5%.
Results
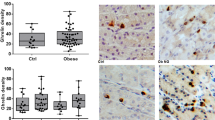
Mean total ghrelin plasma levels decreased sharply from 1,676 ± 470 pg/ml before to 1,179 ± 188 pg/ml when measured 3 months after surgery (a 27% drop; P < 0.00001) (Fig. 1). Mean BMI decreased from 46 ± 6 to 38 ± 5 kg/m2 in the same period. However, we found no correlation between the decline in plasma ghrelin levels and BMI in the postoperative measurement (R 2 = 0.0426) (Fig. 2).
Ghrelin mRNA was readily detectable in all examined regions of the stomach with gradually decreasing expression from the fundus distally. The RQ of ghrelin mRNA expression was 0.043 ± 0.016 at the fundus, 0.026 ± 0.01 at the body, and 0.015 ± 0.006 in the pre-antral region. Thus, the fundus expressed ghrelin 1.7- and 2.9-fold more than the body and pre-antral stomach, respectively. The difference between the fundus and pre-antral region was statistically significant (P = 0.05) (Fig. 3). We found that the RT-PCR amplification plot of ghrelin was roughly only four cycles behind that of ribosomal gene S18, a known highly expressed protein, demonstrating the high expression of ghrelin in the stomach of our obese subjects.
The prevalence of ghrelin-producing cells as detected by immunohistochemical staining followed the same pattern, with the greatest percentage of cells staining positive for ghrelin in the fundus, intermediate in the body, and fewest in the pre-antral area. Average ghrelin cell counts were 60 ± 40, 45 ± 20, and 39 ± 13 cells/high power field in the fundus, body, and pre-antral region, respectively. This trend was not statistically significant (P = 0.08) (Figs. 4 and 5).
Distribution of ghrelin immunoreactive cells in the fundic gland. Ghrelin immunoreactive cells were recognized mainly near the lamina muscularis. Arrows indicate ghrelin immunoreactive cells (×40). Immunoperoxidase of monoclonal antibody to human ghrelin on formalin-fixed paraffin-embedded human stomach
Discussion
In this work, mapping of ghrelin expression and cell distribution was studied in resected stomach segments of morbidly obese individuals following SG. Ghrelin mRNA expression was threefold higher in the fundus than in the pre-pyloric area. The same pattern was also seen in ghrelin cell distribution.
Plasma ghrelin levels decrease after gastric bypass and lose their meal-associated variance but rise after gastric banding in a way similar to dieting, maintaining this variance [12]. After SG, levels have been shown to decrease up to 5 years after surgery [11, 13]. Substantial weight loss has been well documented for these procedures implying that the specific operative manipulation is responsible for the changes observed in circulating ghrelin levels. We found that the total plasma ghrelin levels significantly decrease 3 months after surgery, and a simultaneous significant weight loss was observed in morbidly obese patients 3 months after surgery. However, there was no distinct correlation between these two parameters. This is consistent with our previous findings implying that ghrelin is but one of the myriad of factors contributing to the success of this procedure [10] and that weight loss per se is not responsible for ghrelin level change.
Ghrelin is produced by oxyntic cells along the entire gastrointestinal tract but its main site of production is the stomach [1]. It is therefore not surprising that ghrelin levels decrease immediately after SG and have been shown to remain low for up to 5 years [11, 14].
The total plasma ghrelin level found in obese patients prior to surgery was quite high even though fasting plasma ghrelin concentration is negatively correlated with body mass index. Nevertheless, our study design included measurement of total plasma ghrelin levels. Active ghrelin was not measured and might have been lower. In addition, higher expression of ghrelin mRNA was found (when compared to the highly expressed ribosomal control gene) in all specimens. High levels of mRNA do not always correlate with final protein levels, and this may be true for ghrelin as well. It has recently been shown that in ob/ob and in diet-induced obese mice, there was a significant increase in preproghrelin mRNA levels while tissue and plasma proghrelin-derived peptide levels were significantly reduced [8]. This discrepancy between ghrelin mRNA expression and circulating ghrelin levels has also been demonstrated in humans. Gravid women with gestational diabetes show no association between circulating ghrelin and the higher ghrelin mRNA expression in their placental tissue [15]. The decrease of ghrelin cell count along the stomach is consistent with similar findings demonstrating higher levels of expression in endoscopically obtained samples from the fundus and antrum of healthy patients [16] and surgically obtained specimens from gastric cancer patients [17].
The efficacy of SG in long-term weight reduction is still under scrutiny. Five- and 6-year excess weight loss results range from 55% to 64%, showing a distinct weight regain after a nadir at 2–3 years [11, 18]. Ghrelin levels seem to increase slowly as well, though still remaining significantly lower than prior to surgery [11]. Some authors have demonstrated the “neo-fundus” in radiological evaluation of patients after SG, attributing weight regain to this stretching and enlargement of the superior portion of the sleeve. Ghrelin levels in this subset of patients were not addressed [18–20].
Obestatin is a peptide originally isolated from the rat stomach, derived from proghrelin by post-transcriptional processing. Though sharing the same precursor with ghrelin, it has been shown to have opposite effects, namely inducing diminished food intake, reducing gut motility, and promoting weight loss, albeit in rodents [21]. Obestatin levels were not obtained in this study but it would be interesting to look at the relationship between ghrelin and obestatin in the future.
The presence of ghrelin-producing cells in the antrum as documented in this study is of special interest. There is an ongoing debate regarding the need of antrum removal and the relationship between its removal and long-term weight loss. Some authors strongly recommend protecting the antrum due to its importance as a pumping mechanism for gastric emptying and also stated that resecting part of the antrum will not significantly change the remaining volume of the stomach [22, 23]. Others argue that leaving an intact antrum leaves room for sleeve dilatation, loss of restriction, and concomitant weight regain and suggest to resect closer to the pylorus about 3–4 cm and not 7 cm [24]. This study proposes that, though to a lesser degree, removal of antral tissue facilitates a more complete removal of ghrelin-producing cells. This too should be taken into account in addition to volume reduction, when discussing the surgical technique of sleeve gastrectomy.
Utilizing large caliber bougies to create the sleeve correlates with poorer weight loss results [25], when compared with thinner bougies, perhaps attributable to both reduced restriction and a reduced hormonal effect with more ghrelin cells remaining. Gut peptides seem to play a crucial role in the weight reduction achieved by bariatric surgery. Ghrelin is the only orexigenic gut peptide described to date. Its levels decrease after SG and may contribute to the impressive outcome of this procedure. To the best of our knowledge, this is the first work looking into specific ghrelin cell distribution and expression in resected stomachs of morbidly obese individuals after SG. Extra care should be taken to resect as much ghrelin-producing tissue during SG, and this study, which helps define the borders of this tissue, should assist in optimizing this procedure to achieve more weight loss.
References
Ariyasu H, Takaya K, Tagami T, et al. Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J Clin Endocrinol Metab. 2001;86:4753–8.
Kojima M, Hosoda H, Date Y, et al. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–60.
Tschop M, Wawarta R, Riepl RL, et al. Post-prandial decrease of circulating human ghrelin levels. J Endocrinol Invest. 2001;24:RC19–21.
Date Y, Murakami N, Toshinai K, et al. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology. 2002;123:1120–8.
Cummings DE, Purnell JQ, Frayo RS, et al. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–9.
Shiiya T, Nakazato M, Mizuta M, et al. Plasma ghrelin levels in lean and obese humans and the effect of glucose on ghrelin secretion. J Clin Endocrinol Metab. 2002;87:240–4.
Date Y, Kojima M, Hosoda H, et al. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology. 2000;141:4255–61.
Simonsson M, Eriksson S, Hakanson R, et al. Endocrine cells in the human oxyntic mucosa. A histochemical study. Scand J Gastroenterol. 1988;23:1089–99.
Roa PE, Kaidar-Person O, Pinto D, et al. Laparoscopic sleeve gastrectomy as treatment for morbid obesity: technique and short-term outcome. Obes Surg. 2006;16:1323–6.
Yehoshua RT, Eidelman LA, Stein M, et al. Laparoscopic sleeve gastrectomy—volume and pressure assessment. Obes Surg. 2008;18:1083–8.
Bohdjalian A, Langer FB, Shakeri-Leidenmuhler S, et al. Sleeve gastrectomy as sole and definitive bariatric procedure: 5-year results for weight loss and ghrelin. Obes Surg. 2010;20:535–40.
Cummings DE, Weigle DS, Frayo RS, et al. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med. 2002;346:1623–30.
Langer FB, Reza Hoda MA, Bohdjalian A, et al. Sleeve gastrectomy and gastric banding: effects on plasma ghrelin levels. Obes Surg. 2005;15:1024–9.
Frezza EE, Chiriva-Internati M, Wachtel MS. Analysis of the results of sleeve gastrectomy for morbid obesity and the role of ghrelin. Surg Today. 2008;38:481–3.
Telejko B, Kuzmicki M, Zonenberg A, et al. Ghrelin in gestational diabetes: serum level and mRNA expression in fat and placental tissue. Exp Clin Endocrinol Diabetes. 2010;118:87–92.
Gnanapavan S, Kola B, Bustin SA, et al. The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. J Clin Endocrinol Metab. 2002;87:2988.
Tanaka-Shintani M, Watanabe M. Distribution of ghrelin-immunoreactive cells in human gastric mucosa: comparison with that of parietal cells. J Gastroenterol. 2005;40:345–9.
Himpens J, Dobbeleir J, Peeters G. Long-term results of laparoscopic sleeve gastrectomy for obesity. Ann Surg. 2010;252:319–24.
Goitein D, Goitein O, Feigin A, et al. Sleeve gastrectomy: radiologic patterns after surgery. Surg Endosc. 2009;23:1559–63.
Iannelli A, Schneck AS, Noel P, et al. Re-sleeve gastrectomy for failed laparoscopic sleeve gastrectomy: a feasibility study. Obes Surg. 2010;21(7):832–5.
Zhang JV, Ren PG, Avsian-Kretchmer O, et al. Obestatin, a peptide encoded by the ghrelin gene, opposes ghrelin’s effects on food intake. Science. 2005;310:996–9.
Weiner RA, Weiner S, Pomhoff I, et al. Laparoscopic sleeve gastrectomy—influence of sleeve size and resected gastric volume. Obes Surg. 2007;17:1297–305.
Givon-Madhala O, Spector R, Wasserberg N, et al. Technical aspects of laparoscopic sleeve gastrectomy in 25 morbidly obese patients. Obes Surg. 2007;17:722–7.
Baltasar A, Serra C, Perez N, et al. Re-sleeve gastrectomy. Obes Surg. 2006;16:1535–8.
Gagner M. Leaks after sleeve gastrectomy are associated with smaller bougies: prevention and treatment strategies. Surg Laparosc Endosc Percutan Tech. 2010;20:166–9.
Conflict of Interest
David Goitein MD, Doron Lederfein PhD, Ronit Zioni MA, Haim Berkenstadt MD, Moris Venturero MD, and Moshe Rubin MD have no conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Goitein, D., Lederfein, D., Tzioni, R. et al. Mapping of Ghrelin Gene Expression and Cell Distribution in the Stomach of Morbidly Obese Patients—a Possible Guide for Efficient Sleeve Gastrectomy Construction. OBES SURG 22, 617–622 (2012). https://doi.org/10.1007/s11695-011-0585-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-011-0585-9