Abstract
Background
The purpose of this study was to determine if the routine use of postoperative continuous positive airway pressure (CPAP) in patients undergoing laparoscopic Roux-en-Y gastric bypass (RYGB) is associated with an increase in transmural gastric pouch pressure, which may create the risk for anastomotic leak.
Methods
Transmural gastric pressures (difference between gastric pouch and bladder pressures) were measured postoperatively [post-anesthesia recovery care unit (PACU) arrival (prior to initiation of CPAP), 5 min, 30 min, and PACU discharge] in 28 patients (19 patients used CPAP, 9 patients did not) following laparoscopic RYGB. Changes in pressure over time were assessed using a generalized estimating equation, taking into account the repeated measurements obtained for each subject. In all cases, two-tailed P values ≤0.05 were considered statistically significant.
Results
Among patients that used CPAP, there were no changes in transmural pouch pressure from baseline at any point in time (P = 0.628). However, in patients that did not use CPAP, there was a trend towards increased transmural gastric/pouch pressure (P = 0.053), which could be attributed to a transient decrease in bladder pressure at the 5-min measurement interval.
Conclusions
Application of CPAP did not increase transmural gastric pouch pressure in our bariatric patients; therefore, its use in the post-RYGB patients does not pose a risk for pouch distension, which could lead to the disruption of anastomotic integrity.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Perioperative management of bariatric patients presents unique challenges to the healthcare team [1]. Obstructive sleep apnea (OSA) may be present in up to 80% of bariatric patients [2, 3]. The presence of OSA can increase the likelihood of postoperative respiratory complications [4]. Noninvasive ventilatory devices (continuous positive airway pressure [CPAP] and bilevel positive airway pressure [BiPAP]) can be used to reduce the incidence of postoperative respiratory complications in patients with OSA including obese patients undergoing bariatric surgery [5–7]. We recently demonstrated that, when bariatric patients were evaluated preoperatively by polysomnography and managed accordingly with noninvasive ventilation postoperatively, the severity of OSA was not associated with the increased rate of postoperative complications [8]. The American Society of Anesthesiology recommends that patients with OSA who preoperatively use CPAP or BiPAP should have it reinstated as soon as feasible after tracheal extubation [9].
A serious postoperative complication of bariatric surgery is the development of an anastomotic leak. Prevalence of this complication ranges from 0.5% to 2% [7, 10–12].
It is believed that aerophagia, i.e., forcing of air into the stomach and bowel, may be a relatively frequent event during noninvasive positive pressure ventilation [13]. Several isolated reports [14–16] described severe gastric distension while using noninvasive ventilatory devices in both postoperative and nonoperative settings, which have resulted in calls for caution regarding the use of CPAP or BiPAP in the postoperative period in bariatric postsurgical patients [15, 17, 18].
To our knowledge, there is no published data on the effects of CPAP on gastric pouch pressure in the postoperative setting in patients who have undergone Roux-en-Y gastric bypass (RYGB). Theoretically, an increase in pouch pressure could increase the risk of leakage from the newly formed gastrojejunostomy. However, it is unknown whether the use of CPAP results in increases in pouch pressure in patients undergoing RYGB. The purpose of this prospective study is to assess and quantify gastric pouch pressures during the use of CPAP following RYGB operation.
Methods and Materials
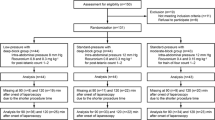
The Mayo Clinic Institutional Review Board approved this investigation, and each subject gave written informed consent. We enrolled in the study 20 competent adult subjects that used CPAP to treat OSA and 10 subjects that did not use CPAP. All our patients underwent first-time laparoscopic RYGB performed by two surgeons (JMS and MK) who used identical surgical techniques. Exclusion criteria included: open RYGB, revision RYGB, or prior esophageal or gastric surgery, contraindication to the placement of a nasogastric catheter, history of significant pulmonary disease other than OSA, a history of neurologic disorders that could affect the upper airway/esophageal/lower gastrointestinal muscle tone or breathing pattern, use of rescue CPAP in the post-anesthesia recovery care unit (PACU) in patients that did not use CPAP preoperatively. Patients that used CPAP were instructed to bring their home CPAP device to the hospital and this device was used postoperatively.
Perioperative Management
Patients underwent a standardized general anesthetic used at the Mayo Clinic for the anesthetic management of bariatric surgery. Intravenous induction consisted of fentanyl 1–3 μg/kg and propofol 2–3 mg/kg, both according to the ideal body weight. Placement of an endotracheal tube was performed after induction under direct laryngoscopy facilitated by succinylcholine 1.0–1.5 mg/kg ideal body weight (with a maximum of 140 mg in any patient). Anesthesia was maintained with desflurane in a mixture of oxygen and air, resulting in an inspired oxygen concentration of 50%. After induction, the bladder was catheterized. Oxymorphone was used for additional analgesia and, usually, ketorolac 15 mg was administered at the end of the operation. Muscle relaxation was provided with vecuronium. Neuromuscular blockade was reversed with neostigmine 5 mg and glycopyrrolate 1 mg at the end of the operation. Since nausea and vomiting can affect abdominal (and gastric) pressure, all our patients received triple antiemetic prophylaxis before emerging from anesthesia (droperidol, dexamethasone, and ondansetron). The patient’s trachea was extubated at the end of the surgery in the operating room with the patient in a semi-sitting position with the head elevated to 30°, and all patients were provided with supplemental oxygen during transport to the PACU, again in the semi-sitting position. For patients that used CPAP preoperatively, their personal CPAP device was placed after arrival to the PACU after the first set of gastric pouch and bladder pressure measurements were done.
Data Collection
Before the end of the operation, while still under general anesthesia, an esophageal pressure balloon catheter was inserted nasally into the gastric pouch under visual guidance with an endoscope. This gastric pouch catheter was constructed using standard technique from PE200 Intramedic Polyethylene tubing (internal diameter of 1.3 mm) with the distal 4 cm of the catheter perforated and covered with a small latex balloon [19]. Before clinical use, the physiologic characteristics of the balloon (compliance and distending pressure characteristics) were validated in the laboratory. After insertion, the catheter was attached to a pressure transducer [modified by sealing the flushing port] (Pressure Monitoring Set PX-MK053, Edwards Lifesciences, Irvine, CA, USA) and the balloon was filled with 0.6 mL of air. Bladder pressures are used as proxy for intra-abdominal pressure [20]. Bladder pressure measurements were obtained using a Foley catheter placed intraoperatively using standard technique with a pressure transducer (Pressure Monitoring Set PX-MK053, Edwards Lifesciences, Irvine, CA, USA). A bladder volume of 50 mL of 0.9% saline was used for each measurement [20]. Analog output from both pressure transducers were converted to digital output using a commercially available analog/digital converter (PowerLab 8/30 ML870, ADInstruments, Bella Vista, Australia) and acquired by the CardioCap5 Monitor (GE Datex Ohmeda, Madison, WI, USA). This digital waveform was recorded by the Chart 5 software (Chart 5, version 5.5.6, ADInstruments, Bella Vista, Australia), which was calibrated for the expected physiological pressure ranges. Output was measured in millimeters of mercury and converted into centimeters of water (1 mmHg = 1.36 cm H2O). Both transducers (gastric pouch and bladder) were zeroed in the usual fashion and secured with tape (≈5 cm above and 10 cm lateral from the umbilicus) on abdominal wall. After obtaining stable waveforms, the values for both pouch and bladder pressures were calculated as a mean of each waveform for five respiratory cycles. For patients that use CPAP, intragastric pouch and bladder pressure measurements were made immediately before and after the initiation of CPAP, 30 min after CPAP has been initiated, and prior to PACU discharge. In patients who did not use CPAP, these measurements were obtained upon arrival to the PACU, 5 and 30 min after arrival to PACU, and prior to PACU discharge. All measurements in all patients were done in a semi-sitting position with the head elevated to 30° and the pressure transducers were kept in constant position. The gastric catheter was removed at the time of PACU discharge.
Statistics
Mean pouch and bladder pressures from each subject were calculated from measurements during five respiratory cycles as outlined above. The differences between gastric pouch and bladder pressures were calculated to estimate the transmural pressure across the gastric/pouch wall (P Tm = P Ga − P Ab). Repeated-measures analyses were performed for each pressure characteristic using a generalized estimating equation (GEE). In all cases, an initial analysis was performed, which included time (baseline, 5 min, 30 min, discharge) and CPAP use (yes, no) as explanatory variables. The time-by-CPAP interaction effect was included to assess whether changes in pressure over time were dependent on CPAP use. Subsequent repeated-measures analyses were performed to assess changes in pressure separately for each CPAP group. In all cases, two-tailed P values ≤0.05 were considered statistically significant.
Results
Thirty subjects that underwent laparoscopic RYGB were enrolled in the study, 20 that used CPAP preoperatively and 10 that did not use CPAP preoperatively. Two patients did not complete the study. In one patient that used CPAP, the gastric catheter became dislodged during tracheal extubation. The other patient that did not use CPAP requested that the gastric catheter be removed immediately upon arrival to the PACU. Among the patients who used CPAP, all used nasal CPAP except for two patients that used a full-face mask. The mean CPAP pressure was 10 ± 4 cm H2O. All other patients tolerated the gastric catheter well. The clinical and demographic characteristics of patients who completed the study were similar except all the patients that did not use CPAP were female (Table 1). None of the patient in this study reported nausea and none required antinausea medications while in PACU
From repeated-measures analysis, there was some evidence (P = 0.055) of a two-way interaction suggesting that transmural gastric pouch pressure changed differentially over time for patients with vs. without CPAP. From subsequent analyses performed separately for each patient group, CPAP patients were not found to have a change in transmural gastric pouch pressures with the application of CPAP (P = 0.628), but there was some evidence (P = 0.053) suggesting that non-CPAP patients had modest changes, which could be attributed primarily to variations in bladder pressure. Specifically, in these patients, mean bladder pressure at 5 min was slightly lower, and with unchanged pouch pressure, the calculated transmural pressure was higher (mean of 2.5 cm H2O or 1.8 mmHg). By 30 min, bladder pressures in this group were not different from measurements taken either at arrival or discharge from PACU (Table 2). No statistically significant differences in pouch pressure or bladder pressure over time were noted in either group (all P > 0.05).
Discussion
Postoperative application of CPAP in patients undergoing laparoscopic RYGB did not significantly affect intragastric (pouch) pressure or transmural pouch pressure. Although these results are obtained on a relatively small number of RYGB patients, they are reassuring given concerns regarding the possibility of excessive gastrointestinal distension with noninvasive ventilation that may lead to anastomotic disruption.
Postoperative anastomotic leak following bariatric surgery is a dreaded complication resulting in increased hospitalization, utilization of intensive care unit resources, and increased mortality [10, 21]. In one series of laparoscopic RYGB, the development of gastrojejunostomy leak resulted in 22% mortality [22]. Based on several case reports of significant gastric distension that occurred during the use of BiPAP, caution has been advised with the use of noninvasive ventilatory devices in patients after bariatric surgery [15, 17, 18]. Vasquez et al. [15] reported about two patients who used BiPAP after open RYGB and developed massive bowel distension and subsequent anastomotic leaks 1 week after surgery. It remains questionable whether gastric distension in these patients could be attributed to the use of BiPAP or rather to the postoperative paralytic ileus. Severe gastric distension has been reported with the use of noninvasive positive pressure ventilation in the nonoperative setting for the treatment of respiratory failure [14, 16]. Yamada et al. [16] reported a case of massive gastric distension with the use of BiPAP in a patient with amyotrophic lateral sclerosis. Severe neurologic diseases including spinal cord injury may be associated with dysphagia, altered esophageal motility, and altered lower esophageal sphincter (LES) tone, and all may predispose patients to aerophagia. Another report described a patient with respiratory failure who developed acute gastric overdistension while using BiPAP; the large increase in intra-abdominal pressure (patient developed abdominal compartment syndrome) caused cardiovascular collapse, which resolved with gastrointestinal gas evacuation with gastric tube [14]. In a small series of 40 patients with chronic respiratory failure treated with noninvasive positive pressure ventilation (BiPAP used in 85% of patients), 13% of the patients did complain of aerophagia [13]. It is possible that patients in respiratory distress [13, 14] or those with neurologic conditions (amyotrophic lateral sclerosis) [16] may be more predisposed to aerophagia due to desynchronization between breathing and swallowing. The negative results from our study are in agreement with observations in larger series of patients undergoing bariatric surgery where the use of CPAP was well tolerated and not associated with an increased rate of anastomotic leaks [6–8]. Finally, balance of compliances between chest wall and abdomen can be responsible for the direction of air (lungs vs. stomach) during CPAP breathing, and we postulate that low abdominal compliance in morbidly obese patients may be a protective factor against gastric insufflation.
While we observed no changes in pouch pressures with the implementation of CPAP, Shepherd et al. [23] demonstrated that gastric pressure modestly increased with increasing CPAP setting in awake, normal-weight, healthy adults. The maximum CPAP pressure of 15 cm H2O resulted in an increase of 3.4 cm H2O over baseline, while the increases in gastric pressures at 5 and 10 cm H2O were 1.2 and 2.0 cm H2O, respectively. Regardless of the fact that these pressure increases were statistically significant, they are not clinically significant. At the end of the bariatric surgery, we routinely test the potential for gastrojejunal anastomotic leak by distally occluding the Roux limb, submerging the anastomosis under saline, and insufflating oxygen via endoscope into the gastric pouch, allowing it to achieve full distension. While this pressure is substantial, we did not directly measure it. In clinical practice, colorectal anastomoses are tested by distending it with an effective distending pressure of 30 cm H2O [24], which is threefold greater than the pouch distending pressure in our patients (approximately 10 cm H2O). An animal study showed that a properly closed gastrostomy can withstand high testing pressures [25].
It is interesting to note that the mean baseline pouch pressures in our study were higher (21.2 cm H2O) than that reported by Shepherd (6.4 cm H2O for normal-weight individuals) [23], which may reflect primarily differences in body habitus between the two cohorts (normal weight vs. obese). Pandolfino et al. [26] demonstrated that intragastric pressure is significantly higher in obese patients (14 mmHg or 19 cm H2O) compared to those with a normal BMI (6 mmHg or 8 cm H2O). In addition, the shape of the container where the pressure is measured may affect pressure measurement. More specifically, compared to intact gastric cavity, we measured pressure in the gastric pouch, which is a smaller-radius chamber, and therefore, according to the Law of Laplace would be expected to develop higher pressures for a given volume of air.
It has been shown that CPAP improves symptoms of nocturnal gastroesophageal reflux disease (GERD) in patients with OSA [27] and reduces episodes of low esophageal pH [28, 29]. Furthermore, in normal subjects, the application of CPAP increases the tone of LES [23, 30] and decreases the duration of LES relaxation during swallowing [23]. Because the LES tone is the primary barrier between stomach and esophagus, these reflexes represent a potential mechanism how CPAP may mitigate both the reflux and air ingress during CPAP use. In our series, 58% of patients that used CPAP had also been diagnosed with GERD. We did not find any change in pouch pressures after the application of CPAP in patients with and without GERD (P ≥ 0.60) or in those with and without CPAP. Because our study was not designed to evaluate the function of LES tone, we cannot comment on the potential role the CPAP may have had on the integrity of LES in our patients. The question of air ingress during CPAP may be even more complicated because the gastric pouch is not a closed container but rather has a fully open passage to the Roux jejunal limb; therefore, even if the air enters at an increased rate with CPAP breathing, it may not be detected. At the same time, if the air ingress was substantial, one would detect it as a gastrointestinal distension that would lead to an increase in abdominal pressure, which was not confirmed in our study.
This study has several limitations. First, we cannot generalize our conclusions to all forms of noninvasive ventilation and different types of patients and surgeries. For example, our cohort did not include patients treated with BiPAP and anecdotal reports of clinically relevant complications related to aerophagia that occurred in patients using this ventilatory device [15]. All the patients included in this study used their own individual CPAP equipment. At our institution, patients considering bariatric surgery that have OSA have their CPAP equipment carefully adjusted under the guidance of a pulmonologist specialized in sleep medicine. Thus, our findings cannot be generalized to patients that use CPAP where settings have not been individualized. Furthermore, we performed experiments on bariatric patients undergoing laparoscopic RYGB and our conclusions have limited applicability to patients undergoing celiotomy, thoracotomy, and esophagectomy, as well as patients with neurologic diseases where esophageal integrity may be altered. Second, from the unchanged pouch pressures, we cannot conclude that the ingress of air does not occur during the use of CPAP because the pouch has a wide open communication to the jejunal limb where the air may readily escape. At the same time, if this had occurred, one would expect it to be associated with the increase in bladder (abdominal) pressure, which we did not confirm. Finally, we studied a limited number of patients, and the safety of any method can be declared only after examining a larger population sample.
In conclusion, changes in transmural gastric pouch pressure with the application of CPAP did not occur in our bariatric patients. Furthermore, abdominal pressure did not increase, suggesting that the gas was not entering the gastrointestinal system passing the pouch in any clinically significant amounts. This suggests that substantial aerophagia leading to pouch distension does not occur routinely during postoperative use of CPAP in patients after laparoscopic RYGB. Larger studies are needed to examine the safety of different noninvasive ventilation devices (BiPAP) in the perioperative setting.
References
Pieracci FM, Barie PS, Pomp A. Critical care of the bariatric patient. Crit Care Med. 2006;34:1796–804.
Frey WC, Pilcher J. Obstructive sleep-related breathing disorders in patients evaluated for bariatric surgery. Obes Surg. 2003;13:676–83.
O'Keeffe T, Patterson EJ. Evidence supporting routine polysomnography before bariatric surgery. Obes Surg. 2004;14:23–6.
Gupta RM, Parvizi J, Hanssen AD, et al. Postoperative complications in patients with obstructive sleep apnea syndrome undergoing hip or knee replacement: a case–control study. Mayo Clin Proc. 2001;76:897–905.
Rennotte MT, Baele P, Aubert G, et al. Nasal continuous positive airway pressure in the perioperative management of patients with obstructive sleep apnea submitted to surgery. Chest. 1995;107:367–74.
Huerta S, DeShields S, Shpiner R, et al. Safety and efficacy of postoperative continuous positive airway pressure to prevent pulmonary complications after Roux-en-Y gastric bypass. J Gastrointest Surg. 2002;6:354–8.
Livingston EH, Huerta S, Arthur D, et al. Male gender is a predictor of morbidity and age a predictor of mortality for patients undergoing gastric bypass surgery. Ann Surg. 2002;236:576–82.
Weingarten TN, Flores AS, McKenzie JA, et al. Obstructive sleep apnoea and perioperative complications in bariatric patients. Br J Anaesth. 2011;106:131–9.
Gross JB, Bachenberg KL, Benumof JL, et al. Practice guidelines for the perioperative management of patients with obstructive sleep apnea: a report by the American Society of Anesthesiologists Task Force on Perioperative Management of patients with obstructive sleep apnea. Anesthesiology. 2006;104:1081–93.
Fernandez Jr AZ, Demaria EJ, Tichansky DS, et al. Multivariate analysis of risk factors for death following gastric bypass for treatment of morbid obesity. Ann Surg. 2004;239:698–702. discussion 702–3.
Podnos YD, Jimenez JC, Wilson SE, et al. Complications after laparoscopic gastric bypass: a review of 3464 cases. Arch Surg. 2003;138:957–61.
Yeats M, Wedergren S, Fox N, et al. The use and modification of clinical pathways to achieve specific outcomes in bariatric surgery. Am Surg. 2005;71:152–4.
Criner GJ, Brennan K, Travaline JM, et al. Efficacy and compliance with noninvasive positive pressure ventilation in patients with chronic respiratory failure. Chest. 1999;116:667–75.
De Keulenaer BL, De Backer A, Schepens DR, et al. Abdominal compartment syndrome related to noninvasive ventilation. Intensive Care Med. 2003;29:1177–81.
Vasquez TL, Hoddinott K. A potential complication of bi-level positive airway pressure after gastric bypass surgery. Obes Surg. 2004;14:282–4.
Yamada S, Nishimiya J, Kurokawa K, et al. Bilevel nasal positive airway pressure and ballooning of the stomach. Chest. 2001;119:1965–6.
Frangos SG, Schwartz DR. Continuous positive airway pressure and postoperative hypoxemia. JAMA. 2005;293:2714. author reply 2714–5.
Deutzer J. Potential complications of obstructive sleep apnea in patients undergoing gastric bypass surgery. Crit Care Nurs Q. 2005;28:293–9.
Schilder DP, Hyatt RE, Fry DL. An improved balloon system for measureing intraesophageal pressure. J Appl Physiol. 1959;14:1057–8.
Fusco MA, Martin RS, Chang MC. Estimation of intra-abdominal pressure by bladder pressure measurement: validity and methodology. J Trauma. 2001;50:297–302.
Hamilton EC, Sims TL, Hamilton TT, et al. Clinical predictors of leak after laparoscopic Roux-en-Y gastric bypass for morbid obesity. Surg Endosc. 2003;17:679–84.
Madan AK, Lanier B, Tichansky DS. Laparoscopic repair of gastrointestinal leaks after laparoscopic gastric bypass. Am Surg. 2006;72:586–90. discussion 590–1.
Shepherd KL, Holloway RH, Hillman DR, et al. The impact of continuous positive airway pressure on the lower esophageal sphincter. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1200–5.
Wheeler JM, Gilbert JM. Controlled intraoperative water testing of left-sided colorectal anastomoses: are ileostomies avoidable? Ann R Coll Surg Engl. 1999;81:105–8.
Willingham FF, Turner BG, Gee DW, et al. Leaks and endoscopic assessment of break of integrity after NOTES gastrotomy: the LEAKING study, a prospective, randomized, controlled trial. Gastrointest Endosc. 2010;71:1018–24.
Pandolfino JE, El-Serag HB, Zhang Q, et al. Obesity: a challenge to esophagogastric junction integrity. Gastroenterology. 2006;130:639–49.
Green BT, Broughton WA, O'Connor JB. Marked improvement in nocturnal gastroesophageal reflux in a large cohort of patients with obstructive sleep apnea treated with continuous positive airway pressure. Arch Intern Med. 2003;163:41–5.
Kerr P, Shoenut JP, Millar T, et al. Nasal CPAP reduces gastroesophageal reflux in obstructive sleep apnea syndrome. Chest. 1992;101:1539–44.
Tawk M, Goodrich S, Kinasewitz G, et al. The effect of 1 week of continuous positive airway pressure treatment in obstructive sleep apnea patients with concomitant gastroesophageal reflux. Chest. 2006;130:1003–8.
Kerr P, Shoenut JP, Steens RD, et al. Nasal continuous positive airway pressure. A new treatment for nocturnal gastroesophageal reflux? J Clin Gastroenterol. 1993;17:276–80.
Acknowledgment
We are thankful to Minelle Hulsebus for the assistance in the construction of gastric pouch pressure measurement catheters.
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Funding Sources
Support was provided by the Department of Anesthesiology, College of Medicine, Mayo Clinic, Rochester, MN 55905, USA.
Rights and permissions
About this article
Cite this article
Weingarten, T.N., Kendrick, M.L., Swain, J.M. et al. Effects of CPAP on Gastric Pouch Pressure After Bariatric Surgery. OBES SURG 21, 1900–1905 (2011). https://doi.org/10.1007/s11695-011-0419-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-011-0419-9