Abstract
Purpose
Glucagon-like peptide-1 (GLP-1) is a hormone widely studied in the short-term postoperative follow-up of Roux-en-Y gastric bypass due to its elevation and association with improvement of the glucose metabolism, but there are few studies in 10 years after RYGB follow-up with the same patient.
Methods
Twenty morbidity obesity patients were submitted to RYGB; these patients were divided into two groups: normal glucose-tolerant morbidly obese patients (NGT) 11 patients and abnormal glucose metabolism morbidly obese patients (AGM) 9 patients. Oral glucose tolerance test (OGTT) was done during four different periods: T1 (first evaluation), T2 (pre-surgery), T3 (9 months after surgery) and T4 (10 years after surgery).
Results
Groups were matched for age and gender, and as NGT and AGM had BMI of 46.31 ± 5.03 kg/m2 and 50.87 ± 10.31 kg/m2. After 10 years of RYGB, they were obesity grade I with BMI for NGT 32.45 ± 4.99 kg/m2 and AGM 34.85 ± 4.46 kg/m2. Plasma glucose levels decreased NGT group at T4 period had a significant reduction at 120 min after OGTT for NGT 55.49 ± 17.15 mg/dL (p˂0.001). Insulin levels changed from T1 to T4 for the NGT group. GLP-1 curves were statistically different between the NGT and AGM groups. The AGM group had a higher mean for GLP-1 secretion at T4 period and at 30 min of OGTT 63.85 ± 37.98 pmol/L when compared to NGT 50.73 ± 24.82 pmol/L with AGM > NGT with p˂0.001.
Conclusion
Evaluation of the same patient during 4 different periods shows that, even with weight regain, after 10-years of RYGB high levels of GLP-1 remained which can be associated with metabolic improvement especially at the NGT group.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Roux-en-Y gastric bypass (RYGB) has been the most efficient method to help morbidity obesity patients to lose weight [1, 2]. The success of the surgery is due to the rapid weight loss that is a consequence of diet restriction, gastric reduction, duodenal exclusion, and fast delivery of nutrients to the ileum, which, combined, contribute to the metabolic improvement of the patient [3, 4]. This surgical method results in weight reduction along with improvement in the quality of life and most associated comorbidities, such as diabetes, systemic arterial hypertension, and cardiovascular dysfunction [5].
Most studies have shown that the metabolic improvement is due to the secretions of some incretins and these can be of particular relevance for the antidiabetic effect of the RYGB [6,7,8]. An important incretin that acts in insulin secretion is glucagon-like peptide-1 (7–36) amide (GLP-1), a peptide secreted by L-cells from the distal ileum after proglucagon cleavage. This peptide, in response to food intake, potentiates insulin glucose-stimulated secretion, inhibits the action of glucagon, and delays gastric emptying [9,10,11]. Studies have also shown that GLP-1 is responsible for increased insulin secretion as a result of food intake [12, 13].
Additionally, GLP-1 is a gut-derived hormone usually associated with the improvement of blood glucose after RYGB, since it has been observed that high levels of GLP-1 are secreted from patients after RYGB surgery [14, 15]. Several studies have shown this secretion of GLP-1 in short-term RYGB follow-ups [16,17,18], with most of them being 2–5 years long. Other studies that did 10-year follow-up of RYGB did not keep track of the same patients [19, 20].
Therefore, this article aims to show changes during 10-year follow-up of RYGB from the same patients during 4 different periods, focusing glucose metabolism, GLP-1 secretion, insulin secretion, and loss or regain of weight.
Materials and methods
Study design and participants
This study was done at the Metabolic Unit, Faculty of Medical Sciences, University of Campinas, through the follow-up evaluations of patients treated at the Bariatric Surgery Ambulatory beginning in August 2005 and concluded in July 2017.
The evaluations were as follows: T1—the first evaluation occurred when the patient arrived at the clinic for the first medical appointment and preparation for surgery, without previous treatment or nutritional monitoring; T2—the second occurred after the patient reached a 10% weight loss with diet, average of 5–7 months after the initial assessment; T3—9 months after the RYGB bariatric surgery; and the last evaluation T4—10 years after the RYGB bariatric surgery.
After 18 months of the RYGB surgery, the patient’s body weight was measured to calculate the percentage of excess weight loss (%EWL). The data were obtained through analysis of patient records, and the 18-month mark was chosen based on scientific reports that demonstrated that the higher amount of weight loss happens after 18 months of surgery [20, 21].
The patients were recruited at the Bariatric Surgery Ambulatory of the Faculty of Medical Sciences University of Campinas and underwent the 4 evaluations: T1, T2, T3, and T4. The data assessments were done within 3–5 months of the end of the data collection for each period.
At the Bariatric Surgery Ambulatory, all patients are screened to participate in the surgery groups. When a patient starts a group, he must follow a protocol to lose 10% of body weight so he can be ready for bariatric surgery. If a patient fails to do so, they will not undergo RYGB.
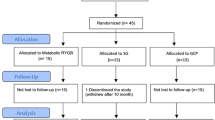
The study started in 2005, period T1, with 87 patients, but only 19 patients lost 10% body weight and went to the second evaluation (T2), before RYGB. These same 19 patients were also evaluated at period T3. This first part of the study, covering periods T1 to T3, was the research of a master’s degree thesis and was published as an article [14]. During these 3 periods, the patients had frequent visits to the Bariatric Surgery Ambulatory under the supervision of doctors, nutritionists, and psychologists.
After 10 years, period T4, these 19 patients of Bariatric Surgery Ambulatory were contacted but only 13 fit the inclusion criteria of the research. These 13 patients evaluated at T4 were not going often to the ambulatory, and they were having a regular lifestyle without any diet restrictions or use of weight loss medications.
During the T1, T2, and T3 periods, 11 morbidly obese patients with normal glucose tolerance (NGT group) and 8 patients who, besides obesity, had diabetes or glucose intolerance (abnormal glucose metabolism; AGM group) were evaluated and monitored. At T4, 9 individuals of the NGT group and 4 in the AGM group were assessed. In both groups, there were difficulties in the follow-up process, either due to patient health issues or due to lack of up-to-date information on patients. In the AGM group, one patient died of cancer and three could not retake the exams because they were using antidepressant medications that could tamper the evaluation results. Similarly, in the NGT group, three patients could not be re-evaluated since their contact information was not up to date and, therefore, the residential addresses and telephone numbers on file did not belong to these patients anymore.
The periods T1, T2, and T3 of the study had the protocol approved by the Research Ethics Committee of University of Campinas-UNICAMP, School of the Faculty of Medical Sciences (FCM-UNICAMP).
The period T4 of the study had the protocol approved by the Research Ethics Committee of University of Campinas-UNICAMP, School of the Faculty of Medical Sciences (FCM-UNICAMP) (number 31989314.3.0000.5404).
All volunteers included were previously informed about the study and signed an informed consent form at 2 different moments; the first informed consent was signed at T1 period and the second informed consent was signed at T4 period (10 years).
Patients who agreed to participate in the study were between 18 and 60 years old; BMI > 40 kg/m2 for obese and BMI ≥ 35 kg/m2 for diabetic obese. Exclusion criteria were as follows: individuals with chronic diseases such as heart disease, nephropathy, or thyroid disorder; patients on hormone replacement therapy, corticosteroid treatment, and renal or hepatic impairment. Patients who were using drugs that could interfere with metabolism such as antidepressants, corticosteroids, and diuretics were excluded.
Diabetic patients were classified according to the revised American Diabetes Association (ADA) criteria 2018 [22].
Assessments
At each of these periods, all patients that accepted to participate in the study underwent the Oral Glucose Tolerance Test (OGTT) Association (ADA) criteria 2018 [22]. The patients drank the 75 g of glucose; after surgery, they drank slowly than before, and they took almost 1 to 3 min to drink all the glucose.
During OGTT, blood samples were collected at 0, 30, 60, 90, and 120 min for quantification of plasma glucose and insulin, being considered as time 0 the final intake of 75 g of glucose. At times 0, 30, and 60 min of OGTT, samples were collected for later dosages of active GLP-1, and DPP-IV inhibitor was added to the blood samples.
All collected blood samples were centrifuged for serum, aliquoted, and stored in a freezer at − 80 °C for later dosing. All samples were dosed within a period of 3–5 months after the samples were collected.
Laboratory methods
Plasmatic glucose: Dosed in duplicate by the enzymatic method automated in the YSI 2300-Stat equipment; YSI, Yekkiw Springs, OH, USA.
Insulin: Quantified in duplicate by the enzyme-linked immunosorbent assay (ELISA) using Merck Millipore (Billerica, MA, USA) high-sensitivity human-specific kit (sensitivity: 1µU/mL).
Plasma active GLP-1: Measured by a fluorescence immunoassays method using high-sensitivity specific ELISA kit, sensitivity: 2 pM, Merck Millipore (Billerica, Ma, USA) [23,24,25].
Anthropometric measurements
Weight and height: we used a 100-g division mechanical scale for weight measurement (standardization: wearing light clothes and no shoes), and a portable stadiometer (Secca brand) with 1-mm division for height evaluation (participant without shoes and the five body points against a wall without skirting board).
Body composition assessment
Body composition was assessed by bioelectrical impedance using the Biodynamics Model 310 appliance after 4-h fasting [26]. All participants were instructed not to drink alcohol, coffee, coke, guarana, and tea (mate or black), and not to exercise the day before the test.
All participants were evaluated for anthropometric, body composition, and biochemical measurements.
Data analysis
The patients were classified according to weight loss sustenance that was calculated by the relationship between the %EWL (ideal weight based on the BMI of 25 kg/m2) at the last weight assessment and the maximum %EWL reached [1 − (last%EWL/maximum%EWL)] [20], which in fact represents the amount of weight regain of the maximum weight lost, expressed as percentages. Classifications: A: successful weight loss sustenance after the initial successful weight loss (maximum %EWL > 50%) and maintaining up to 50% of the weight lost—A1: excellent, with regain of 50% of the weight lost; A2: regular, with regain corresponding to 10–50% of the weight lost; B: unsatisfactory weight loss sustenance after the initial successful weight loss, with regain of > 50% of the weight lost. The maximum weight loss was obtained at 18 months after RYGB [20, 21]. Body weight at 18 months was obtained through analysis of patient records.
HOMA-IR was calculated as fasting glucose in mmol/L times the fasting insulin in µU/mL divided by 22.5 (fasting insulin vs. fasting glucose)/22.5 [27,28,29,30].
Statistical analysis
Descriptive statistics of numerical variables were calculated to describe the sample profile according to the variables under study, with mean values, standard deviation (SD), and standard error (SE).
To study the parameters over time, the generalized estimating equation (GEE) method was used. The estimates were calculated by maximum likelihood for treating losses over time. The data were transformed into ranks due to the lack of normal distribution. No covariate was analysed, only the comparison between the AGM X NGT groups [31,32,33,34,35,36,37].
For comparison of groups, the Mann–Whitney test was used. The significance level adopted for this study was 5% (p ˂0.05).
Software: The SAS System for Windows (Statistical Analysis System), version 9.4. SAS Institute Inc, 2002–2012, Cary, NC, USA [38].
All numeric data are given as mean ± SD, with only the graphics at Fig. 1 showing means and standard error bars in order to better illustrate the results.
OGTT curves. (A, B) Plasma glucose levels during OGTT for groups NGT and AGM respectively during periods T1, T2, T3, and T4; (C, D) Plasma insulin levels during OGTT for groups NGT and AGM respectively during periods T1, T2, T3, and T4; (E, F) Plasma insulin levels during OGTT for groups NGT and AGM respectively during periods T1, T2, T3, and T4; NGT, group normal-glucose tolerance; AGM, group abnormal-glucose metabolism. Numeric data are expressed as means ± SD and graphic data are expressed as means ± SE bars, black line and symbol (T1 period: first evaluation 11 patients NGT; 9 patients AGM), red line and symbol (T2 period: pre-surgery 11 patients NGT; 9 patients AGM), blue line and symbol (T3 period: 9 months after RYGB 11 patients NGT; 9 patients AGM), and pink line and symbol (T4 period: 10 years after RYGB 9 patients NGT; 4 patients AGM)
Results
Groups were matched for age and gender; as shown in Table 1, NGT and AGM initially presented BMI of 46.31 ± 5.03 kg/m2 and 50.87 ± 10.31 kg/m2, respectively, and a high percentage of body fat of 44.29 ± 4.27% and 47.33 ± 5.07% for NGT and AGM, respectively. Both groups showed an increase in BMI after 10 years and remained obesity grade I with BMI for NGT 32.45 ± 4.99 kg/m2 and AGM 34.85 ± 4.46 kg/m2.
The waist measurement comparing T3 and T4 periods showed an increase, 6.98% for the NGT group, and 15.16% for the AGM group. Regarding the body fat mass percentage between the T1 and T3 periods, the NGT group showed a reduction from 44.29 ± 4.27 to 28.26 ± 4.72, and a fat mass regain of 28.26 ± 4.72 at T3 and 37.17 ± 6.41 at T4. The AGM group showed a reduction in body fat mass percentage from 47.33 ± 5.07 at T1 to 32.74 ± 5.92 at T3, and a regain at T4 38.20 ± 4.94.
The mean weight in kilogrammes at T1 was NGT 122.84 kg ± 23.82 and AMG 114.86 kg ± 16.35. After 18 months of RYGB (moment of maximum loss), the mean was NGT 66.60 kg ± 12.49 and AMG 68.60 kg ± 16.36. The percentage of loss between these two moments was NGT 54.64% ± 7.23 and AMG 60.64% ± 11.54. And 10 years after RYGB, the mean weight was NGT 83.48 kg ± 17.055 and AMG 85.23 kg ± 13.75 and the percentage of weight regain between 18 months and 10 years after RYGB was NGT 26.37% ± 23.26 and AMG 26.34% ± 11.75.
Regarding the HOMA-IR and ΔHOMA-IR evaluations, Table 2 shows that NGT and AGM presented a significant reduction of the values over time. AUGC total showed a decrease from T1 to T3 periods for both groups, and at T4 period, the values return to be similar to T1. Fasting insulin (Table 2) in NGT and AMG have levels compatible with IR at T1, and at T4 period, fasting insulin levels became normality. The HbA1c at T4 for the NGT group was 5.5 ± 0.31% and the AMG group was 6.4 ± 1.29.
According to the plasma glucose curves of NGT (Fig. 1A) and AGM (Fig. 1B), patients presented an average glycaemic level of 117.8 ± 17.53 mg/dL and 202.07 ± 32.82 mg/dL respectively at 120 min for T1 period. When analysed separately, these groups showed a significant reduction in the mean plasma glucose levels after RYGB T4 period at 120 min after OGTT with NGT 55.49 ± 17.15 mg/dL and AMG 124.58 ± 60.94 mg/dL (p˂0.001), with glycaemic levels of the NGT and AGM groups statistically different at 120 min. Observing glucose curve of the AMG group (Fig. 1B), the glycaemia means at t30, t60, and t90 min are above 200 mg/dL. For the NGT group (Fig. 1A), it is noted that the mean 120-min glycaemia at T4 period is below 60 mg/dL.
For the primary analysis using GEE model when comparing NGT (Fig. 1A) with AGM (Fig. 1B), there was a significant change (p < 0.0001) between means of T1 vs. T2, T1 vs. T3, T2 vs. T3, T3 vs. T4, time intervals between 0 vs. all times (30, 60, 90, and 120 min), 30 min vs. 120 min, 60 min vs. 90 min and 120 min, and 90 min vs. 120 min.
Fig. 1C and D show the mean plasma insulin levels during OGTT for the NGT and AGM groups performed at T1, T2, T3, and T4. There was a change from T1 to T3 at the NGT and AGM groups, and a significant reduction from the first hour of OGTT, including fasting values. At T4 period, the NGT group had mean insulin levels increased at t30, t60 and t0, and t90 and t120 which were lower compared to T1. However, the AMG group had mean insulin levels at T4 lower than those at T1 and reduction at t90 and t120 min
Fig. 1E and F show the results of mean plasma levels of GLP-1 during the OGTT. The GLP-1 curves are statistically different (p = 0.001) for the NGT and AGM groups. From T3 to T4 periods, there is a significant increase (p < 0.0001) in GLP-1 secretion, at times 30 and 60 min. The AGM group at T4 had a higher increase in GLP-1; t30 of OGTT for AMG was 63.85 ± 37.98 pmol/L (Fig. 1F) when compared to NGT and t30 OGTT was 50.73 ± 24.82 pmol/L (Fig. 1E)
Discussion
Currently, RYGB is one of the most effective methods for treating morbid obesity, especially when the patient is unsuccessful after trying clinical, nutritional, and drug treatment [39,40,41,42].
Postoperatively, there are several long-term benefits for patients, such as control of systolic and diastolic pressures; normalization of serum levels of total cholesterol, triglycerides, uric acid and HDL cholesterol fraction, and decreasing cardiovascular risk; reduction in cases of asthma and obstructive sleep apnea, improvement in glycaemic levels and, consequently, carbohydrate metabolism, as well as decreased risk of type 2 diabetes; and psychological improvement, influencing self-esteem, social relationship, and reducing anxiety and depression [43, 44].
In our study, at T4 period, the BMI was NGT 32.45 ± 4.99 kg/m2 and AGM 34.85 ± 4.46 kg/m2, despite the weight regain that the patients had; according to Magro [21], there was no surgical failure (the failure is considered when the patient has BMI > 35 kg/m2). And even with weight and waist regain, these values remained lower in T4 when compared to T1; so, it is important to note that even with this weight recovery, NGT still have an improvement of metabolic levels.
Another factor evaluated was GLP-1 levels, this hormone has been studied after RYGB and it is related to the increase or even restoration of glucose-induced insulin secretion [19, 45]. Roux-en-Y gastric bypass (RYGB) is a bariatric surgery developed by Mason and Ito in 1967 for the management of obesity [46]. It is well known that GLP-1 mechanism and its potential increased levels in patients after RYGB [19, 45]. It has been hypothesized that the early delivery of nutrients to the distal gut may lead to improvement in diabetes after RYGB, the exact mechanism by which GLP-1 levels increase after RYGB remains unclear. In the hindgut hypothesis, secretions and nutrients reach the distal ileum and colon more quickly following gastric bypass, which may lead to more stimulation of L-cells and therefore a higher level of GLP-1 [47]. A recent [48] publication demonstrated that GLP-1 expressing cells are located in the stomach and contribute to the active levels of GLP-1 in the portal vein. These cells undergo changes in plasticity after bariatric surgeries in rats and obese individuals and can modulate gastric functions locally, contributing to the control of glucose homeostasis.
At T4 period, in the NGT group, GLP-1 levels increased at t30 and t60 min of OGTT and this fact is concomitant with the increase of insulin that occurs at t30–t60 min of OGTT. After this period, both levels decreased. However, the AGM group had at T4 expressive levels of GLP-1; they did not show corresponding insulin levels (Supplementary 1), probably this happens because this group patients were DM2 or were progressing to the clinical picture of DM2, so we must consider a possible beta cell degeneration process or beta cell loss (a probable pancreatic insufficiency) [49] so in our study there was no coincidence of the GLP-1 and insulin curves in the AMG after 10 years of RYGB; also, the AGM group at T4 had HbA1c 6.4 ± 1.29%. Another point we must consider is that we did not do the OGTT with an antagonist of GLP-1, like Jørgensen et al. [7] did to better elucidated the relation of GLP-1 and insulin levels.
The role of GLP-1 in T2DM remission after bariatric surgery is difficult to determinate in humans, because of other predictive factors such as duration of disease and degree of b-cell destruction before surgery; those may be more critical in determining whether b-cells can recover sufficiently to resolve T2DM [49]. These findings could be an explanation for maintaining normalization of blood glucose and insulin levels as well as part of the resolution of type 2 diabetes after the RYGB [50, 51]. In a recent study, Mingrone [52] was found that metabolic surgery resulted in continuous remission of DM2 within 10 years in 37.5% of patients with advanced type 2.
Improvement in glucose metabolism after weight loss occurs in either type 2 diabetes, carbohydrate-intolerant, or healthy oral glucose-tolerant patients, and in some cases, they have drug doses reduction [49, 53]. We observed a significant improvement in the blood glucose levels after OGTT specially at the NGT group. The AGM group presented levels higher than the NGT at T4. AMG also at T4 had mean glucose levels above T1, so these mean levels at T4 were still compatible with DM2 levels as ADA18 [22]. At the NGT group, even though had weight regain, the HOMA-IR levels are below value considered reference for IR diagnosis [54], demonstrating evidence of metabolic improvement for NGT after 10 years of RYGB. Moreover, in a previous study, this same group of morbidly obese individuals undergoing RYGB has demonstrated increased GLP-1 secretion at 9 months after RYGB and an improvement of insulin sensitivity, a fact that was reinforced by findings in other studies [55] and a study made by our group; postoperative RYGB patients were evaluated by a hyperinsulinemic-euglycemic clamp, and it was found that weight loss was associated with increased insulin sensitivity and decreased basal insulin secretion, with patients reaching values similar to the control group [56, 57].
It was also observed at the NGT group that even though there was weight regain at T4, HOMA-IR decreased throughout periods with a statistical difference between them; and at T4, the levels were lower compared to T1. The AGM group showed an important reduction in HOMA-IR when compared to the NGT group, but there was a decrease in the mean insulin levels also at T4 which may have decreased without leading an improvement in DM2; and at T4, the AMG group still has HbA1c 6.4%, according to ADA 18 [22], lower value for DM2 diagnosis.
We also observed that 55% of patients in the NGT group at T4 period had blood glucose lower than 60 mg/dL. Between these patients, the mean of blood glucose was 42 mg/dL at 120 min of OGTT, which was accompanied by symptoms such as nausea, vomiting, diarrhoea, sweating, and headache in some of them. This picture was promptly reverted with intravenous glucose infusion, which was made after the last collected blood sample at time 120 min of the OGTT. Also, at the 30 min of OGTT, GLP-1 secretion in T4 was 12.3 times higher than T1 in the NGT group. One study evaluated postoperative patients and observed very high levels of GLP-1 after RYGB, and such patients presented severe hypoglycaemia after RYGB with GLP-1 concentrations 10 to 20 times higher than usual [58, 59]. Moreover, at T4, the NGT group had glucose levels NGT = 55.49 ± 17.15 mg/dL, so a plausible explanation that hypoglycaemia presented by several patients undergoing this surgical procedure could be due to elevated GLP-1 levels.
It is important to note that several other authors have evaluated the consequences of the RYGB but most of them in the early postoperative period (2–5 years). PubMed survey [6, 60,61,62] and very few articles [19, 20] have shown GLP-1 after 10 years of RYGB, with our study exclusively assessing the same patients before and after 10 years of the RYGB.
This study has shown that after 10 years of RYGB, patients had regained weight, but the metabolic improvement remained specially at the NGT group; this fact can be attributed to the high levels of GLP-1 that these patients presented even after 10 years of RYGB. In order to better understand the benefits and consequences of this surgery through insulin secretion, glucose, and GLP-1 levels, further research is needed.
Study limitations
An important limitation of this study refers to the sample size, which is presented in a small number, allowed to consider the results found only in the population in question.
In addition, the fact that OGTT was not performed with an antagonist of GLP-1, like Jørgensen et al. did [7] to better understand long-term DM2 reversal, and also, we did not measure the C-peptide to assess insulin secretion.
However, with the absence of long-term follow-up articles using the same patients, the results of this research can be further complemented with future studies that will focus on the long-term consequences of a RYGB surgical procedure.
Conclusion
According to the results found in this study, it was noted by the evaluation of the same patients during 4 different times in the 10-year post-operatory RYGB period; even with weight regain, high levels of GLP-1 remained which can be associated with metabolic improvement specially at normal glucose-tolerant obese. Therefore, long-term follow-up after RYGB studies, using the same patients, is needed to better understand the mechanisms involved in the metabolic improvement.
References
Sjöström L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B et al (2004) Lifestyle, diabetes and cardiovascular risk factor ten years after bariatric surgery. N Engl J Med 351:2683–2693
Adams TD, Gress RE, Smith SC, Halverson RC, Simper SC, Rosamond WD et al (2007) Long-term mortality after gastric bypass surgery. N Engl J Med 357:753–761
Kashyap SR, Bhatt DL, Wolski K, Watanabe RM, Abdul-Ghani M, Abood B et al (2013) Metabolic effects of bariatric surgery in patients with moderate obesity and type 2 diabetes: analysis of a randomized control trial comparing surgery with intensive medical treatment. Diabetes Care 36:2175–2182
Holst J, Madsbad S (2016) Mechanisms of surgical control of type 2 diabetes: GLP-1 is key factor. Surg Obes Relat Dis 12:1236–1242
Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K et al (2004) Bariatric surgery: a systematic review and meta-analysis. JAMA 292:1724–1737
Laferrère B, Heshka S, Wang K, Khan Y, McGinty J, Teixeira J, Hart AB, Olivan B (2007) Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care 30:1709–1716
Jørgensen NB, Dirksen C, Bojsen-Møller KN, Jacobsen SH, Worm D, Hansen DL, Kristiansen VB, Naver L, Madsbad S, Holst JJ (2013) Exaggerated glucagon-like peptide 1 response is important for improved β-cell function and glucose tolerance after Roux-en-Y gastric bypass in patients with type 2 diabetes. Diabetes 62(9):3044–3052. https://doi.org/10.2337/db13-0022
Dirksen C, Bojsen-Møller KN, Jørgensen NB, Jacobsen SH, Kristiansen VB, Naver LS, Hansen DL, Worm D, Holst JJ, Madsbad S (2013) Exaggerated release and preserved insulinotropic action of glucagon-like peptide-1 underlie insulin hypersecretion in glucose-tolerant individuals after Roux-en-Y gastric bypass. Diabetologia 56(12):2679–2687. https://doi.org/10.1007/s00125-013-3055-1
Bojanowska E (2005) Physiology and pathophysiology of glucagon-like peptide-1 (GLP-1): the role of GLP-1 in the pathogenesis of diabetes mellitus, obesity, and stress. Med Sci Monit 11:RA271–RA278
Gutzwiller JP, Degen L, Heuss L, Beglinger C (2004) Glucagon-like peptide 1 (GLP-1) and eating. Physiol Behav 82:17–19
Knop FK, Aaboe K, Vilsbøll T, Vølund A, Holst JJ, Krarup T et al (2012) Impaired incretin effect and fasting hyperglucagonaemia characterizing type 2 diabetic subjects are early signs of dysmetabolism in obesity. Diabetes Obes Metab 14:500–510
Holst JJ (2007) The physiology of glucagon-like peptide 1. Physiol Rev 87:1409–1439
Jiménez A, Casamitjana R, Flores L, Delgado S, Lacy A, Vidal J (2013) GLP-1 and the long-term outcome of type 2 diabetes mellitus after Roux-en-Y gastric bypass surgery in morbidly obese subjects. Ann Surg 257:894–899
de Carvalho CP, Marin DM, de Souza AL, Pareja JC, Chaim EA, de Barros MS et al (2009) GLP-1 and adiponectin: effect of weight loss after dietary restriction and gastric bypass in morbidly obese patients with normal and abnormal glucose metabolism. Obes Surg 19:313–320
Dirksen C, Jørgensen NB, Bojsen-Møller KN, Jacobsen SH, Hansen DL, Worm D, Holst JJ, Madsbad S (2012) Mechanisms of improved glycaemic control after Roux-en-Y gastric bypass. Diabetologia 55(7):1890–1901. https://doi.org/10.1007/s00125-012-2556-7
Kashyap SR, Daud S, Kelly KR et al (2010) Acute effects of gastric bypass versus gastric restrictive surgery on beta-cell function and insulinotropic hormones in severely obese patients with type 2 diabetes. Int J Obes (Lond) 34(3):462–471. https://doi.org/10.1038/ijo.2009.254
Holdstock C, Zethelius B, Sundbom M, Karlsson FA, Edén EB (2008) Postprandial changes in gut regulatory peptides in gastric bypass patients. Int J Obes (Lond) 32(11):1640–1646. https://doi.org/10.1038/ijo.2008.157
Jørgensen NB, Bojsen-Møller KN, Dirksen C et al (2019) Sustained Improvements in Glucose Metabolism Late After Roux-En-Y Gastric Bypass Surgery in Patients with and Without Preoperative Diabetes. Sci Rep 9:15154. https://doi.org/10.1038/s41598-019-51516-y
Dar MS, Chapman WH 3rd, Pender JR, Drake AJ 3rd, O’Brien K, Tanenberg RJ et al (2012) GLP-1 response to a mixed meal: What happens ten years after Roux-en Y Gastric Bypass (RYGB)? Obes Surg 22:1077–1083
Santo MA, Riccioppo D, Pajecki D, Kawamoto F, de Cleva R, Antonangelo L et al (2016) Weight regain after gastric bypass: influence of gut hormones. Obes Surg 26:919–925
Magro DO, Geloneze B, Delfini R, Pareja BC, Callejas F, Pareja JC (2008) Long-term Weight Regain after Gastric bypass: A 5-year Prospective Study. Obes Surg 18:648–651
American Diabetes Association Report ADA (2018) Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2018. Diabetes Care 2018(41):S13–S27
Kahles F, Meyer C, Möllmann J, Diebold S, Findeisen HM, Lebherz C, Trautwein C, Koch A, Tacke F, Marx N, Lehrke M (2014) GLP-1 Secretion Is Increased by Inflammatory Stimuli in an IL-6–Dependent Manner, Leading to Hyperinsulinemia and Blood Glucose Lowering. Diabetes 63(10):3221–3229
de Souza AH, Tang J, Yadev AK et al (2020) Intra-islet GLP-1, but not CCK, is necessary for β-cell function in mouse and human islets. Sci Rep 10:2823
Ellingsgaard H, Hauselmann I, Schuler B et al (2011) Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells. Nat Med 17:1481–1489
Fulcher GR, Farrer M, Walker M, Rodham D, Clayton B, Alberti KM (1991) A comparison of measurements of lean body mass derived by bioelectrical impedance, skinfold thickness and total body potassium. A study in obese and non-obese normal subjects. Scand J Clin Lab Invest 51(3):245–253. https://doi.org/10.3109/00365519109091611
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment; insulin resistance and beta cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419
McAuley KA, Williams SM, Mann JI, Walker RJ, Lewis-Barned NJ, Temple LA et al (2001) Diagnosing insulin resistance in the general population. Diabetes Care 24:460–464
Radziuk J (2000) Insulin sensitivity and its measurement: structural commonalities among the methods. J Clin Endocrinol Metab 85:4426–4433
Wallace TM, Levy JC, Matthews DR (2004) Use and abuse of HOMA modeling. Diabetes Care 27:1487–1495
Conover WJ (1998) Practical nonparametric statistics. New York: Wiley
Conover W, Iman R (1981) Rank Transformations as a Bridge Between Parametric and Nonparametric Statistics. Am Stat 35(3):124–129. https://doi.org/10.2307/2683975
Liang K, Zeger S (1986) Longitudinal Data Analysis Using Generalized Linear Models. Biometrika 73(1):13–22. https://doi.org/10.2307/2336267
Allison PD (2012) Handling missing data by maximum likelihood. Keynote presentation at the SAS Global Forum. Retrieved from http://support.sas.com/resources/papers/proceedings12/312–2012.pdf
Stokes ME, Davis CS, Koch GG (2000) Categorical data analysis using SAS system 2nd edition. Cary
Paul S, Zhang X (2014) Small sample GEE estimation of regression parameters for longitudinal data. Stat Med 33:3869–3881. https://doi.org/10.1002/sim.6198
Li Z, McKeague IW (2013) Power and Sample Size Calculations for Generalized Estimating Equations via Local Asymptotics. Stat Sin 23(1):231–250. https://doi.org/10.5705/ss.2011.081
SAS INSTITUTE Inc. SAS/STAT. User’s guide, version 9.4. Cary: SAS Institute, 2002–2012
Zilberstein B, Galvão Neto M, Ramos AC (2002) O papel da cirurgia no tratamento da obesidade. Rev Bras Med São Paulo 59(4):258–264
Picot J, Jones J, Colquitt JL, Gospodarevskaya E, Loveman E, Baxter L, Clegg AJ (2009) The clinical effectiveness and cost-effectiveness of bariatric (weight loss) surgery for obesity: a systematic review and economic evaluation. Health Technol Assess 13(41):1-190-215–357, iii–iv. https://doi.org/10.3310/hta13410
Costa JV, Duarte JS (2006) Tecido adiposo e adipocinas. Acta Med Port 19:251–256
Sjöström L (2013) Review of the key results from the Swedish Obese Subjects (SOS) trial - a prospective controlled intervention study of bariatric surgery. J Intern Med 273:219–234
Mango VL, Frishman WH (2006) Physiologic, psychologic, and metabolic consequences of bariatric surgery. Cardiol Rev 14(5):232–237
Sjöström L, Peltonen M, Jacobson P, Sjöström CD, Karason K, Wedel H et al (2012) Bariatric surgery and long-term cardiovascular events. JAMA 307:56–65
Drucker DJ (2001) Minireview: the glucagon-like peptides. Endocrinology 142:521–527
Mason EE, Ito C (1967) Gastric bypass in obesity. Surg Clin North Am 47:1345–1351
Nausheen S, Shah IH, Pezeshki A et al (2013) Effects of sleeve gastrectomy and ileal transposition, alone and in combination, on food intake, body weight, gut hormones, and glucose metabolism in rats. Am J Physiol Endocrinol Metab 305:E507–E518. https://doi.org/10.1152/ajpendo.00130.2013
Ribeiro-Parenti L, Jarry AC, Cavin JB et al (2021) Bariatric surgery induces a new gastric mucosa phenotype with increased functional glucagon-like peptide-1 expressing cells. Nat Commun 12:110. https://doi.org/10.1038/s41467-020-20301-1
Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Leccesi L et al (2012) Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med 366:1577–1585
Hickey MS, Pories WJ, MacDonald KG Jr, Cory KA, Dohm GL, Swanson MS et al (1998) A new paradigm for type 2 diabetes mellitus: could it be a disease of the foregut? Ann Surg 227:637–643. discussion 643-44
Nannipieri M, Baldi S, Mari A, Colligiani D, Guarino D, Camastra S, Barsotti E, Berta R, Moriconi D, Bellini R, Anselmino M, Ferrannini E (2013) Roux-en-Y gastric bypass and sleeve gastrectomy: mechanisms of diabetes remission and role of gut hormones. J Clin Endocrinol Metab 98(11):4391–4399
Mingrone G, Panunzi S, De Gaetano A et al (2021) Metabolic surgery versus conventional medical therapy in patients with type 2 diabetes: 10-year follow-up of an open-label, single-centre, randomised controlled trial. Lancet 397:293–304. https://doi.org/10.1016/S0140-6736(20)32649-0
Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Brethauer SA, Navaneethan SD et al (2014) Bariatric surgery versus intensive medical therapy for diabetes – 3-year outcomes. N Engl J Med 370:2002–2013
Geloneze B, Tambascia MA (2006) Avaliação laboratorial e diagnóstico da resistência insulínica. Arq Bras Endocrinol Metab 50:208–215
Jorgensen NB et al (2012) Acute and long-term effects of Roux-en-Y gastric bypass on glucose metabolism in subjects with Type 2 diabetes and normal glucose tolerance. Am J Physiol Endocrinol Metab 303:E122-131
Marin DM (2007) Resistencia a insulina e função da celula ‘beta’: efeito da perda de peso após bypass gastrico. 188p. Tese (doutorado) - Universidade Estadual de Campinas, Faculdade de Ciências Médicas, Campinas, SP. Available : http://repositorio.unicamp.br/jspui/handle/REPOSIP/311986 Access: Mar 2021
Bojsen-Møller KN, Dirksen C, Jørgensen NB et al (2014) Early enhancements of hepatic and later of peripheral insulin sensitivity combined with increased postprandial insulin secretion contribute to improved glycemic control after Roux-en-Y gastric bypass. Diabetes 63:1725–1737
Patti ME, McMahon G, Mun EC, Bitton A, Holst JJ, Goldsmith J et al (2005) Severe hypoglycaemia post-gastric bypass requiring partial pancreatectomy: evidence for inappropriate insulin secretion and pancreatic islet hyperplasia. Diabetologia 48:2236–2240
Kandel D, Bojsen-Moller KN, Svane MS et al (2019) Mechanisms of action of a carbohydrate-reduced, high-protein diet in reducing the risk of postprandial hypoglycemia after Roux-en-Y gastric bypass surgery. Am J Clin Nutr 110:296–304
Korner J, Bessler M, Inabnet W, Taveras C, Holst JJ (2007) Exaggerated glucagon-like peptide-1 and blunted glucose-dependent insulinotropic peptide secretion are associated with Roux-en-Y gastric bypass but not adjustable gastric banding. Surg Obes Relat Dis 3:597–601
le Roux CW, Aylwin SJ, Batterham RL, Borg CM, Coyle F, Prasad V et al (2006) Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg 243:108–114
Whitson BA, Leslie DB, Kellogg TA, Maddaus MA, Buchwald H, Billington CJ et al (2007) Entero-endocrine changes after gastric bypass in diabetic and nondiabetic patients: a preliminary study. J Surg Res 141:31–39
Acknowledgements
We thank all those who somehow contributed to this project. We specially thank Daniel Henrique do Amaral Corrêa, Ph.D., for the bibliographical references, organization, and statistical analysis.
Author information
Authors and Affiliations
Contributions
Conception or design of the work: Camila Carvalho, Elintom Adami Chaim, and Sarah Monte Alegre. Data collection: Camila Carvalho, Joelma Silvia Giorgetti, Viviane Bressane Claus Molina, Gisele Almeida Batista, Louise Franciscato Trivelato Duran, and Débora Puzzi Fernandes. Data analysis and interpretation: Aglécio Luiz de Souza, Sarah Monte Alegre, and Camila Carvalho. Drafting the article: Camila Carvalho, Elintom Adami Chaim, Sarah Monte Alegre, and Raquel Gonçalves. Critical revision of the article: Camila Carvalho, Elintom Dami Chaim, Sarah Monte Alegre, and Aglécio Luiz de Souza. Final approval of the version to be published: Camila Carvalho, Aglécio Luiz de Souza, Gisele Almeida Batista, Louise Franciscato Trivelato Duran, Débora Puzzi Fernandes, Viviane Bressane Claus Molina, Raquel Gonçalves, Joelma Silvia Giorgetti1, Elintom Adami Chaim, and Sarah Monte Alegre.
Corresponding author
Ethics declarations
Ethics approval
The study had protocol approved by the Research Ethics Committee of University of Campinas-UNICAMP, School of the Faculty of Medical Sciences (FCM-UNICAMP) number 31989314.3.0000.5404.
Ethical statement
All procedures performed in this study were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments. This study was approved by the institutional review board.
Informed consent statement
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Carvalho, C., de Souza, A.L., Batista, G.A. et al. GLP-1: 10-year follow-up after Roux-en-Y gastric bypass. Langenbecks Arch Surg 407, 559–568 (2022). https://doi.org/10.1007/s00423-021-02341-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00423-021-02341-3