Abstract
Background
A simple model to predict nonalcoholic steatohepatitis (NASH) in patients with nonalcoholic fatty liver disease is desirable to optimize the selection of patients for liver biopsy. We investigated a large group of morbidly obese patients to derive a scoring system based on simple clinical and laboratory variables.
Methods
Consecutive subjects undergoing bariatric surgery and without evidence of other liver disease or significant alcohol use underwent intraoperative liver biopsy. Demographic, clinical, and biochemical variables were collected. A scoring model was derived using variables found to be independent predictors of NASH. The scores were divided into four risk categories (low, intermediate, high, and very high). Positive and negative predictive values (PPV/NPV) were derived for each category and the area under the receiver operator curve (AUROC) was calculated.
Results
A total of 253 subjects were included: 52 (20.6%) had NASH, 116 (45.8%) had simple steatosis, and 85 (33.6%) had normal liver histology. Only ten subjects (19% of NASH group) had significant (≥ stage 2) fibrosis. Multivariate analysis identified diabetes, abnormal ALT, and hypertriglyceridemia as independent predictors of NASH. Sleep apnea showed a strong trend toward significance and was also included in the model. This model showed a NPV of 89.7% in the low risk category and a PPV of 75% in the very high risk category, with AUROC of 0.76.
Conclusions
A simple scoring system performs well in predicting NASH and can be used in the clinic to optimize the selection of morbidly obese patients for liver biopsy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With the increasing prevalence of obesity, diabetes, and metabolic syndrome in the general population, nonalcoholic fatty liver disease (NAFLD) has become the most common cause of chronic liver disease in the USA [1, 2]. Nonalcoholic steatohepatitis (NASH) is an advanced NAFLD phenotype that can progress to cirrhosis with associated increase in the risk of hepatocellular carcinoma and liver-related death.[3–5]
As NASH is a histological diagnosis, liver biopsy is needed to definitively establish or rule it out. However, liver biopsy is an invasive procedure and is associated with an appreciable expense and significant complication rate of around 1% [6]. Therefore, noninvasive means of selecting only the patients at the highest risk for NASH and/or fibrosis to undergo biopsy are highly desired. Alternatively, if the lowest-risk patients are reliably identified noninvasively, unnecessary liver biopsies could be avoided.
Several prior studies have identified clinical risk factors for NASH and fibrosis in different populations of patients [7–10]. However, these risk factors vary by study and their use in objectively quantifying patients’ risk of having NASH is unclear. Our aim was to investigate a large group of morbidly obese patients undergoing bariatric surgery in order to identify predictors of NASH and derive a scoring system based on simple clinical and laboratory variables which would help in selecting patients for liver biopsy.
Methods
Study Population
The study included all patients undergoing bariatric surgery as treatment of morbid obesity at the Medical College of Wisconsin between June 2006 and June 2008. Data was collected retrospectively using patient records and laboratory values obtained at the time of surgery, which were standardized for all patients. A liver biopsy was performed on all patients during the bariatric procedure.
In order to be considered for surgery, patients had to have a BMI ≥40 kg/m2 or a BMI >35 kg/m2 with other obesity-related co-morbidities. All patients had failed to lose weight or maintain weight loss with nonsurgical weight reduction methods. Those patients who were <18 years old, were HIV positive, had alcohol intake >20 g/day, known liver disease, or were taking medications known to cause NAFLD (e.g., steroids, amiodarone, valproate, or methotrexate) were excluded from the study. All subjects had serum hepatitis C antibody, hepatitis B surface antigen, and iron stain of the biopsy performed. Ceruloplasmin levels (in subjects younger than 35 years), anti-nuclear antibodies, anti-smooth muscle antibodies, anti-mitochondrial antibodies, and alpha-1-antitrypsin level were checked in patients who were found to have more than simple steatosis on biopsy. Those who had other causes of liver disease as determined by positive serologic testing and supportive liver biopsy findings were also excluded from the study.
A liver biopsy was performed during surgery. The tissue specimen was placed in formalin and stained with H&E, Masson trichrome, Perl’s, and reticulin stains. All liver biopsy samples were read by an expert GI and liver pathologist (R.K.). The pathologist semi-quantitatively scored the individual histological features/sub-phenotypes, including steatosis, lobular and portal inflammation, hepatocellular ballooning, Mallory’s hyaline and fibrosis according to the scoring system suggested by the NIH NASH Clinical Research Network working group [11]. Using a strict pathologic protocol which is based on criteria used by Dixon [8] to define NASH, each liver biopsy specimen was classified as one of four categories: (1) hepatic steatosis alone, (2) possible NASH (>5% steatosis plus one of the following zone 3 centrilobar findings: lobular inflammation, hepatocyte ballooning with or without Mallory’s hyaline, pericellular/perisinusoidal fibrosis), (3) definite NASH (>5% steatosis plus two of the following zone 3 centrilobar findings: lobular inflammation, hepatocyte ballooning with or without Mallory’s hyaline, pericellular/perisinusoidal fibrosis), or 4) normal. Patients classified into groups 2 and 3 were considered to have NASH for the purposes of the analysis. Each biopsy was assigned a NAFLD Activity Score (NAS) based on the sum of steatosis grade, lobular inflammation, and hepatocyte ballooning scores, as described by Kleiner et al. [11].
Variables of Interest
Medical records were reviewed, and the following variables were specifically sought and recorded, to be considered as possible predictors of NASH in the analysis: demographics (sex, age, race), history of alcohol use, type 2 diabetes mellitus (DM), hypertension (HTN), hyperlipidemia, obstructive sleep apnea (OSA), all medications being taken at the time of surgery as well as height, weight, and body mass index (BMI) measurements before the surgery. DM was defined by having a documented diagnosis in the patient’s medical record and/or being on anti-diabetic medications. Hypertension was defined by having a documented diagnosis in the patient’s medical record and/or being on anti-hypertensive medications. Hyperlipidemia was defined by laboratory findings fulfilling the ATP III criteria and/or being on hypolipidemic medications. Obstructive sleep apnea was defined by having a documented diagnosis in the patient’s medical record and/or diagnostic sleep study in our records. The medications recorded were: (1) metformin, (2) sulfonylureas, (3) thiazolidinediones, (4) insulin, (5) ACE inhibitors, (6) angiotensin receptor blockers, (7) hydrochlorothiazide, (8) fibrates, (9) statins, (10) nonsteroidal anti-inflammatory agents, (11) vitamins E and C, and (12) multivitamins. Laboratory testing included serum aminotransferases (ALT and AST), bilirubin, albumin, platelets, fasting glucose, total cholesterol, triglycerides (TG), and in most diabetic patients, hemoglobin A1C level (HgbA1c). The cutoffs used in our laboratory for normal ALT and AST levels are 40and 45 U/L, respectively.
Statistical Analysis
Statistical analysis was performed using Stata 9.2 (Statacorp, College Station, TX, USA). Our primary outcome of interest was the presence of NASH on liver biopsy. Continuous variables were summarized using means and standard deviation (median for skewed variables) and categorical variables were expressed in proportions. Continuous variables were compared using the t test while categorical variables were compared using the chi-square test with the Fisher’s exact test being used when appropriate. As the initial step in our analysis, univariate logistic regression was performed to identify factors associated with NASH. Predictor variables that were significant in the univariate analysis at p < 0.1 were included in the final multivariate model. Multivariate logistic regression with stepwise backward selection was then used to identify independent predictors of NASH. A p value <0.05 was considered significant in this analysis. The multivariate model was then repeated and included only the significant variables. The likelihood ratio test was used to arrive at the most parsimonious model with the highest discriminative capacity in distinguishing patients with NASH. The Hosmer–Lemeshow test was used to confirm goodness of fit. The area under the receiver operating curve (AUROC) was calculated for each model along with its 95% confidence interval.
The second part of the analysis consisted of developing the NASH risk score. For this analysis, we utilized the variables found to be significant in the final multivariate analysis. The weight for each risk factor was arrived at by dividing the regression coefficient for each of the variables by the standard error for the coefficients. The cumulative risk score was then calculated by summing up the presence of each significant risk factor multiplied by its numeric weight. Based on inspection of the risk of NASH with each risk score, the overall cumulative risk score then stratified into four strata based on low, intermediate, high, and very high likelihood of NASH on the liver biopsy. The AUROC was then calculated for the regression model utilizing the risk score strata and compared to the AUROC for the model adjusting for all the variables individually.
The study was approved by the Institutional Review Board of the Medical College of Wisconsin.
Results
Study Population
In all, 269 patient records were examined. Six subjects were excluded on the basis of having other causes of liver disease [2] or being on medications with potential for causing liver damage [4]. Ten subjects were excluded for not having complete records. A total of 253 subjects were included in the study. The demographic characteristics of the subjects are shown in Table 1. The average age was 43.2 years, and most subjects were female (86.6%) and Caucasian (80.0%). There were 52 (20.6%) subjects with NASH [41 (16.3%) with possible NASH, 11 (4.3%) with definite NASH], 85 (33.6%) with normal liver histology, and 116 (45.8%) with simple steatosis. Ten subjects (19% of NASH cohort) had significant (≥ stage 2) fibrosis, four of them with cirrhosis. The average NAS for subjects without NASH was significantly lower than for subjects with NASH (0.59 vs. 2.29, p < 0.001). Subjects with NASH were significantly older (46.6 vs. 42.3 years, p = 0.02). There were no significant gender or racial differences in the prevalence of NASH, nor was there a difference in BMI.
Univariate Analysis
When various co-morbid conditions were examined as predictors of NASH in a univariate model, DM, HTN, and OSA were found to be significantly associated with NASH (Table 1). After a univariate analysis of laboratory values (Table 2), triglyceride level, as well as ALT and AST were found to be significantly higher in NASH subjects. Significantly more patients with NASH had abnormal ALT values than subjects without NASH (23.1% vs. 6.5%, p = <0.001). The same was not true for AST, however (7.7% vs. 3.5%, p = 0.185). The prevalence of hypertriglyceridemia, as defined by TG level >150 mg/dL, was also significantly greater in NASH subjects (57.7% vs. 31.8% p = 0.001).
Interestingly, HgbA1c levels, which were available in 78/80 (97.5%) diabetic subjects, were found to be significantly higher in those with NASH than in those without NASH (7.62 vs. 6.91, p = 0.05).
Among medications being taken at the time of biopsy, only ACE inhibitors (ACE-I) showed a significant association with NASH, in that 29.8% of patients on ACE-I had NASH vs. 15.1% of patients not on the medication who also had NASH (p = 0.005).
Multivariate Analysis and NASH Risk Score
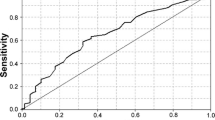
When the predictors found to have significance in the univariate analysis were included in the multivariable model, DM, abnormal ALT, and hypertriglyceridemia were found to be independently associated with the risk of NASH (Table 3), with OSA showing a trend toward statistical significance. A point score was then assigned to each variable, equal to its regression coefficient divided by the standard error (Table 3). This gave us a scoring range of 0–5. The predictive value of each score or score range is represented by a category of risk (low, intermediate, high, and very high). Prevalence of NASH at each level of risk, representing the positive predictive value (PPV), is presented in Table 4, with its complement representing the negative predictive value (NPV). Therefore, the NPV of the low risk category is 89.7% and the PPV of the very high risk category is 75%. The AUROC for the model was 0.76.
Discussion
This study was able to produce a simple scoring model based on four readily available variables that can be used clinically to assist in selection of morbidly obese subjects with suspected NAFLD for liver biopsy. The performance of this model was good despite relatively low prevalence of NASH in our cohort.
The results of our univariate analysis support results of other previous studies which showed a strong relationship between manifestations of the metabolic syndrome, such as diabetes mellitus, hypertension, hyperlipidemia, and NASH. [7, 8, 11] Only DM and hypertriglyceridemia were independently predictive of NASH in our cohort. OSA, which was found to be an independent risk factor in some studies, [12] but not others [13], showed a trend toward being an independent predictor of NASH in our study, but the trend was not statistically significant after correction for other contributing factors.
It is well-known that aminotransferase levels in many (if not most) patients with NASH are often normal or only slightly elevated [12, 14, 15]. This was also true in our cohort, where only 23.1% of NASH subjects had elevated ALT. However, that proportion was significantly higher than in subjects without NASH. NASH subjects had significantly higher mean transaminase values, and abnormal ALT, but not AST, was an independent predictor of NASH in the multivariate model. Another way of using transaminase values to predict histology is to calculate the AST to ALT ratio, with values >1 having been previously associated with NASH [16]. We did not find this association in our cohort, however (data not shown).
The variables used in our scoring model were the three independent predictors of NASH derived in the multivariate analysis (DM, hypertriglyceridemia, and abnormal ALT) in addition to OSA, which, while not being an independent predictor in the multivariate analysis, nevertheless, showed a strong trend toward statistical significance. Addition of OSA significantly improved the discriminative ability of the model, thus further justifying the inclusion of this variable in calculating the risk score. We then divided the possible scores into risk categories based on the predictive value of each score. This model, while being simple to use and incorporating variables easily obtained in the clinic, performed well in predicting the existence or absence of NASH in subjects falling into the high, very high, and low categories, with PPV for the high and very high scorers being 60% and 75%, respectively, and NPV for the low category being 89.7%. Overall, the model had a very reasonable AUROC of 0.76. We believe that patients falling into the high and very high risk categories should be recommended to undergo a liver biopsy to confirm the diagnosis and stage the possible concomitant fibrosis. On the other hand, patients falling into the low and intermediate categories could safely be followed clinically and avoid the risks associated with biopsy.
Several earlier studies investigated the clinical predictors of fibrosis in NASH subjects, and some constructed good predictive models based on those factors [17–19]. As the prevalence of significant fibrosis was low in our cohort (4%), this model could not be used to reliably predict the existence or severity of fibrosis. However, several investigators did seek to create clinical predictive models specifically for NASH. A study by Dixon et al. [8] constructed a predictive model of NASH using the insulin resistance index (IR), ALT >40 and hypertension in a group of morbidly obese patients. This model had a specificity of 0.89 and sensitivity of 0.80, with an AUROC of 0.90 for predicting NASH. While performing very well, the system used IR index, which requires laboratory values such as C-peptide and insulin levels that are not usually obtained in routine clinical practice, and thus would be difficult to apply outside of a research setting. Another well-designed study, by Campos et al. [12] identified six independent clinical predictors of NASH and used their regression coefficients to develop risk scores, with different risk categories based on those scores. The model had an AUROC of 0.80, which is comparable to our study. Having fewer variables, we believe our model would be easier to use in a busy clinical practice, however. Finally, in a paper by Gholam et al., a signal detection model was constructed using AST and DM to differentiate NASH from steatosis with nonspecific inflammation, thus demonstrating how simple clinical variables could be used reliably to predict NASH in morbidly obese patients [20].
There were other interesting findings in this study. The prevalence of NASH in our study (combining NASH and possible NASH) was only 20.6%, with 19% of those having significant fibrosis (≥ stage 2). These numbers, particularly for definite NASH prevalence, are lower than those reported in some studies of similar patient populations [10, 21, 22]. However, in the literature on fatty liver disease in bariatric surgery patients, there is a wide range of reported NASH prevalence, ranging from 9.8% [13] to 72.5% [23]. We believe that the reasons for this variability lie in the fact that the criteria for histologic diagnosis of NASH and defining its lesions (e.g., ballooning, lobular inflammation) are still debated, are often pathologist-dependent, and vary among NAFLD studies. For example, we defined subjects with >5% steatosis plus one of the following: hepatocyte ballooning, lobular inflammation, or fibrosis as having possible NASH, while other authors classified these subjects as having definite NASH [3, 21, 23]. The introduction of the NAFLD Activity Score (NAS) by Kleiner et al. [11] provided a more quantitative way to evaluate NAFLD histological activity in clinical trials. In our study, subjects with NASH had significantly higher NAS than non-NASH subjects (2.29 vs. 0.59, p < 0.001). However, the diagnosis of NAFLD phenotypes was not based on the NAS score but on the pathologist’s use of strict criteria for defining each phenotype. Indeed, Dr. Kleiner and colleagues cautioned against using the NAS numerical score instead of the pathologist’s assessment to diagnose steatohepatitis, further supporting our approach to NAFLD histological phenotyping.
Diabetes has been repeatedly shown to be strongly associated with NASH [9, 24, 25]. In addition to confirming this association, our study showed that diabetic patients with NASH had significantly worse glycemic control than diabetic patients without NASH, as demonstrated by increased HgbA1c levels (7.62 vs. 6.91, p = 0.05). This has also been previously shown by Gholam et al. [20] and may support the notion that more severe hyperglycemia may be involved in the progression of hepatic steatosis to NASH.
Currently, there are no clearly defined medication regimens for the treatment of NASH, although several agents, most notably thiazolidinediones, are actively being investigated. Ours was one of the first studies to record all medications being actively taken by the subjects at the time of liver biopsy in order to examine any potential protective or NASH-promoting effects of these drugs. A recent paper by Brunt et al. [26] showed a significant association between increased chronic portal inflammation and medications used to treat HTN, DM, and NAFLD. In our analysis, only ACE inhibitors showed a significant association with NASH, with greater proportion of patients taking these medications (29.8%) having NASH than in those not taking them (15.1%, p = 0.005). Rather than being a reflection of a detrimental effect, the higher frequency of ACE-I in NASH subjects may be a reflection of the higher percentage of DM and hypertension in the group with NASH that represent guideline recommended indications for the use of these agents. No association for thiazolidinediones, metformin, vitamin E, statins, or hydrochlorothiazide was noted with NASH in our cohort.
Our study was limited by the retrospective design, and certain important measures such as insulin resistance and length of time subjects were exposed to medications could not be obtained. However, the clinical data on each patient were collected in a standardized fashion, with each patient having a complete history obtained and the same set of laboratory values drawn right before surgery and liver biopsy. Our cohort was also among the largest reported from a single institution, making the findings more robust. While the generalizability of our findings may be limited by including only morbidly obese patients undergoing bariatric surgery, the proportion of obese individuals is growing in the United States and around the globe, which makes this data applicable to an ever growing segment of population. Moreover, the risk factors associated with NASH in this population, such as hypertriglyceridemia and diabetes, were also found to be associated with NASH in moderately obese and nonobese populations [7, 19, 27, 28] thus making it likely that our scoring system would be relevant in most patients being evaluated for the possibility of having NASH. Further validation of our risk score in an independent population is necessary, as is an analysis of its performance in a nonobese population.
In conclusion, this study shows that a simple scoring system which incorporates diabetes, OSA, ALT, and TG performs well in predicting NASH and can be used in the clinic to optimize the selection of morbidly obese patients for liver biopsy.
References
Charlton M. Nonalcoholic fatty liver disease: a review of current understanding and future impact. Clin Gastroenterol Hepatol. 2004;2(12):1048–58.
Lazo M, Clark JM. The epidemiology of nonalcoholic fatty liver disease: a global perspective. Semin Liver Dis. 2008;28(4):339–50.
Matteoni CA, Younossi ZM, Gramlich T, et al. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116(6):1413–9.
Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346(16):1221–31.
Bugianesi E, Leone N, Vanni E, et al. Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology. 2002;123(1):134–40.
Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med. 2001;344(7):495–500.
Marchesini G, Bugianesi E, Forlani G, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37(4):917–23.
Dixon JB, Bhathal PS, O'Brien PE. Nonalcoholic fatty liver disease: predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterology. 2001;121(1):91–100.
Ong JP, Elariny H, Collantes R, et al. Predictors of nonalcoholic steatohepatitis and advanced fibrosis in morbidly obese patients. Obes Surg. 2005;15(3):310–5.
Spaulding L, Trainer T, Janiec D. Prevalence of non-alcoholic steatohepatitis in morbidly obese subjects undergoing gastric bypass. Obes Surg. 2003;13(3):347–9.
Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–21.
Campos GM, Bambha K, Vittinghoff E, et al. A clinical scoring system for predicting nonalcoholic steatohepatitis in morbidly obese patients. Hepatology. 2008;47(6):1916–23.
Harnois F, Msika S, Sabate JM, et al. Prevalence and predictive factors of non-alcoholic steatohepatitis (NASH) in morbidly obese patients undergoing bariatric surgery. Obes Surg. 2006;16(2):183–8.
Wong VW, Wong GL, Tsang SW, et al. Metabolic and histological features of non-alcoholic fatty liver disease patients with different serum alanine aminotransferase levels. Aliment Pharmacol Ther. 2009;29(4):387–96.
Fracanzani AL, Valenti L, Bugianesi E, et al. Risk of severe liver disease in nonalcoholic fatty liver disease with normal aminotransferase levels: a role for insulin resistance and diabetes. Hepatology. 2008;48(3):792–8.
Sorbi D, Boynton J, Lindor KD. The ratio of aspartate aminotransferase to alanine aminotransferase: potential value in differentiating nonalcoholic steatohepatitis from alcoholic liver disease. Am J Gastroenterol. 1999;94(4):1018–22.
Angulo P, Keach JC, Batts KP, et al. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology. 1999;30(6):1356–62.
Hossain N, Afendy A, Stepanova M, et al. Independent predictors of fibrosis in patients with non-alcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009 Jun;7:1224–9.
Amarapurka DN, Amarapurkar AD, Patel ND, et al. Nonalcoholic steatohepatitis (NASH) with diabetes: predictors of liver fibrosis. Ann Hepatol. 2006;5(1):30–3.
Gholam PM, Flancbaum L, Machan JT, et al. Nonalcoholic fatty liver disease in severely obese subjects. Am J Gastroenterol. 2007;102(2):399–408.
Abrams GA, Kunde SS, Lazenby AJ, et al. Portal fibrosis and hepatic steatosis in morbidly obese subjects: a spectrum of nonalcoholic fatty liver disease. Hepatology. 2004;40(2):475–83.
Kallwitz ER, Guzman G, TenCate V, et al. The histologic spectrum of liver disease in African-American, non-Hispanic White, and Hispanic obesity surgery patients. Am J Gastroenterol. 2009;104(1):64–9.
Sorrentino P, Tarantino G, Conca P, et al. Silent non-alcoholic fatty liver disease—a clinical-histological study. J Hepatol. 2004;41(5):751–7.
Clark JM. The epidemiology of nonalcoholic fatty liver disease in adults. J Clin Gastroenterol. 2006;40 Suppl 1:S5–10.
Younossi ZM, Gramlich T, Matteoni CA, et al. Nonalcoholic fatty liver disease in patients with type 2 diabetes. Clin Gastroenterol Hepatol. 2004;2(3):262–5.
Brunt EM, Kleiner DE, Wilson LA, et al. Portal chronic inflammation in nonalcoholic fatty liver disease (NAFLD): a histologic marker of advanced NAFLD-clinicopathologic correlations from the nonalcoholic steatohepatitis clinical research network. Hepatology. 2009;49(3):809–20.
Friis-Liby I, Aldenborg F, Jerlstad P, et al. High prevalence of metabolic complications in patients with non-alcoholic fatty liver disease. Scand J Gastroenterol. 2004;39(9):864–9.
Sung KC, Ryan MC, Wilson AM. The severity of nonalcoholic fatty liver disease is associated with increased cardiovascular risk in a large cohort of non-obese Asian subjects. Atherosclerosis. 2009;203(2):581–6.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ulitsky, A., Ananthakrishnan, A.N., Komorowski, R. et al. A Noninvasive Clinical Scoring Model Predicts Risk of Nonalcoholic Steatohepatitis in Morbidly Obese Patients. OBES SURG 20, 685–691 (2010). https://doi.org/10.1007/s11695-010-0118-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-010-0118-y