Abstract
Purpose
The aim of this study was to explore risk factors of NASH and then develop a non-invasive scoring model in Chinese patients with obesity. A scoring system was then applied to assess the effect of sleeve gastrectomy on NASH.
Methods
A total of 243 patients with obesity were included and divided into NASH group and non-NASH group according to the pathological results of liver biopsy. Logistic regression was used to determine risk factors of NASH. A scoring model was derived by risk factors of NASH. Then, postoperative follow-up was performed in 70 patients.
Results
Among the 243 patients, 118 (48.56%) patients showed NASH. Multivariate logistic regression identified aspartate aminotransferase (AST) (>21.50 IU/L), high-density lipoprotein cholesterol (HDL-C) (<1.155mmol/L), and homeostasis model assessment (HOMA-IR) (>9.368) as independent risk factors of NASH. The model included above risk factors showed a negative predictive value (NPV) of 70.38% in the low-risk category and a positive predictive value (PPV) of 85.71% in the high-risk category, with the area under the receiver operator curve (AUROC) of 0.737. Bariatric surgery resulted in a sharp decline in AST and HOMA-IR and a significant increase of HDL-C. The points of scoring model were improved at 6 months after surgery.
Conclusion
A non-invasive scoring model was derived by the risk factors of NASH included AST, HDL-C, and HOMA-IR and applied to the postoperative follow-up. After sleeve gastrectomy, the above risk factors and points of scoring model were significantly improved.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The latest national statistics (2015–2019) show that the overweight rate of adults according to the Chinese standard is 34.3% and the obesity rate is 16.4% [1]. The incidence and growth rate of overweight and obesity in China ranks first in the world, and China has become the country with the largest number of overweight and obesity [2]. It is estimated that by 2030, the prevalence of overweight and obesity in Chinese adults will reach 61% [3]. Obesity has gradually become a serious public health problem in China, which is strongly associated with metabolic diseases such as type 2 diabetes, hypertension, hyperlipidemia, non-alcoholic fatty liver disease (NAFLD), and obstructive sleep apnea syndrome.
NAFLD, the manifestation of metabolic syndrome in the liver, has become one of the most common chronic liver diseases in the world [4]. NASH is a progressive inflammatory subtype of NAFLD, characterized by steatosis, hepatocyte ballooning, and intralobular inflammation, presence or absence fibrosis [5]. Obesity, one of the most critical risk factors for metabolic syndrome, has a prevalence of NAFLD and NASH as high as 91% and 37% [6]. Because most NASH patients are asymptomatic or only have atypical symptoms such as fatigue or vague abdominal pain, it is not easy to attract attention in time. But over time, NASH can develop into cirrhosis, end-stage liver disease, and even a liver transplant [7]. Therefore, early identification and treatment of patients with NASH are essential.
The gold standard for NASH diagnosis is liver biopsy, but an invasive intervention limits its widespread use as a screening and follow-up method [8]. With the surge in obesity, given the severity and the high prevalence of NASH among the obese population, identifying risk factors for NASH development to drive a non-invasive scoring system is essential. Previous studies have identified several biological and clinical risk factors for NAFLD or NASH in patients with obesity. Older age [9], abnormal liver function tests [10], body mass index (BMI) [10], systemic hypertension [11], insulin resistance [12], or type 2 diabetes mellitus (T2DM) [13] have been considered as risk factors associated with NASH or fibrosis.
Lifestyle changes have little effect on improving NASH, and there are no effective drugs for the clinical treatment of NASH [14]. Currently, the most effective treatment for improving metabolic syndrome in patients with severe obesity is bariatric surgery [15]. Therefore, bariatric surgery is considered one of the most promising treatment strategies for the treatment of metabolic syndrome, including NASH. The 1-year follow-up study confirmed that nearly 85% of patients with NASH had significant improvement [16]. Thus, bariatric surgery not only improves the histopathological manifestations of NASH, but also provides the opportunity for laparoscopic liver biopsy for NASH screening.
Despite NASH’s high prevalence and prone to progress to liver cirrhosis and liver cancer, it is under attention in clinical practice. Therefore, the aim of this study was to investigate risk factors of NASH to develop a scoring system in a cohort of Chinese patients with severe obesity. Furthermore, the effects of sleeve gastrectomy on NASH were assessed in 6 months after the surgery by the changes of risk factors and points of scoring model.
Patients and Methods
Study Design
This study included patients with obesity undergoing bariatric surgery at the General Surgery Department of Tianjin Medical University General Hospital. The same surgical team performs all surgeries. This study was conducted following the principles of the Declaration of Helsinki. The Tianjin Medical University General Hospital Institutional Review Board approved the study protocol (IRB2020-YX-029-01).
Study Population
In this study, we included a total of 30 patients with obesity underwent laparoscopic sleeve gastrectomy with intraoperative liver biopsy in the Department of General Surgery, Tianjin Medical University General Hospital between January 2020 and April 2022. We further investigated 70 patients with obesity who had undergone laparoscopic sleeve gastrectomy and performed a follow-up visit at 6 months.
Inclusion and Exclusion Criteria
Indications for bariatric surgery followed the standards established by the Chinese Society of Metabolic and Bariatric Surgery [17] as follows: Chinese patients with a BMI greater than 32.5kg/m2 or greater than 27.5kg/m2 with obesity-associated comorbidities, non-surgical treatment did not show any improvement. Inclusion criteria: aged 18 to 60 (including both ends) when signing the informed consent form; meeting the indications for laparoscopic sleeve gastrectomy; being able to comprehend and sign the informed consent. Exclusion criteria included alcohol intake greater than 210 g/week in men and 140 g/week in women, taking hepatotoxic medications; combined with other liver diseases, such as autoimmune hepatitis, hemochromatosis, Wilson disease, or alpha1-antitrypsin deficiency; and malignant disease. Due to the effect of exogenous insulin on HOMA-IR values, we excluded patients receiving insulin therapy. Figure 1 shows the flow chart of identification of the study population.
Liver Biopsy and Histologic Examination
A laparoscopic guided wedge-shaped liver biopsy of the left liver lobe was performed during bariatric surgery with two experienced surgeons. All patients provided informed consent for liver biopsy. Intraoperative and postoperative liver biopsy-related complications were recorded. The liver tissue sample was stained with hematoxylin-eosin for histopathological interpretation. All liver biopsy specimens were interpreted by an experienced pathologist, unaware of participants’ clinical and laboratory data. According to the NASH Clinical Research Network NAFLD Activity Score (NAS), three key histological features of NAFLD, including steatosis, hepatocyte ballooning, and lobular inflammation, were scored and staged [18].
The degree of steatosis <5%, 5–33%, 34–66%, and >66% was scored as 0, 1, 2, or 3, respectively. Lobular inflammation was scored as 0, 1, 2, or 3 for no inflammation, <2 lesions, 2–4 lesions, and >4 lesions/×20 fields, respectively. Ballooning was scored as 0, 1, and 2 for no ballooning, few cells, and prominent cells with ballooning, respectively. Add the scores for all three components to obtain the overall NAS. NAFLD was defined as the presence of grade 1 or more significant steatosis. NASH was diagnosed when steatosis, ballooning, and lobular inflammation each had at least one grade [18].
Data Collection
The preoperative assessment included standard clinical data, demographic information (age, sex), anthropometric measurements (weight, height, waist circumferences, and hip circumferences), blood pressure measurements, and presence of coexisting comorbidities (type 2 diabetes mellitus, systemic hypertension, hyperlipidemia). BMI was calculated as weight (in kilograms) divided by height (in m2). Waist-height ratio (WHR) was calculated as waist circumferences (cm) divided by height (cm). Hip-height ratio (HHR) was calculated as hip circumferences (cm) divided by height (cm). Waist-hip ratio (WHR) was calculated as waist circumferences (cm) divided by hip circumferences (cm). Details of alcohol consumption and use of hepatotoxic drugs were recorded.
Following an overnight fasting period of 10h, blood samples were obtained between 08:00 and 10:00 a.m. before the bariatric surgery. Laboratory testing included platelets, albumin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), γ-glutamyl transpeptidase (GGT), alkaline phosphatase (ALKP), total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), uric acid, fasting plasma glucose, fasting blood insulin, and hemoglobin A1c (HbA1c). Iron studies and hepatitis B and C serologies were also performed. Insulin resistance (IR) was assessed using the Homeostasis Model Assessment (HOMA-IR) described by Matthews [19]. The equation is the following: HOMA-IR=(insulin×glucose)/22.5, where glucose was expressed in mmol/L and insulin in uU/mL.
Postoperatively, patients were provided diet and exercise recommendations, postoperative prescriptions, and outpatient follow-up. In addition, we collected clinical data and laboratory testing results from 70 patients with obesity at 6 months after sleeve gastrectomy.
Statistical Analysis
Descriptive results were expressed as mean±standard deviation and median (interquartile range) for data with normal and non-normal distribution respectively, and categorical variables were expressed as proportions. Continuous variables were compared using the independent t-test (two-tailed) for normally distributed and the Mann-Whitney U test for not normally distributed. In contrast, categorical variables were compared using the chi-square test with the Fisher’s exact test being used when appropriate. Appropriate cutoffs for continuous variables at P<0.1 in univariate analysis were assessed using receiver operating characteristic (ROC) curves, converted to dichotomous variables and included in the final multivariate logistic regression with stepwise forward selection to identify risk factors for NASH. A non-invasive scoring system was derived according to the odds ratio value of risk factors. The points of scoring system were divided into three risk categories (low, intermediate, high). The model calibration degree was evaluated by Homer-Lemeshow test and calibration curve, and the Bootstrap resampling method was used for internal model verification and Calibration curve plotting.
We studied changes in risk factors and points of scoring system from presurgery baseline to 6 months after bariatric surgery using the Wilcoxon’s signed rank tests for ordinal variables or continuous variables when the change was not normal, or using the paired Student t test otherwise. Statistical analyses were performed using SPSS Statistics version 25.0 (IBM Corp., Armonk, NY) and R software (version 4.2.0, R Foundation). P-value <0.05 was considered significant in the study.
Results
Characteristics of Patients Undergoing Bariatric Surgery
Clinical, demographic, anthropometric, and biochemical characteristics of the study subjects are provided in Table 1. A total of 243 subjects were included in the study. There were 67 (27.57%) males and 176 (72.43%) females. NASH was diagnosed in 118 of 243 Chinese patients with severe obesity, with a prevalence of 48.56%. There was no significant difference in the prevalence of NASH between men and women (P=0.200).
The subjects were divided into two groups based on the presence or absence of NASH. Compared with the non-NASH group, the level of ALT, AST, GGT, HbA1c, TG, fasting plasma glucose, fasting blood insulin, HOMA-IR, and prevalence of type 2 diabetes were markedly elevated in the NASH group (P<0.05). The level of HDL-C in the NASH group was substantially lower than that in the non-NASH group (P<0.05). As compared with the non-NASH group, the NASH group had slightly higher body weight, BMI, waist circumstance, hip circumstance, and WHR, but the differences were not statistically significant (P>0.05). However, there was no significant difference between non-NASH and NASH groups for age, gender, the prevalence of systemic hypertension, and dyslipidemia.
Multivariate Analysis
The appropriate cutoff for the continuous variables, including ALT, AST, GGT, TG, HDL-C, HbA1c, fasting plasma glucose, fasting blood insulin, and HOMA-IR, was assessed by the ROC curves (supplementary table 1). From multivariate analysis, the AST (>21.50IU/L), HDL-C (<1.155mmol/L), and HOMA-IR (>9.368) were independent risk factors associated with NASH. A score was then assigned to each variable according to its odds ratio (Table 2).
A Non-invasive Scoring Model Development and Validation
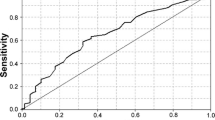
We developed a scoring system ranging of 0–9 and defined 0–3 as low risk, 4–6 as intermediate risk, and 7–9 as high risk of NASH. As shown in Table 3, prevalence of NASH at each level of risk represented the positive predictive value (PPV), while its complement represented the negative predictive value (NPV). Therefore, the NPV of the low-risk category was 70.38% and the PPV of the very high-risk category was 85.71%. The AUROC for the model was 0.737 (95%CI 0.675–0.800).
Plot the calibration curve with 1000 bootstrap resampling to evaluate the calibration of the scoring system and perform a Hosmer-Lemeshow test. The calibration curve shows that the probability estimates for NASH are in good agreement with those for a histopathological diagnosis (Fig. 2). The Hosmer-Lemeshow test was not statistically significant (P = 0.857), indicating a good fit and no deviation from the perfect fit.
Application of Non-invasive Scoring Model in Follow-up After Sleeve Gastrectomy
Comparison of risk factors and points of scoring model of 70 (21 men and 49 women, mean age was 31.21 ± 6.81 years) patients with obesity between preoperative and 6 months after sleeve gastrectomy was conducted (supplement table 2). AST level was significantly declined 6 months after surgery (25.00 (17.00–50.25) vs 16.00 (13.75–18.25), P=0.000, Fig. 3A). The percentage of AST >21.50IU/L decreased from 64.3 to 8.6% at postsurgery (Fig. 4A). However, HDL-C level was markedly increased from to 1.08 ±0.19 to 1.27±0.21 (P=0.000, Fig. 3B), accompanying with the increased percentage of HDL-C<1.155mmol/L from 65.7 to 30.0% (Fig. 4B).
AST (A), HDL-C (B), and HOMA-IR (C) levels and points of scoring model (D) before and 6 months after bariatric surgery. This figure shows a significant decrease in AST, HOMA-IR, and points of scoring model, while HDL-C levels increased significantly after bariatric surgery (both P<0.001). AST, aspartate aminotransferase; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homeostasis model assessment of insulin resistance
Percentage of AST >21.50IU/L (A), HDL-C<1.155mmol/L (B), HOMA-IR >9.368 (C) and low-, intermediate-, high-risk category (D) before and 6 months after bariatric surgery. This figure shows a significant decrease in percentage of AST >21.50IU/L, HDL-C<1.155mmol/L, and HOMA-IR >9.368, after bariatric surgery. The percentage of low risk significantly increased, while the percentage of intermediate and high risk markedly decreased after surgery. AST, aspartate aminotransferase; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homeostasis model assessment of insulin resistance
HOMA-IR improved 6 months after surgery (5.65 (4.55–9.19) vs 2.09 (1.35–2.76), P=0.000, Fig. 3C), and the percentage of HOMA-IR >9.368 was significantly reduced from 22.9 to 0% (Fig. 4C).
The points of scoring model were significantly decreased (2.50 (2.00–5.00) at preoperative vs 0.00 (0.00–3.00) at 6 months postoperative, P=0.000, Fig. 3D). The percentage of low risk (0–3) increased from 62.9 to 95.7% at postoperative, while the percentage of intermediate risk (4–6) decreased from 30.0 to 4.3%. The proportion of high risk (7–9) decreased from 7.16% before surgery to 0% after surgery (Fig. 4D).
Discussion
The present study reported the prevalence and risk factors of NASH in the severely obese Chinese population undergoing bariatric surgery. Then we investigated a non-invasive scoring model using the risk factors of NASH and observed the effect of sleeve gastrectomy on it. The prevalence of NASH in this study was 48.56%, similar to the data reported in Taiwan, China (50.5%) [10], and the same pathological diagnosis criteria of NASH were used in both studies. Yosuke Seki et al. reported the prevalence of NASH was 77.5% in Japanese patients with obesity undergoing bariatric surgery [12]. Similar studies have confirmed that 45 (33.6%) showed NASH in 134 South Indian patients with obesity undergoing bariatric surgery [13].
This study found that the prevalence of NASH in Chinese patients with obesity was much higher than expected, so early identification and intervention are needed. Although the gold standard for NASH diagnosis is liver biopsy, its invasiveness limits its use in clinical work as a routine screening method for NASH. Therefore, previous studies have identified BMI [10], insulin resistance [11], TG [6], hypertension [11], diabetes [20,21,22,23], age [9, 24], and abnormal liver function [11, 22, 23] as predictors of NASH. In our study, we explored the relationship of clinical, anthropometric, and laboratory characteristics with NASH. Ultimately, we determined that AST, HDL-C, and HOMA-IR were independent predictors of NASH.
Elevated transaminases were thought to result from hepatocyte damage in patients with NASH. Similar to previous findings, ALT, AST, and GGT in the NASH group were significantly higher than those in the non-NASH group in this study [22, 25]. Although NASH patients have higher ALT, AST, and GGT levels than non-NASH patients, the median AST and GGT values were within the normal range, except for ALT in NASH, which was elevated out of the normal range. Using the current upper limit of normal liver enzymes may underestimate the presence of NASH, so a lower cutoff may be more beneficial for predicting NASH. However, how to determine the cutoff value of transaminases as a predictor of NASH remains controversial [26, 27]. In our study, we found the cutoff values of liver enzymes for predicting NASH by ROC curve.
Unlike previous studies [10, 12, 28], AST was an independent risk factor for NASH, not ALT, in the multivariate logistic regression of this study. A possible explanation is that NASH is a progressive inflammatory from of NAFLD. In the liver, ALT is located only in the cytoplasm, while AST is 20% in the cytoplasm and 80% in the mitochondria. Due to the higher AST concentrations in the liver acinar region 3, damage to this region, either by ischemia or toxicity, may have resulted in more pronounced changes in AST levels [29]. Lipid overload, oxidative stress, and inflammation of hepatocytes act together in NASH progression, leading to hepatocyte death, which leads to liver damage, further inflammation, and tissue fibrosis [30]. It is speculated that AST is an independent risk factor for NASH in this study, which may be related to the significant increase in AST caused by severe hepatic cell damage in NASH.
As the hepatic manifestation of metabolic syndrome, NASH is closely linked to insulin resistance, type 2 diabetes, and dyslipidemia [7]. Due to the close relationship between insulin resistance and NASH, insulin resistance-related indicators may serve as predictors of NASH. Most scoring systems for predicting NAFLD use serum fasting insulin levels, serum fasting c-peptide levels, HOMA-IR, or T2DM [10, 11, 31]. Insulin resistance as an essential mechanism of NASH development was highlighted by a recent review [32]. Insulin resistance indicators were also introduced as a predictor in the non-invasive diagnostic model of NASH [12].
In addition, the incidence of comorbid diabetes is high in NASH patients [33], so diabetes should be a screening priority. Glycated hemoglobin, which reflects poor glycemic control, has also been reported as a predictor of NAFLD by previous studies [28, 34]. Consistent with the findings of Alex ulitsky et al. [28], the NASH group subjects had a higher prevalence of type 2 diabetes but not hypertension and dyslipidemia compared with subjects without NASH. In univariate analysis, the levels of fasting blood glucose, fasting plasm insulin, glycosylated hemoglobin, and HOMA-IR in the NASH group were significantly higher than those in the non-NASH group. Furthermore, our study provides further evidence that HOMA-IR is an independent predictor of NASH. This was also confirmed by Yosuke Seki et al. that HOMA-IR may be involved in NASH progression in Japanese patients with severe obesity who underwent bariatric surgery [12].
TG appears to predict NASH and advanced fibrosis [35], but remains controversial. From a pathophysiological point of view, peripheral blood insulin resistance leads to increased adipose tissue lipolysis and de novo adipogenesis in hepatocytes, resulting in elevated TGs [36]. In a non-invasive NASH scoring system created by Adrian T. Billeter et al. [37], TG was the predictor of NASH. In our study, the NASH group had markedly lower HDL-C and higher TG levels than the non-NASH group. A counterintuitive finding in multivariate logistic regression analysis study suggested that HDL-C but not TG was an independent risk factor for NASH. Possible explanations could be that in subjects with “metabolic/obese NAFLD,” the liver is insulin resistant, resulting in overproduction of glucose and VLDL [38]. In turn, glucose triggers hyperinsulinemia by stimulating insulin secretion. An increase in VLDL value leads to a decrease in HDL-C concentration [39]. HDL-C as a predictor of NASH has not been elucidated in previous studies and requires further study. Regardless, dyslipidemia is inevitably associated with the occurrence and development of NASH.
Until now, liver biopsy remains the gold standard for diagnosing NASH, but its invasive nature limits its use in screening and follow-up for NASH. Therefore, a non-invasive scoring model for NASH based on the risk factors is highly desired. Alex Ulitsky et al. [28] derived a non-invasive clinical scoring model predicts the risk of NASH in patients with obesity. The scoring model included diabetes, sleep apnea, abnormal ALT, and hypertriglyceridemia, which showed a NPV of 89.7% in the low-risk category and a PPV of 75% in the very high-risk category, with AUROC of 0.76. Similarly, a simple clinical scoring system including BMI, ALT, and triglycerides was developed by Tai CM et al. with AUROC of 0.80 [10]. In our study, we developed a scoring model including AST >21.50 IU/L, HDL-C <1.155mmol/L, and HOMA-IR >9.368. This scoring model showed a NPV of 70.38% in the low-risk category and a PPV of 85.71% in the high-risk category, with AUROC of 0.737.
There is strong evidence confirmed that bariatric surgery improved the biochemical and histologic features of NAFLD and slowed the progression of NASH [40]. The benefits and harms of bariatric surgery for NASH cannot be assessed at this time due to insufficient evidence. The invasive nature of liver biopsy limits it as a routine follow-up method for NASH after bariatric surgery. However, scoring models compensate for the shortcomings of liver biopsy with the advantages of non-invasive, inexpensive, and repeatability. In our study, we assessed the effect of sleeve gastrectomy on NASH by a non-invasive model and confirmed risk factors and points of scoring model were significantly improved postoperative. Consistent with the results of the Chi-Ming Tai et al. [41], we conformed that risk factors of NASH were significantly improved and the points of the scoring system were markedly reduced after the surgery.
This study developed a non-invasive scoring model for NASH in patients with severe obesity and applied to assess the effect of sleeve gastrectomy on NASH. Undoubtedly, our study had several limitations. First, this study was conducted by a single-center and conducted in patients with obesity, who may not be representative of the entire NAFLD patient population, and obesity is a cause of NAFLD, leading to a decisive confounding variable. Therefore, a limitation of this study is that the results only apply to the Chinese population undergoing bariatric surgery and may not be generalized to the general population. Another limitation of this study is that only internal validation of the scoring model was performed in this study, further external validation of our scoring model in independent populations is necessary.
Conclusion
In this study, we found a high prevalence of NASH among severely obesity patients undergoing bariatric surgery in China and risk factors of NASH including AST, HDL-C, and HOMA-IR. A new non-invasive scoring system developed by the risk factors for predicting NASH is a useful assessment tool for the evaluation of NASH treatment. Bariatric surgery is effective in improving risk factors and points of scoring model of NASH which suggesting remission of NASH postoperative.
References
Pan XF, Wang L, Pan A. Epidemiology and determinants of obesity in China. Lancet Diabetes Endocrinol. 2021;9(6):373–92.
Wang Y, Wang L, Qu W. New national data show alarming increase in obesity and noncommunicable chronic diseases in China. Eur J Clin Nutr. 2017;71(1):149–50.
Wang YF, Sun MX, Xue H, et al. Interpretation of the blue book on obesity prevention and control in China and recommendations for obesity prevention and control measures in China. Chinese J Prev Med. 2019;09:875–84. (In Chinese)
Younossi Z, Anstee QM, Marietti M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15(1):11–20.
Matteoni CA, Younossi ZM, Gramlich T, et al. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116(6):1413–9.
Machado M, Marques-Vidal P, Cortez-Pinto H. Hepatic histology in obese patients undergoing bariatric surgery. J Hepatol. 2006;45(4):600–6.
Sheka AC, Adeyi O, Thompson J, et al. Nonalcoholic steatohepatitis: a review. JAMA. 2020;323(12):1175–83.
Buchwald H, Williams SE. Bariatric surgery worldwide 2003. Obes Surg. 2004;14(9):1157–64.
Crespo J, Fernández-Gil P, Hernández-Guerra M, et al. Are there predictive factors of severe liver fibrosis in morbidly obese patients with non-alcoholic steatohepatitis. Obes Surg. 2001;11(3):254–7.
Tai CM, Yu ML, Tu HP, et al. Derivation and validation of a scoring system for predicting nonalcoholic steatohepatitis in Taiwanese patients with severe obesity. Surg Obes Relat Dis. 2017;13(4):686–92.
Dixon JB, Bhathal PS, et al. Nonalcoholic fatty liver disease: predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterology. 2001;121(1):91–100.
Seki Y, Kakizaki S, Horiguchi N, et al. Prevalence of nonalcoholic steatohepatitis in Japanese patients with morbid obesity undergoing bariatric surgery. J Gastroenterol. 2016;51(3):281–9.
Praveenraj P, Gomes RM, Kumar S, et al. Prevalence and predictors of non-alcoholic fatty liver disease in morbidly obese South Indian patients undergoing bariatric surgery. Obes Surg. 2015;25(11):2078–87.
Sumida Y, Nakajima A, Itoh Y. Limitations of liver biopsy and non-invasive diagnostic tests for the diagnosis of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J Gastroenterol. 2014;20(2):475–85.
Jorge JM, Golas A, Patel N, et al. Management of nonalcoholic fatty liver disease and the role of bariatric surgery: a brief review for surgeons. Surg Obes Relat Dis. 2020;16(5):699–703.
Lassailly G, Caiazzo R, Buob D, et al. Bariatric surgery reduces features of nonalcoholic steatohepatitis in morbidly obese patients. Gastroenterology. 2015;149(2):379-388; quiz e15-6.
Wang Y, Wang CC, Zhu SH, et al. Guidelines for the surgical treatment of obesity and type 2 diabetes in China (2019 edition). Chinese J Pract Surg. 2019;39(4):301–6. (In Chinese)
Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–21.
Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9.
Beymer C, Kowdley KV, Larson A, et al. Prevalence and predictors of asymptomatic liver disease in patients undergoing gastric bypass surgery. Arch Surg. 2003;138(11):1240–4.
Abrams GA, Kunde SS, Lazenby AJ, et al. Portal fibrosis and hepatic steatosis in morbidly obese subjects: a spectrum of nonalcoholic fatty liver disease. Hepatology. 2004;40(2):475–83.
Spaulding L, Trainer T, Janiec D. Prevalence of non-alcoholic steatohepatitis in morbidly obese subjects undergoing gastric bypass. Obes Surg. 2003;13(3):347–9.
Udelsman BV, Corey KE, Lindvall C, et al. Risk factors and prevalence of liver disease in review of 2557 routine liver biopsies performed during bariatric surgery. Surg Obes Relat Dis. 2019;15(6):843–9.
Sepúlveda-Flores RN, Vera-Cabrera L, Flores-Gutiérrez JP, et al. Obesity-related non-alcoholic steatohepatitis and TGF-beta1 serum levels in relation to morbid obesity. Ann Hepatol. 2002;1(1):36–9.
Ong JP, Elariny H, Collantes R, et al. Predictors of nonalcoholic steatohepatitis and advanced fibrosis in morbidly obese patients. Obes Surg. 2005;15(3):310–5.
Mofrad P, Contos MJ, Haque M, et al. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology. 2003;37(6):1286–92.
Wong VW, Wong GL, Tsang SW, et al. Metabolic and histological features of non-alcoholic fatty liver disease patients with different serum alanine aminotransferase levels. Aliment Pharmacol Ther. 2009;29(4):387–96.
Ulitsky A, Ananthakrishnan AN, Komorowski R, et al. A noninvasive clinical scoring model predicts risk of nonalcoholic steatohepatitis in morbidly obese patients. Obes Surg. 2010;20(6):685–91.
Giannini EG, Testa R, Savarino V. Liver enzyme alteration: a guide for clinicians. CMAJ. 2005;172(3):367–79.
Huby T, Gautier EL. Immune cell-mediated features of non-alcoholic steatohepatitis. Nat Rev Immunol. 2022;22(7):429–43.
Pulzi FB, Cisternas R, Melo MR, et al. New clinical score to diagnose nonalcoholic steatohepatitis in obese patients. Diabetol Metab Syndr. 2011;3(1):3.
Cotter TG, Rinella M. Nonalcoholic fatty liver disease 2020: the state of the disease. Gastroenterology. 2020;158(7):1851–64.
Williams R, Karuranga S, Malanda B, et al. Global and regional estimates and projections of diabetes-related health expenditure: results from the International Diabetes Federation Diabetes Atlas (9th edition). Diabetes Res Clin Pract. 2020;162:108072.
Sheth H, Bagasrawala S, Shah M, et al. The HAALT non-invasive scoring system for NAFLD in obesity. Obes Surg. 2019;29(8):2562–70.
Wong RJ, Tran T, Kaufman H, et al. Increasing metabolic co-morbidities are associated with higher risk of advanced fibrosis in nonalcoholic steatohepatitis. PLoS One. 2019;14(8):e0220612.
Jornayvaz FR, Shulman GI. Diacylglycerol activation of protein kinase Cε and hepatic insulin resistance. Cell Metab. 2012;15(5):574–84.
Billeter AT, Wloka S, Behnisch R, et al. Development and validation of a novel scoring system for noninvasive nonalcoholic steatohepatitis detection in bariatric patients. Obes Facts. 2021;14(5):490–500.
Yki-Järvinen H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014;2(11):901–10.
Kotronen A, Westerbacka J, Bergholm R, et al. Liver fat in the metabolic syndrome. J Clin Endocrinol Metab. 2007;92(9):3490–7.
Tai CM, Huang CK, Hwang JC, et al. Improvement of nonalcoholic fatty liver disease after bariatric surgery in morbidly obese Chinese patients. Obes Surg. 2012;22(7):1016–21.
Tan CH, Al-Kalifah N, Ser KH, et al. Long-term effect of bariatric surgery on resolution of nonalcoholic steatohepatitis (NASH): an external validation and application of a clinical NASH score. Surg Obes Relat Dis. 2018;14(10):1600–6.
Funding
This study was funded by Tianjin Key Medical Discipline (Specialty) Construction Project (TJYXZDXK-030A) and Tianjin Health Science and Technology Project (TJWJ2022MS002).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Approval
The study was approved by the Tianjin Medical University General Hospital Institutional Review Board (approval number IRB2020-YX-029-01) and performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed Consent
Informed consent does not apply.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Points
The prevalence of NASH in Chinese patients with obesity was 48.56%.
A non-invasive scoring model included AST, HDL-C, and HOMA-IR.
The points of scoring model were improved at 6 months after sleeve gastrectomy.
Supplementary Information
ESM 1
(DOCX 16 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xiao, J., Zhang, X., Zhu, C. et al. Development, Validation, and Application of a Scoring Model for Non-alcoholic Steatohepatitis. OBES SURG 33, 3246–3255 (2023). https://doi.org/10.1007/s11695-023-06804-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-023-06804-y