Abstract
Background
We recently identified differences in abdominal subcutaneous adipose tissue (SAT) from insulin-resistant (IR) as compared to obesity-matched insulin sensitive individuals, including accumulation of small adipose cells, decreased expression of cell differentiation markers, and increased inflammatory activity. This study was initiated to see if these changes in SAT of IR individuals were present in omental visceral adipose tissue (VAT); in this instance, individuals were chosen to be IR but varied in degree of adiposity. We compared cell size distribution and genetic markers in SAT and VAT of IR individuals undergoing bariatric surgery.
Methods
Eleven obese/morbidly obese women were IR by the insulin suppression test. Adipose tissue surgical samples were fixed in osmium tetroxide for cell size analysis via Beckman Coulter Multisizer. Quantitative real-time polymerase chain reaction for genes related to adipocyte differentiation and inflammation was performed.
Results
While proportion of small cells and expression of adipocyte differentiation genes did not differ between depots, inflammatory genes were upregulated in VAT. Diameter of SAT large cells correlated highly with increasing proportion of small cells in both SAT and VAT (r = 0.85, p = 0.001; r = 0.72, p = 0.01, respectively). No associations were observed between VAT large cells and cell size variables in either depot. The effect of body mass index (BMI) on any variables in both depots was negligible.
Conclusions
The major differential property of VAT of IR women is increased inflammatory activity, independent of BMI. The association of SAT adipocyte hypertrophy with hyperplasia in both depots suggests a primary role SAT may have in regulating regional fat storage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The association between obesity and insulin resistance was described many years ago [1, 2]. However, since not all obese individuals are insulin resistant (IR) [3, 4], fat mass, per se, can not be the sole determinant of insulin resistance. We recently demonstrated two mechanisms by which biologic activity of adipose tissue may contribute to insulin resistance. Compared to equally obese, insulin sensitive (IS) individuals, we found that subcutaneous adipose tissue (SAT) of IR individuals had a higher ratio of small to large adipocytes and was associated with evidence of decreased expression of genes related to adipose cell differentiation [5]. These results suggest that a defect in cell differentiation or impaired fat storage capacity may contribute to insulin resistance. We subsequently observed that inflammatory activity was modestly upregulated in SAT of IR as compared with equally obese, IS women [6], representing yet another mechanism by which adipose tissue may contribute to differences in insulin sensitivity, independent of obesity. As an extension of these studies, we sought to elucidate whether these changes in biologic activity observed in SAT of IR individuals are also seen in visceral adipose tissue (VAT) of equally IR individuals, particularly as others have suggested that an excess of VAT is associated with adverse metabolic consequences [7, 8]. In contrast to our earlier studies [5, 6] in which we matched for adiposity, we wanted to compare VAT and SAT in individuals matched for degree of insulin sensitivity over a range of obesity. To accomplish this goal, we recruited a population of obese to morbidly obese individuals in whom direct quantification of insulin resistance was performed. We then compared measures of cell size distribution, genes related to adipocyte differentiation and inflammation, and inflammatory cell presence in SAT and VAT.
Subjects and Methods
Subjects
Eleven obese/morbidly obese women were recruited from Stanford’s Bariatric Surgery Program and determined to be IR, as defined by the criteria described below. All participants were non-diabetic, as defined by a fasting plasma glucose <7.0 mmol/l. Patients were excluded if they had coronary artery disease, hepatic or renal disease, cancer, and use of medications intended for weight loss or known to influence insulin sensitivity. This study was approved by the Stanford University Human Subjects Committee and the National Institute of Digestive Diseases and Kidney Disease (NIDDK) Institutional Review Board. All subjects gave written informed consent.
Quantification of Insulin-mediated Glucose Disposal
Insulin-mediated glucose disposal was quantified by a modification of the insulin suppression test [9], as originally described and validated by our research group [10, 11]. Briefly, subjects were infused for 180 min with octreotide to suppress endogenous insulin secretion, insulin, and glucose. Body surface area was adjusted to account for the disproportionately high ratio of fat to lean body mass [12]. Blood was sampled every 10 min from 150–180 min to measure plasma glucose and insulin concentrations. The mean of these four values represents the steady-state plasma insulin (SSPI) and glucose (SSPG) concentrations for each individual. Because SSPI concentrations are similar for all individuals, SSPG concentration provides a direct measure of the ability of insulin to mediate disposal of an infused glucose load; the higher the SSPG, the more insulin-resistant the individual. Measurements of insulin-mediated glucose uptake using the insulin suppression test were shown to be essentially identical to those obtained using the hyperinsulinemic euglycemic clamp method [10]. Based on prior studies showing increased risk of clinical events in the subgroup in the upper tertile of SSPG concentration, we defined cut-off values for insulin resistance using an SSPG value ≥9.44 mmol/l (170 mg/dl) [13, 14].
Plasma glucose, insulin, and lipid/lipoprotein concentrations were measured after an overnight fast as described previously [11, 15]. Other experimental measurements such as weight (measured in light clothing after an overnight fast), height, body mass index (BMI) calculated as weight in kilograms/(height in meters) square, waist circumference (measured as the midpoint between iliac crest and rib cage at end-expiration), race/ethnicity, and blood pressure (measured as average of three readings performed after a 5-min rest) were performed.
Adipose Tissue Harvesting and Cell Size Analysis
Ten grams of SAT from the mid-anterior abdominal and 10 g of VAT from the omental region were obtained during bariatric surgery and processed immediately. Three and a half grams were flash frozen in liquid nitrogen for gene expression analyses of tissue. These samples were stored at −80°C for subsequent analysis. Two samples of 30 mg of tissue from SAT and VAT were immediately fixed in osmium tetroxide and incubated in a water bath at 37°C for 48 h as described previously [16], and then adipose cell size was determined by a Beckman Coulter Multisizer III with a 400-μm aperture as congruent with our previous study [5]. The range of cell sizes that can effectively be measured using this aperture is 20–240 μm. The instrument was set to count 6,000 particles, and the fixed-cell suspension was diluted so that coincident counting was <10%. After collection of pulse sizes, the data were expressed as particle diameters and displayed as histograms of counts against diameter using linear bins and a linear scale for the x-axis.
As previously described [5], for each participant, analysis of adipose cell size distribution from Multisizer graphs entailed identification of the nadir, defined as the low point between the two cell populations. The number of adipose cells above and below this point was calculated by the Multisizer software and expressed as the ‘percent above’ and ‘percent below’ the nadir, as well as the ratio of small to large cells. In addition, the ‘peak diameter’ of the large adipose cells was defined as the mean diameter at which the frequency of the large cell population reached a maximum. We compared this method with the mathematical modeling technique used as described previously [5] and found that the two methods generally gave concordant results. Minor discrepancies between the two methods were attributed to imprecision of the measurements. For simplicity of reporting, we chose to include results generated from the first method only, acknowledging that the methods used may be somewhat subjective descriptions of the observations made. Finally, the Multisizer software calculated the mean, median, and mode of the overall cell sizes for each subject.
Gene Expression Analysis of Adipose Tissue
Total RNA was extracted from the adipose tissue using Qiazol and the Adipose Tissue RNAeasy kit (Qiagen, Valencia, CA, USA) according to the manufacturers’ instructions. cDNA was synthesized from total RNA using the SuperScript II kit (Invitrogen, Carlsbad, CA, USA). Taqman primer/probe sets for mRNA transcripts of the following were purchased from Applied Biosystems (Foster City, CA, USA): leptin, adiponectin, peroxisome proliferator-activated receptor γ 1/2 (PPAR γ 1/2), GLUT4, sterol receptor element binding protein 1c (SREBP1c), monocyte chemotactic protein-1 (CCL2, also known as MCP-1), IL-6, IL-8, visfatin, CD68, TNF-α, plasminogen activator inhibitor-1 (PAI-1), and 18S ribosomal RNA as a control probe. Amplification was carried out in triplicate on an ABI Prism 7700 sequence detection system at 50°C for 2 min and 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. A threshold cycle (Ct value) was obtained from each amplification curve, and a ΔCt value was first calculated by subtracting the Ct value for 18S ribosomal RNA from the Ct value for each sample. A ΔΔCt value was calculated by subtracting the ΔCt value of a SAT sample (control), chosen arbitrarily as the most IS subject. Fold changes compared with the control were then determined by calculating 2−ΔΔCt.
Immunohistochemistry
Adipose tissue samples from SAT and VAT were fixed with 10% formalin and embedded in paraffin. Five-micron sections underwent immunohistochemical analysis using primary antibodies for CD68 and CD45 (Novocastra Laboratories, Newcastle, UK) and visualization with standard avidin-biotin-peroxidase technique (Vector Laboratories, Burlingame, CA, USA). All sections were counterstained with Harris hematoxylin. Inflammatory cells present per high power field (HPF = 40x) were recorded and averaged over each tissue section. On average, 86 high power fields were counted per section. Tissue sections were also observed for presence of crown-like structures, described as macrophages localized around dead adipocytes [17].
Statistical Analysis
Student’s paired t tests were used to compare characteristics between SAT and VAT, including cell size distribution variables, gene expression, and relative number of cells counted via immunohistochemical staining. Where data were not normally distributed, values for gene expression were logarithmically transformed prior to analysis. Outliers were also removed if they were greater than or equal to two standard deviations above or below the mean. Data were expressed as mean ± standard error of the mean. Simple and partial correlation coefficients were calculated between participant characteristics, multi-sizer variables, and gene expression data. p value <0.05 was taken to indicate statistical significance. These analyses were performed using Statistical Package for the Social Sciences software 15.0 (Chicago, IL, USA).
Results
Participant Characteristics
Table 1 shows the characteristics of the 11 IR subjects. Their mean age was 41 years, they were obese to morbidly obese with an average BMI of 45.3 kg/m2 and had average fasting concentration of insulin 185 pmol/L. By selection, they were IR, with an average SSPG value of 13.4 mmol/l.
Cell Size Distribution and Variables
Fig. 1 illustrates the Multisizer adipose cell size profiles of the 11 subjects undergoing bariatric surgery in (a) SAT and (b) VAT. Similar to our previously reported findings [5], we found that each subject had a bimodal distribution of adipose cells, present in both tissue depots. They had roughly equivalent proportions of small cells in the range of 20–50 μm and larger cells in the 80–130 μm range. We previously showed photographic examples of small adipose cells using scanning electron microscopy of paraformaldehyde-fixed tissue [5], supporting that the small cells identified by Multisizer were indeed adipose cells. This was also quantitatively confirmed by subjecting adipose tissue to collagenase digestion and comparing Multisizer curves of isolated adipose cells with that of intact adipose tissue [5], which was also performed and confirmed in the present study with all SAT and VAT samples (data not shown).
Table 2 compares the adipose cell size variables in SAT and VAT. The two tissue depots differed in that the mean size of cells was larger in SAT than VAT (p < 0.001), and the nadir (defined as the low point between the two cell populations) was significantly higher in SAT than VAT (p < 0.05). Most notable was that the peak diameter of the large cells was significantly greater in SAT than VAT (126 ± 3.9 versus 104 ± 4.7 μm, p < 0.01). However, the percent of cells below nadir (i.e., percent of small cells) or ratio of small to large cells did not differ between the two tissue depots.
Correlations between BMI and peak large cell diameters of SAT and VAT are shown in Fig. 2, demonstrating lack of statistical significance for both relationships (r = −0.14, p = 0.67; r = 0.14, p = 0.67, respectively). When BMI was compared with the other cell size variables including percentage of cells below nadir and ratio of small to large cells in SAT and VAT, no statistically significant correlations emerged (data not shown).
The relationships between SAT peak diameter and percentage of small cells or ratio of small to large cells in SAT and VAT are depicted in Fig. 3. As Pearson’s correlation coefficients (r) and p values did not differ substantially when calculated with or without adjustment for BMI, values are reported without adjustment for BMI. Peak diameter of large cells in SAT was highly correlated with accumulation of small cells in both SAT and VAT. As such, increasing peak diameter in SAT was associated with accumulation of small cells in SAT as represented by percentage of small cells (r = 0.77, p < 0.01; Fig. 3a) and ratio of small to large cells (r = 0.85, p = 0.001; Fig. 3b). Peak diameter of SAT also highly correlated with percentage of small cells (Fig. 3c) and ratio of small to large cells in VAT (Fig. 3d; r = 0.63, p < 0.05; r = 0.72, p = 0.01, respectively).
Graphic representation of the relationships between peak diameter of large cells in subcutaneous adipose tissue (SAT) versus (a) percentage of small cells in SAT, (b) ratio of small to large cells in SAT, (c) percentage of small cells in visceral adipose tissue (VAT), and (d) ratio of small to large cells in VAT
By contrast, the results in Fig. 4 reveal that there were no significant relationships between peak diameter of VAT and any cell size variables in SAT and VAT. Fig. 4a and b show VAT peak diameter versus percentage of small cells (r = 0.39, p = 0.24) and ratio of small to large cells in VAT (r = 0.35, p = 0.29), respectively. There was also no association between VAT and SAT peak diameter (r = 0.24, p = 0.49) as seen in Fig. 4c.
Additionally, there did appear to be a relationship between the ratio of small to large cells in SAT and VAT (r = 0.74, p < 0.01), whereas correlation between percentage of small cells in SAT and VAT did not reach statistical significance (r = 0.50, p = 0.12; figures not shown).
Cell Differentiation and Inflammatory Gene Expression
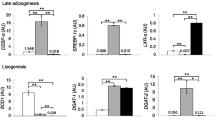
Expression of genes related to cell differentiation and inflammation were quantified in SAT and VAT. While markers of cell differentiation including adiponectin, PPARγ, and SREBP1c did not differ between the two tissue depots (Fig. 5a), there was greater expression of leptin in SAT than in VAT (p < 0.01).
Five out of the seven inflammatory genes had greater expression in VAT than SAT including: MCP-1, IL-6, IL-8, visfatin, and PAI-1 (p < 0.05; Fig. 5b). Other markers of inflammation including CD68 and TNF-α were not differentially expressed.
Relationships between BMI and gene expression levels in SAT and VAT were evaluated, and no statistically significant correlations were observed (data not shown). Additionally, no other relevant correlations were observed between other clinical characteristics and SAT or VAT gene expression data.
Immunohistochemistry
Tissue sections were evaluated for cells that stained positive for CD68 (macrophage marker) and CD45 (leukocyte marker). There was not a statistically significant difference in number of CD45- and CD68-containing cells between SAT and VAT (Table 3). BMI was not associated with number of CD45-containing or CD68-containing cells in SAT or VAT. Fig. 6 depicts representative slides of SAT and VAT sections stained for CD68 and CD45 antigens. Of note, we infrequently observed the presence of macrophages surrounding single adipocytes in crown-like structures; out of the total of 11 SAT and VAT sections, a single crown-like structure was observed in three SAT and two VAT sections, and two crown-like structures were seen in three additional SAT sections.
Discussion
Several key points can be elaborated from the relationships we found between inter-depot cell size characteristics among the IR women. First, these two adipose tissue depots did not differ in proportion of small adipose cells. Second, large adipose cells in SAT had a greater peak diameter than those in VAT, and finally, increasing size of SAT large cells was associated positively with the accumulation of small cells in SAT and VAT, a property not observed with VAT large cells. Importantly, BMI, per se, was not associated with cell size variables in either depot, indicating that our results varied as a function of the adipose tissue depots themselves.
These findings add to our previous data [5], in which we showed that the proportion of small to large adipocytes was increased in SAT of IR as compared with IS individuals. This enhanced proportion of small cells was also associated with decreased expression of genetic markers of adipocyte differentiation [5]. In the present study, we detected no differences in small cell proportion or expression of genes upregulated during adipogenesis between the two adipose tissue depots, supporting the hypothesis that differential small cell accumulation, as a plausible manifestation of impaired adipogenesis, varies as a function of insulin resistance rather than of regional deposition of fat tissue.
On the other hand, peak diameter of large adipose cells appears to be regionally determined. Regional differences in adipose cell size have been previously reported using measures of ‘mean’ cell diameter [18–20]; however, prior methods using photographic visualization are limited to a representative sample of cells and may not sufficiently account for adipose cell size spread. By contrast, Multisizer technology enables use of intact whole adipose tissue specimens for cell size characterization, providing accurate determination of adipose cell diameters and distribution. This method permits discrimination between large cells and the discrete population of small adipocytes that would otherwise have been overlooked [18–20].
Our comparison of adipose tissue depots in a population of IR women over a range of obesity also reveals that BMI, per se, was not associated with the differences found in cell size variables between SAT and VAT. Other studies that have documented parallel increases in adipocyte size with increasing fat mass importantly did not control for insulin resistance, included mixed populations of lean and obese individuals that likely varied in insulin resistance, and used other methods as mentioned above to quantify and report cell size [20, 21]. Our study underscores the concept that further increases in adipose cell size do not occur above a threshold BMI in both SAT and VAT of obese, IR women. While it is possible that addition of subjects who were even more severely obese (e.g., BMI > 60 kg/m2) would have revealed a positive correlation, we did not observe a trend in that direction with the present data. Moreover, to the best of our knowledge, our study may be the first to document, using Multisizer technology, that increasing cell size in SAT is associated with accumulation of small cells in both SAT and VAT. In contrast, increasing size of large cells in VAT did not confer any association with cell size variables in either depot, suggesting that VAT may be metabolically less relevant as a site of preferential fat storage. In other words, these findings could be interpreted to mean that SAT may serve as the primary site of triglyceride deposition wherein once cellular hypertrophy reaches a maximal state, recruitment of smaller adipocytes, or fat cell hyperplasia, ensues in both adipose tissue depots. This postulation is supported by earlier studies that suggested that adipose cells enlarge to a finite degree in the setting of overfeeding in humans [22] and rats [23]. Cell culture data have furthermore demonstrated that human subcutaneous and omental preadipocytes harbor discrete characteristics, including a greater capacity for lipid accumulation in the subcutaneous cultured preadipocytes [24]. A previous study reported findings somewhat discrepant from our results, including the predominance of adipocyte hyperplasia and increased expression of adipogenic genes in SAT [21]. Nonetheless, these differences are possibly accounted for by their use of a patient population that combined lean and obese subjects, absence of insulin sensitivity measures, and photographic visualization of cell sizes. Further studies are necessary to clarify the biologic properties of small and large adipocytes, as well as the clinical significance regional fat tissue may confer upon triacylglycerol storage or utilization.
The present study also indicates that a defining property of VAT in IR subjects is its pro-inflammatory propensity. Our study adds to the growing literature supporting that gene expression levels of IL-6, IL-8, MCP-1, and PAI-1 are increased in VAT [25–29] by controlling for insulin resistance. Published studies on differential expression of visfatin in SAT and VAT are more conflicting [30–35] and may be due in part to its putative roles in insulin action or insulin resistance in addition to its pro-inflammatory effects. Visfatin’s preferential expression in VAT in the present study is consistent with upregulation of the other inflammatory cytokines. Similarly, as with adipocyte size, our results demonstrate that above a threshold of BMI, parallel increases in inflammatory gene expression in either depot do not occur with increasing BMI. Again, that other studies have reported otherwise is likely explained by the above-mentioned differences in patient population and cell sizing methodology [26, 27]. Leptin, a satiety factor produced by terminally differentiated adipocytes, was the only genetic marker preferentially expressed in SAT, a finding consistent with previous reports and has been attributed to the relatively greater mass effect of SAT and an increased secretory rate [36, 37]. Our study provides additional information showing that when controlled for insulin resistance, leptin is differentially expressed and does not vary as a function of BMI.
How an inflammatory milieu is generated in VAT, and in particular what role macrophages or other non-adipose cells have in this process, is another point of controversy [38–40]. In that context, we detected no difference between depots in macrophage-specific markers, including CD68 gene expression and immunohistochemical staining for number of CD68- and CD45-containing cells. Additionally, contrary to other studies [17, 41], we did not detect an abundance of crown-like structures, i.e., macrophages localized around adipocytes, in our subjects. These findings are consistent with our previous study in moderately obese women, in whom we detected rare crown-like structures [6]. Our data therefore raise the possibility that cytokine expression by the adipocytes themselves—rather than infiltrating inflammatory cells—mediate the inflammatory environment in VAT. In other words, visceral adipocytes may be responsible for the increased inflammatory activity in VAT relative to SAT. These findings differ from studies in rodent models of obesity [17, 41] and humans [25–27] that suggest non-adipose cells, e.g., macrophages, are primarily responsible for the inflammatory response. Also strikingly discordant is that whereas crown-like structures have been reported to be present in greater density in VAT than SAT of obese mice, we found, if anything, greater predominance in SAT. These discrepant results may be related to inequitable comparisons between our weight-stable obese human subjects versus rodents with rapidly increasing weight.
Overall strengths of our study include direct measurement of insulin resistance in our subjects and use of updated cell sizing technique in combination with gene expression data to characterize the two adipose tissue depots. A limitation of our study is its cross-sectional design; conclusions can therefore not be drawn about causal roles that depot-dependent differences in adipose tissue cell size and gene expression may have in insulin resistance. Further studies comparing depot-dependent differences between IR subjects and equally obese, IS subjects would provide additional useful information. Given the possible gender distinction in adipose cell size reported in the literature, it would also be worthwhile to evaluate men separately and in comparison to women. Depot-specific adipocyte size has been reported in men to not be different, although these studies included either non-obese subjects or men of variable BMI, and insulin resistance was not assessed [19, 42].
In conclusion, our findings demonstrate that SAT and VAT of IR women differ with respect to adipose cell size, relationship between hypertrophy and hyperplasia, and inflammation. These associations are largely independent of BMI, suggesting a threshold of adiposity above which these variables are not affected. While the depots do not differ in percentage of small cells and expression of differentiation gene markers, SAT large cells are larger and highly correlated with hyperplasia in SAT and VAT. Taken together, SAT appears to bear a more dynamic role in fat storage, wherein SAT cell enlargement may be responsible for triggering adipose cell recruitment in both depots. VAT is relatively pro-inflammatory, a state which may be mediated by adipocytes themselves rather than the presence of infiltrating inflammatory cells. Future studies are needed to help elucidate the mechanisms behind these regional fat cell properties and their metabolic consequences.
References
Olefsky J, Reaven GM, Farquhar JW. Effects of weight reduction on obesity. Studies of lipid and carbohydrate metabolism in normal and hyperlipoproteinemic subjects. J Clin Invest. 1974;53:64–76.
Ferrannini E, Natali A, Bell P, et al. Insulin resistance and hypersecretion in obesity. European Group for the Study of Insulin Resistance (EGIR). J Clin Invest. 1997;100:1166–73.
Abbasi F, Brown BW, Lamendola C, et al. Relationship between obesity, insulin resistance, and coronary heart disease risk. J Am Coll Cardiol. 2002;40:937–43.
McLaughlin T, Abbasi F, Lamendola C, et al. Heterogeneity in the prevalence of risk factors for cardiovascular disease and type 2 diabetes mellitus in obese individuals: effect of differences in insulin sensitivity. Arch Intern Med. 2007;167:642–8.
McLaughlin T, Sherman A, Tsao P, et al. Enhanced proportion of small adipose cells in insulin-resistant vs insulin-sensitive obese individuals implicates impaired adipogenesis. Diabetologia. 2007;50:1707–15.
McLaughlin T, Deng A, Gonzales O, et al. Insulin resistance is associated with a modest increase in inflammation in subcutaneous adipose tissue of moderately obese women. Diabetologia. 2008;51:2303–8.
Montague CT, O’Rahilly S. The perils of portliness: causes and consequences of visceral adiposity. Diabetes. 2000;49:883–8.
Pouliot MC, Despres JP, Nadeau A, et al. Visceral obesity in men. Associations with glucose tolerance, plasma insulin, and lipoprotein levels. Diabetes. 1992;41:826–34.
Pei D, Jones CN, Bhargava R, et al. Evaluation of octreotide to assess insulin-mediated glucose disposal by the insulin suppression test. Diabetologia. 1994;37:843–5.
Greenfield M, Doberne L, Kraemer F, et al. Assessment of insulin resistance with the insulin suppression test and the euglycemic clamp. Diabetes. 1981;30:387–92.
Shen SW, Reaven GM, Farquhar JW. Comparison of impedance to insulin-mediated glucose uptake in normal subjects and in subjects with latent diabetes. J Clin Invest. 1970;49:2151–60.
Livingston EH, Lee S. Body surface area prediction in normal-weight and obese patients. Am J Physiol Endocrinol Metab. 2001;281:E586–91.
Yip J, Facchini FS, Reaven GM. Resistance to insulin-mediated glucose disposal as a predictor of cardiovascular disease. J Clin Endocrinol Metab. 1998;83:2773–6.
Facchini FS, Hua N, Abbasi F, et al. Insulin resistance as a predictor of age-related diseases. J Clin Endocrinol Metab. 2001;86:3574–8.
Carantoni M, Abbasi F, Chu L, et al. Adherence of mononuclear cells to endothelium in vitro is increased in patients with NIDDM. Diabetes Care. 1997;20:1462–5.
Hirsch J, Knittle JL. Cellularity of obese and nonobese human adipose tissue. Fed Proc. 1970;29:1516–21.
Cinti S, Mitchell G, Barbatelli G, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46:2347–55.
Edens NK, Fried SK, Kral JG, et al. In vitro lipid synthesis in human adipose tissue from three abdominal sites. Am J Physiol Endocrinol Metab. 1993;265:374–9.
Rebuffe-Scrive M, Anderson B, Olbe L, et al. Metabolism of adipose tissue in intraabdominal depots in severely obese men and women. Metabolism. 1990;39:1021–5.
Tchernof A, Belanger C, Morisset AS, et al. Regional differences in adipose tissue metabolism in women: minor effect of obesity and body fat distribution. Diabetes. 2006;55:1353–60.
Drolet R, Richard C, Sniderman A, et al. Hypertrophy and hyperplasia of abdominal adipose tissues in women. Int J Obes. 2008;32:283–91.
Kashiwagi A, Mott D, Bogardus C, et al. The effects of short-term overfeeding on adipocyte metabolism in Pima Indians. Metabolism. 1985;34:364–70.
Faust IM, Johnson PR, Stern JS, et al. Diet-induced adipocyte number increase in adult rats: a new model of obesity. Am J Physiol. 1978;235:E279–86.
Tchkonia T, Tchoukalova Y, Giorgadze N, et al. Abundance of two human preadipocyte subtypes with distinct capacities for replication, adipogenesis, and apoptosis varies among fat depots. Am J Physiol Endocrinol Metab. 2005;288:E267–77.
Bruun JM, Lihn AS, Madan AK, et al. Higher production of IL-8 in visceral vs. subcutaneous adipose tissue. Implication of nonadipose cells in adipose tissue. Am J Physiol Endocrinol Metab. 2004;286:8–13.
Bruun JM, Lihn AS, Pedersen SB, et al. Monocyte chemoattractant protein-1 release is higher in visceral than subcutaneous human adipose tissue (AT): implication of macrophages resident in the AT. J Clin Endocrinol Metab. 2005;90:2282–9.
Fain JN, Madan AK, Hiler ML, et al. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology. 2004;145:2273–82.
Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab. 1998;83:847–50.
Vohl MC, Sladek R, Robitaille J, et al. A survey of genes differentially expressed in subcutaneous and visceral adipose tissue in men. Obes Res. 2004;12:1217–22.
Jia SH, Li Y, Parodo J, et al. Pre–B cell colony-enhancing factor inhibits neutrophil apoptosis in experimental inflammation and clinical sepsis. J Clin Invest. 2004;113:1318–27.
Moschen AR, Kaser A, Enrich B, et al. Visfatin, an adipocytokine with proinflammatory and immunomodulating properties. J Immunol. 2007;178:1748–58.
Oki K, Yamane K, Kamei N, et al. Circulating visfatin level is correlated with inflammation, but not with insulin resistance. Clin Endocrinol (Oxf). 2007;67:796–800.
Pagano C, Pilon C, Olivieri M, et al. Reduced plasma visfatin/pre-B cell colony-enhancing factor in obesity is not related to insulin resistance in humans. J Clin Endocrinol Metab. 2006;91:3165–70.
van der Veer E, Nong Z, O’Neil C, et al. Pre-B-cell colony-enhancing factor regulates NAD+-dependent protein deacetylase activity and promotes vascular smooth muscle cell maturation. Circ Res. 2005;97:25–34.
Varma V, Yao-Borengasser A, Rasouli N, et al. Human visfatin expression: relationship to insulin sensitivity, intramyocellular lipids, and inflammation. J Clin Endocrinol Metab. 2007;92:666–72.
Montague CT, Prins JB, Sanders L, et al. Depot- and sex-specific differences in human leptin mRNA expression: implications for the control of regional fat distribution. Diabetes. 1997;46:342–7.
Van Harmelen V, Reynisdottir S, Eriksson P, et al. Leptin secretion from subcutaneous and visceral adipose tissue in women. Diabetes. 1998;47:913–7.
Weisberg SP, McCann D, Desai M, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–808.
Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–30.
Weisberg SP, Hunter D, Huber R, et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116:115–24.
Murano I, Barbatelli G, Parisani V, et al. Dead adipocytes, detected as crown-like structures, are prevalent in visceral fat depots of genetically obese mice. J Lipid Res. 2008;49:1562–8.
Boivin A, Brochu G, Marceau S, et al. Regional differences in adipose tissue metabolism in obese men. Metabolism. 2007;56:533–40.
Acknowledgments
Funding for this study was provided by study grants NIH/NIDDK 1 R01 DK071309-01, 5 R01 DK071333-04, and 5 F32 DK079578-02, by the NIDDK Intramural Research Program and supported by Human Health Service grant M01-RR00070.
Conflict of interest
The authors disclose that there was no commercial interest involved in the study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Alice Liu and Tracey McLaughlin contributed equally to this study.
Rights and permissions
About this article
Cite this article
Liu, A., McLaughlin, T., Liu, T. et al. Differential Intra-abdominal Adipose Tissue Profiling in Obese, Insulin-resistant Women. OBES SURG 19, 1564–1573 (2009). https://doi.org/10.1007/s11695-009-9949-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-009-9949-9