Abstract
Background
Although bariatric surgery is currently the most common practice for inducing weight loss in morbidly obese patients (BMI > 40 kg/m2), its effect on the lipid content of adipose tissue and its lipases (lipoprotein lipase [LPL] and hormone-sensitive lipase [HSL]) are controversial.
Methods
We analyzed LPL and HSL activities and lipid content from plasma as well as subcutaneous (SAT) and visceral (VAT) adipose tissue of 34 morbidly obese patients (MO) before and after (6 and 12 months) Roux-en-Y gastric bypass surgery and compare the values with those of normal weight (control) patients.
Results
LPL activity was significantly higher in MO (SAT = 32.9 ± 1.0 vs VAT = 36.4 ± 3.3 mU/g tissue; p < 0.001) than in control subjects (SAT = 8.2 ± 1.4 vs VAT = 6.8 ± 1.0 mU/g tissue) in both adipose depots. HSL activity had similar values in both types of tissue (SAT = 32.8 ± 1.6 and VAT = 32.9 ± 1.6 mU/g) of MO. In the control group, we found similar results but with lower values (SAT = 11.9 ± 1.4 vs VAT = 12.1 ± 1.4 mU/g tissue). Twelve months after surgery, SAT LPL activity diminished (9.8 ± 1.4 mU/g tissue, p < 0.001 vs morbidly obese), while HSL (46.6 ± 3.7 mU/g tissue) remained high. All lipids in tissue and plasma diminished after bariatric surgery except plasma nonesterified fatty acids, which maintained higher levels than controls (16 ± 3 vs 9 ± 0 mg/dL; p < 0.001, respectively).
Conclusions
When obese patients lose weight, they lose not only part of the lipid content of the cells but also the capacity to store triacylglycerides in SAT depots.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adipose tissue mass in mammals is maintained by a dynamic equilibrium between lipid storage, which is mediated by lipoprotein lipase (LPL), and fat mobilization (lipolysis), which is regulated by hormone-sensitive lipase (HSL). Both enzymes play important roles in obesity [1]. However, there is still uncertainty about the importance of each enzyme in the acquisition and maintenance of fat depots, particularly in subcutaneous (SAT) and visceral (VAT) adipose tissue in morbidly obese patients.
LPL activity is related to the liberation of lipolytic products from chylomicra and very-low-density lipoproteins, which are captured by adipocytes and stored as triacylglycerides (TAG). Several authors have found markedly elevated LPL activities in adipose tissue of obese patients, but others have reported contrary results. There are also conflicting data about LPL activity after weight loss [2]. Moreover, there are differences in the TAG uptake capacity between the different adipose depots. According to Marin et al., the uptake of labeled TAG is approximately 50% higher in omental adipose tissue than in subcutaneous abdominal adipose tissue in men with a wide variation of body fat. However, these results did not correlate with LPL activity [3]. The increase in visceral fat mass with increasing total body fat could be explained by an increase in fat cell size only up to a certain adipocyte weight. Further elevations in intraabdominal fat mass with severe obesity appear to be due to higher numbers of adipocytes [4].
The data on obese human HSL activity are also controversial, mainly because of methodological differences among the available studies [5]. Some authors demonstrated that the expression and function of HSL in subcutaneous adipocytes are defective in obese patients. This HSL defect is accompanied by a decreased lipolytic capacity of adipocytes when lipolysis is expressed per gram of lipid in fat cells. Lipolysis per gram of lipid is a better measure of lipolytic capacity than lipolysis per cell, as fat cell volume increases in obesity. Moreover, there is a relationship between cell size and the lipolysis rate per cell but not per gram of lipid [6].
Discrepant data have been reported regarding the effect of weight loss on these enzymes. Bariatric procedures (with or without restrictive components) universally result in an improvement of metabolic syndrome (dyslipidemia, hypertension, insulin resistance, and central obesity). In addition, the procedure also improves hypertension. In light of this marked amelioration of the components of the metabolic syndrome accompanied by reliable maintenance of weight loss, malabsorptive bariatric procedures are the treatment of choice for morbid obesity and its comorbidities [7]. Some authors observed that when obese subjects lost weight and became less hyperinsulinemic, adipose LPL continued to increase. This suggests abnormal LPL regulation independent of insulin [5]. Others reported that weight loss did not change the lipolytic capacity of adipocytes or their capacity for storing TAG [8, 9]. These incongruities may be related to (1) the way the enzyme activities were expressed (tissue weight, protein, DNA, etc.) and (2) the difficulties in measuring LPL activity since part of the enzyme remains buried in the tissue extracts. In contrast, measures of HSL activity give the total amount of the enzyme in tissue [8].
The aim of this study was to determine the LPL and HSL activities in SAT and VAT depots from morbidly obese patients and compare them with a normal weight group (control). In addition, we wanted to study SAT from obese patients after bariatric surgery and compare it with that of the control group.
Methods
Subjects and Sample Acquisition
A group of 34 morbidly obese patients (24 women and ten men) between 27 and 61 years of age with a BMI > 40 kg/m2 were recruited from the Vall d'Hebron Hospital in Barcelona, Spain, in accordance with the Spanish consensus on the diagnosis of obesity [10]. Twenty-five percent of women and 20% of men had type 2 diabetes mellitus. The study protocol was accepted by the hospital's ethics committee, and all subjects gave their written, informed consent to participate.
Two days before the study, all subjects were placed on an isocaloric diet calculated on the basis of individual requirements. The diet was made up of 50% carbohydrate, 20% protein, and 30% fat. Blood samples were taken under fasting conditions between 8:00 and 10:00 a.m. and at the time of surgery. Plasma was separated immediately by centrifugation, and aliquots were frozen at −80°C for subsequent analysis. In obese subjects, subcutaneous abdominal adipose tissue (SAT) and visceral (epiploon) adipose tissue (VAT) biopsies were performed during Roux-en-Y gastric bypass (in tables and graph, obese). Second and third SAT biopsies were obtained six and 16.3 ± 3 months (range, 12–18 months) after bariatric surgery (in tables and graph, 6M or 12M, respectively). The anesthetic procedure was standardized for elective surgery and for the biopsy procedures (1% scandicain was used). Epinephrine was avoided. Tissue samples were quickly minced then frozen in liquid nitrogen and stored at −80°C for further analysis.
Nine euthyroid patients (12 h fasted) who underwent a laparotomy (cholecystectomy or surgery of abdominal hernia) were used as controls. A blood sample and SAT and VAT biopsies were taken simultaneously (in tables and graph, control).
Anthropometric and Body Composition Measurements
Body weight, excess weight, height, and waist and hip circumferences were measured according to standardized procedures [11]. Body mass index (BMI) and the waist-to-hip ratio (WHR) were then calculated. The body fat percentage was calculated from the equation proposed by Deurenberg et al. [12], and the amount of total, subcutaneous, and visceral fat were calculated from the equations proposed by Bonora et al. [13].
Sample Preparation for LPL and HSL Assays
SAT and VAT homogenates for LPL and HSL enzymatic assays were obtained in a Polytron homogenizer at 4°C with 20–30 mg tissue/mL of freshly made buffer at pH 7.4 (10 mM HEPES, 1 mM EDTA, 1 mM DTT) with (for the LPL assay) or without (for the HSL assay) 5 U/mL heparin. Homogenates were centrifuged in a microfuge at 16,100×g for 10 min at 4°C, and the fat-depleted infranatants were used for the enzymatic assays [14, 15].
LPL Activity Assay
Methods involving the use of TAG containing radiolabeled acyl chains are highly specific and sensitive lipase assays [14, 16]. In our experimental procedure, LPL was assayed as previously described by Ballart et al. [17]. The endothelial lipase described has no TAG hydrolase activity when 3–5% serum is present in the assay [18].
HSL Activity Assay
HSL was assayed as previously described by Ramírez et al. and Stam and Hülsmann [19] and [20], respectively]. The recently described enzyme adipose triglyceride lipase (ATGL) showed diminished TAG hydrolase activity in vitro, and its expression was unaffected by obesity and weight reduction [21].
Lipid Extraction and Determination
For the lipid extraction, we applied our recently described method to a small amount of tissue [22]. Cholesterol (free and esterified), phospholipids, nonesterified fatty acids (NEFA), and TAG were measured immediately by enzymatic analyses.
Other Determinations
Protein was determined in both plasma and adipose tissue homogenates using the method developed by Bradford [23]. DNA was quantified in liver biopsies by the fluorimetric method described by Vytasek [24]. Fasting plasma glucose levels were measured enzymatically at the hospital's chemistry laboratory using routine methods. Fasting plasma insulin levels were determined by a commercial radioimmunoassay kit (Amersham, Little Chalfont, UK). The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as previously described [25].
Statistical Analysis
Results were reported as mean ± SEM. Statistical significance of mean value differences for obese or normal weight, 6 or 12 months after surgery (weight loss), was assessed by one-way ANOVA. Individual comparisons were made using the Tukey's multiple comparison test. Correlations between independent variables were determined by Spearman's correlation coefficient. Statistical comparisons were significant when p < 0.05.
All statistical analyses were computed using the GraphPad Prism program, version 4.00 for Windows (GraphPad Software, San Diego, CA, USA, https://doi.org/www.graphpad.com).
Results
Clinical Characteristics of Patients
The characteristics of patients are shown in Table 1. One year after bariatric surgery, the total percentage of weight loss was 37%, excess weight 71%, waist circumference 27%, hip circumference 19%, and WHR 5.6%.
Of the total body weight, 61% corresponded to total fat. Of this, 74% corresponded to subcutaneous fat and 27% to visceral fat. The percentage of fat loss during the first 6 months after bariatric surgery was, approximately, the same for total, subcutaneous, and visceral fat (48%, 43%, and 52%, respectively, all of them with p < 0.001 vs obese). Six months later, the percentage of fat loss was 59%, 55%, and 62% for total, subcutaneous, and visceral fat, respectively (p < 0.001 vs obese).
In morbidly obese cases, body weight and excess weight correlated positively (p < 0.001) with total fat, visceral fat, BMI, as well as hip and waist measurements. Subcutaneous fat correlated positively with body weight (p < 0.05) and excess weight (p < 0.001).
These correlations persisted throughout the weight loss period (p < 0.001). Moreover, WHR correlated positively with body weight (p < 0.001) and excess weight (p < 0.05).
Plasma Biochemical Parameters
As can be seen in Table 2, all parameters except cholesterol in low-density lipoprotein (cLDL) were significantly higher in the plasma of the morbidly obese subjects than in the controls. However, 6 months after surgery, the control values had recovered, except in the case of NEFA, which remained high even 1 year after surgery.
Glycemia and insulin diminished by 29% and 63%, respectively, during the first 6 months. These levels were maintained for the rest of the study period. The reference values for HOMA-IR which indicate insulin resistance, were around 3.5–3.8 [25]. Patients with morbid obesity had values four times higher than controls. Interestingly, 6 months after bariatric surgery, HOMA-IR had diminished by 75% to control values and remained at this level for the rest of the study period.
In morbidly obese subjects, insulin correlated positively with body weight (p < 0.05). After bariatric surgery, insulin and HOMA-IR correlated positively with BMI, body weight, waist, excess weight, and total fat (p < 0.001) as well as with WHR (p < 0.01), hip, and SAT (p < 0.05). Finally, VAT correlated with insulin (p < 0.05) and HOMA-IR (p < 0.01).
Tissue Biochemical Parameters
Table 3 shows a decrease in obese SAT and VAT DNA content per gram of tissue compared with controls (p < 0.01). Additionally, the amount of protein in SAT decreased 50% (p < 0.01) but remained stable in VAT. These data indicate that obese SAT and VAT cells are bigger compared with control ones. This is corroborated by the lipid content per DNA (or per cell), which is higher if it is assessed as total lipid (p < 0.01) in both SAT and VAT, or if we consider each type of lipid separately—except for PL. Taking this into account, VAT cells were smaller than SAT cells in both obese (p < 0.001) and controls (p < 0.05)
During weight loss, obese adipose tissue cells became smaller as their DNA content per gram of tissue increased progressively. Similarly, as the cell decreased in size, its lipid content also diminished. It is noteworthy that NEFA decreased 38% 6 months after surgery, but from 6 to 12 months, the decrease was only about 10%.
LPL Activity
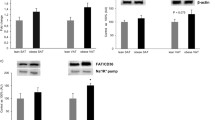
LPL activity in SAT and VAT expressed per gram of tissue was similar (Fig. 1, upper left panel). However, obese SAT values were up to four times higher than those observed in controls (p < 0.001 vs control) despite having less cells per gram of tissue (see Table 3). Moreover, obese subjects also had more LPL activity per cell than controls (p < 0.001, Fig. 1, middle left panel). In VAT, although the cells were smaller, obese subjects had 5.4 times more LPL activity than controls (p < 0.001). If BW was taken into account (Fig. 1, lower left panel, note that activity is expressed in μmol OA body weight/min), SAT from obese patients had an increased TAG storage capacity 7.6 times higher than controls (p < 0.001). In VAT, TAG storage capacity was 4.2 times higher than controls (p < 0.001). Taken together, this could trigger the observed TAG accumulation described in Table 3.
LPL and HSL activity in subcutaneous (SAT) and visceral (VAT) adipose tissue. Activity is expressed as mean ± SEM. C control, OB obese before surgery, 6M obese 6 months after surgery, 12M obese 12 months after surgery. Statistical significance was calculated by one-way ANOVA (upper left corner) and Tukey’s post-test between groups. Differences from controls are shown by asterisks, differences from obese subjects by degree symbols, differences between 6 and 12 months after surgery by plus signs, and between SAT and VAT by multiplication signs. One symbol, p < 0.05; two symbols, p < 0.01; and three symbols, p < 0.001
After bariatric surgery, SAT LPL activity per gram of tissue decreased. At 6M, it was still slightly higher than controls (p < 0.05), but at 12M, it had practically normalized. With weight loss, cells were smaller and exhibited less LPL activity (Fig. 1, middle left panel). These data were reinforced by a 50% reduction in lipid content per cell 12M after surgery (Table 3).
A progressive decrease in TAG storage capacity during the follow-up period could be seen as total LPL at 6M was 69% lower (p < 0.001) and 79% 12M after surgery (p < 0.001 vs OB).
HSL Activity
The right panel of Fig. 1 shows HSL activity in SAT and VAT. Similarly to LPL, HSL values observed in SAT and VAT from obese or control subjects were approximately the same. If we compare this two groups, obese individuals had 2.8 times more activity per gram of tissue than controls (p < 0.001 vs control) in both SAT and VAT, despite having less cells per gram of tissue. Moreover, they also have more HSL activity per cell than controls (p < 0.001, Fig. 1, middle right panel), which increases fatty acid delivery to plasma two-fold (Table 2). This higher lipolytic capacity was also reflected when data were expressed as HSL activity per BW. In both SAT and VAT, obese subjects displayed five times more activity than controls (p < 0.001, Fig. 1, lower right panel).
After bariatric surgery, HSL activity per gram of tissue in SAT showed a linear increase until 12M (r = 0.98, p < 0.001). Thus, lipolytic capacity of obese patients seemed to increase after surgery (p < 0.001, 6M and 12M vs OB). Nevertheless, we must take into account that cells are progressively smaller; thus, HSL activity per cell remained in a steady state. Total HSL activity was also stable during the follow-up period (Fig. 1, lower right panel), which was in agreement with the elevated concentrations of NEFA observed in plasma.
Correlations Between the Lipases and the Parameters Measured
If we consider the obese group, SAT LPL correlated positively with waist (p < 0.05) and negatively with hip (p < 0.05), while no relationship was found between SAT HSL and any of the biochemical or anthropometric parameters measured. In such individuals, VAT LPL correlated positively with weight and hip (p < 0.05), excess weight, BMI, SAT (p < 0.01), and total fat (p < 0.001); however, it correlated negatively with plasma NEFA (p < 0.01). VAT HSL correlated negatively only with waist (p < 0.05) and plasma TAG (p < 0.01).
During the weight loss period, there were positive correlations in SAT LPL with weight, excess weight, BMI, total fat, subcutaneous fat, percent of SAT lipids, SAT TAG, HOMA-IR (p < 0.001), insulin (p < 0.01), and plasma TAG (p < 0.05). Negative correlations were observed of SAT HSL with excess weight, waist and hip (p < 0.01), BMI, total fat, subcutaneous fat, percent of lipids, and TAG in SAT (p < 0.05).
Discussion
In order to adequately explain the observed lipase activities (LPL and HSL) in fat depots (SAT or VAT), it is critical to take note of fat cell size in obese people and its diminution throughout weight loss.
Before Surgery
Cells of both fat depots are larger in morbidly obese subjects compared to the control group, and visceral fat cells are slightly larger than subcutaneous types. We estimated the relative size of fat cells from the amount of DNA in 1 g of tissue. This idea is also supported by the fact that fat cells of obese individuals have an increased ratio of grams of tissue per milligram of protein (calculation devised by Robin and collaborators [26]) and contain more total lipid in both SAT and VAT depots compared with control. However, some authors are of the opinion that no differences exist in the size of subcutaneous and visceral fat cells in morbidly obese individuals [27].
For morbidly obese subjects, LPL activity from SAT and VAT is higher than in control subjects. This finding is in agreement with another study in which the same methods to determine the amount of DNA and LPL activity in biopsies were followed; in that case, the authors also reported higher enzymatic activity in both obese and morbidly obese tested subjects than in control subjects [28]. The LPL activity measured per gram of adipose tissue was also similar to another previous study in which small amounts of human biopsies were utilized [29], as was the case for this study. On the other hand, another group has reported that LPL activity from obese hypertriglyceridemic adipose tissue, when expressed per gram of tissue, was 44% less than in nonobese subjects [30].
In control subjects, LPL activity from SAT and VAT is approximately equal. Cells of both fat depots are extremely similar with respect to lipid quantity, DNA, and protein. This is in agreement with other authors [31] who found no differences between SAT and VAT. However, their LPL values for both depots were very high, on the order of 70,000 mU/g tissue.
In morbidly obese subjects, HSL activity from SAT and VAT is higher than in controls. Thus, as other authors have described [32], basal lipolysis (HSL activity measured in the absence of regulators such as catecholamines, insulin, growth hormone, etc.) generally correlates positively with cell size, i.e., larger adipocytes have higher basal lipolytic activity per cell. However, some authors did not observe differences in the lipolytic capacity of obese and control subjects [9]. In addition, they noticed that HSL activity was not affected by weight loss. In the present study, the basal HSL activity observed in the morbidly obese group, from both SAT and VAT, was up to three times higher than that described by Ramis et al. [33]. In control subjects, the HSL activity from SAT and VAT has approximately the same value.
These data suggest that morbidly obese patients would have an increased capacity to store TAG in their adipose tissue depots.
After Surgery
During the follow-up period up to 1 year after bariatric surgery, subcutaneous fat cells tended to decrease in size. This is supported by an increase in the amount of DNA per gram of tissue and lower amounts of lipid per DNA. There was a decrease in SAT LPL activity that approached control values. This may indicate an impaired ability of TAG to enter into the cell compared with what is seen in obese cases. In contrast, SAT HSL activity in obese individuals remained invariable 1 year after surgery and continues to be significantly higher than in controls. This shows that, despite the conclusions of some authors [9], lipolytic activity does not normalize after weight loss. After surgery, patients followed an equilibrated diet and in most cases increased their physical activity. In this situation, HSL should remain highly active, perhaps due to adrenergic stimulation of lipolysis in adipose tissue reinforced by the reduction in insulin-mediated inhibition of HSL (HOMA-IR improvement). This would lead to the observed continuous release of NEFA into the bloodstream, avoiding an accumulation in adipose tissue. Therefore, plasma NEFA are probably sent to muscles to be oxidized. In fact, some authors [34] observed that the percentage of plasma NEFA captured by muscle during exercise or at rest is on the order of 57% and 40%, respectively, in morbidly obese patients after gastric bypass surgery.
Frayn et al. [8] estimated the rate of action in adipose tissue (HSL/LPL) around 1.5, and it was similar in control and morbidly obese subjects. In contrast, we observed that in the control group, the rates were 1.50 for SAT and 1.76 for VAT, while for obese subjects, the rates were 1.00 and 0.90, respectively. In our study, the differences between storage capacity (subcutaneous LPL activity plus visceral LPL activity) and lipolytic capacity of adipose tissue (subcutaneous HSL activity plus visceral HSL activity) in controls was −9 mU/g tissue and −1196 mU for total body weight; however, the values in obese subjects were +3 mU/g tissue and +644 mU total. During the weight loss period, negative balances were obtained.
In summary, when obese patients lose weight, they lose not only part of the lipid content of the cells but also the capacity to store TAG in SAT depots.
Abbreviations
- HOMA-IR:
-
Homeostasis model assessment of insulin resistance
- NEFA:
-
Nonesterified fatty acid
- LPL:
-
Lipoprotein lipase
- HSL:
-
Hormone-sensitive lipase.
References
Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. 2000;21:697–738.
Kolehmainen M, Vidal H, Ohisalo JJ et al. Hormone sensitive lipase expression and adipose tissue metabolism show gender differences in obese subjects after weight loss. Int J Obes Relat Metab Disord. 2002;26(1):6–16.
Marin P, Andersson B, Ottosson M, et al. The morphology and metabolism of intraabdominal adipose tissue in men. Metabolism. 1992;41:1242–48.
Rebuffe-Scrive M, Andersson B, Olbe L, et al. Metabolism of adipose tissue in intraabdominal depots of nonobese men and women. Metabolism. 1989;38:453–8.
Meisner H, Tenney K. pH as an indicator of free fatty acid release from adipocytes. J Lipid Res. 1977;18:774–6
Large V, Reynisdottir S, Langin D, et al. Decreased expression and function of adipocyte hormone-sensitive lipase in subcutaneous fat cells of obese subjects. J Lipid Res. 1999;40:2059–66.
Hansen EN, Torquati A, Abumrad NN. Results of bariatric surgery. Annu Rev Nutr. 2006;26:481–511.
Frayn KN, Coppack SW, Fielding BA, et al. Coordinated regulation of hormone-sensitive lipase and lipoprotein lipase in human adipose tissue in vivo: implications for the control of fat storage and fat mobilization. Adv Enzyme Regul. 1995;35:163–78.
Lofgren P, Hoffstedt J, Ryden M, et al. Major gender differences in the lipolytic capacity of abdominal subcutaneous fat cells in obesity observed before and after long-term weight reduction. J Clin Endocrinol Metab. 2002;87:764–71.
Sociedad Española para el estudio de la Obesidad (SEEDO). SEEDO'2000 consensus for the evaluation of overweight and obesity and the establishment of criteria for therapeutic intervention. Med Clin. 2000;115:587–97.
(1988) The Airlie (VA) Consensus Conference. In: Loham T, Roche A, Martorel R (eds.) Standardization of anthropometric measurements. Human Kinetics, Champaign, pp. 20–37.
Deurenberg P, Weststrate JA, Seidell JC. Body mass index as a measure of body fatness: age- and sex-specific prediction formulas. Br J Nutr. 1991;65:105–14.
Bonora E, Micciolo R, Ghiatas AA. Is it possible to derive a reliable estimate of human visceral and subcutaneous abdominal adipose tissue from simple anthropometric measurements? Metabolism. 1995;44:1617–25.
Belfrage P, Vaughan M. Simple liquid-liquid partition system for isolation of labeled oleic acid from mixtures with glycerides. J Lipid Res. 1969;10:341–4.
Blay M, Peinado-Onsurbe J, Grasa MM, et al. Effect of oral oleoyl-estrone treatment on plasma lipoproteins and tissue lipase activities of Zucker lean and obese female rats. Int J Obes Relat Metab Disord. 2002;26:618–26.
Briquet-Laugier V, Ben-Zeev O, Doolittle MH. Determining lipoprotein lipase and hepatic lipase activity using radiolabeled substrates. Methods Mol Biol. 1999;109:81–94.
Ballart X, Siches M, Peinado-Onsurbe J, et al. Isoproterenol increases active lipoprotein lipase in adipocyte medium and in rat plasma. Biochimie. 2003;85:971–82.
McCoy MG, Sun GS, Marchadier D, et al. Characterization of the lipolytic activity of endothelial lipase. J Lipid Res. 2002;43:921–9.
Ramírez I, Kryski AJ, Ben-Zeev O, et al. Characterization of triacylglycerol hydrolase activities in isolated myocardial cells from rat heart. Biochem J. 1985;232:229–36.
Stam H, Hülsmann WC. Comparison of heparin-releasable lipase and tissue neutral lipase activity of rat heart. Biochem Int. 1983;7:187–95.
Mairal A, Langin D, Arner P, et al. Human adipose triglyceride lipase (PNPLA2) is not regulated by obesity and exhibits low in vitro triglyceride hydrolase activity. Diabetologia. 2006;49:1629–36.
Rodríguez-Sureda V, Peinado-Onsurbe J. A procedure for measuring triacylglyceride and cholesterol content using a small amount of tissue. Anal Biochem. 2005;343:277–82.
Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54.
Vytasek R. A sensitive fluorometric assay for the determination of DNA. Anal Biochem. 1982;120:243–8.
Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–19.
Robin AP, Greenwood MR, Askanazi J, et al. Influence of total parenteral nutrition on tissue lipoprotein lipase activity during chronic and acute illness. Ann Surg. 1981;194:681–6.
Garaulet M, Hernandez-Morante JJ, Lujan J, et al. Relationship between fat cell size and number and fatty acid composition in adipose tissue from different fat depots in overweight/obese humans. Int J Obes. 2006;30:899–905.
Bullo M, Garcia-Lorda P, Peinado-Onsurbe J, et al. TNFα expression of subcutaneous adipose tissue in obese and morbid obese females: relationship to adipocyte LPL activity and leptin synthesis. Int J Obes Relat Metab Disord. 2002;26:652–8.
Taskinen MR, Nikkila EA, Huttunen JK, et al. A micromethod for assay of lipoprotein lipase activity in needle biopsy samples of human adipose tissue and skeletal muscle. Clin Chim Acta. 1980;104:107–17.
Taskinen MR, Nikkilä EA, Kuusi T. Lipoprotein lipase activity of adipose tissue skeletal muscle and post-heparin plasma in primary endogenous hypertriglyceridaemia: relation to lipoprotein pattern and to obesity. Eur J Clin Inves. 1982;12:433–8.
Panarotto D, Poisson J, Devroede G, et al. Lipoprotein lipase steady-state mRNA levels are lower in human omental versus subcutaneous abdominal adipose tissue. Metabolism. 2000;49:1224–7.
Jacobsson B, Smith U. Effect of cell size on lipolysis and antilipolytic action of insulin in human fat cells. J Lipid Res. 1972;13:651–6.
Ramis JM, Salinas R, Garcia-Sanz JM, et al. Depot- and gender-related differences in the lipolytic pathway of adipose tissue from severely obese patients. Cell Physiol Biochem. 2006;17:173–80.
Thyfault JP, Krauss MR, Hickner RC, et al. Impaired plasma fatty acid oxidation in extremely obese women. Am J Physiol Endocrinol Metab. 2004;287:E1076–81.
Acknowledgments
This research was funded by the Fondo de Investigación Sanitaria del Instituto de Salud Carlos III of the Spanish Ministry for Health and Consumer Affairs (PI030042, PI030024, and PI070079). Both R. Llamas and E. Pardina were awarded grants by the same institution. We express thanks to Professor M. Llobera for his inestimable help.
Conflict of interest
The authors have declared that no conflict of interest exists. The authors who have taken part in this study do not have a relationship, past or present, with the manufacturers of the drugs involved and did not receive funding from the manufacturers to carry out their research.
Writing assistance
English grammar and language have been corrected by American Journal Experts (https://doi.org/www.journalexperts.com).
Author information
Authors and Affiliations
Corresponding author
Additional information
E. Pardina and A. Lecube contributed equally to this study.
J.A. Baena-Fustegueras and J. Peinado-Onsurbe share senior authorship.
Rights and permissions
About this article
Cite this article
Pardina, E., Lecube, A., Llamas, R. et al. Lipoprotein Lipase but Not Hormone-Sensitive Lipase Activities Achieve Normality After Surgically Induced Weight Loss in Morbidly Obese Patients. OBES SURG 19, 1150–1158 (2009). https://doi.org/10.1007/s11695-009-9853-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-009-9853-3