Abstract
Purpose
Side-to-side jejunoileal bypass with proximal loop ligation (SSJIBL) has significant glucose-lowering and weight-control effects; however, no study has elucidated which segment is most effective in SSJIBL. This study investigated the effect of proximal small intestinal bypass (PSIB), middle small intestinal bypass (MSIB), and distal small intestinal bypass (DSIB) on metabolic improvement in streptozotocin (STZ)-induced diabetic rats.
Materials and Methods
STZ-induced diabetic rats were divided into four groups: PSIB, MSIB, DSIB, and sham-operated. The primary outcome measures were body weight, food intake, fasting blood glucose (FBG) levels, oral glucose tolerance (OGTT), insulin tolerance (ITT), serum insulin, gut hormones, serum lipid profile, and liver function levels.
Results
Global body weight in the DSIB group was lower than that in the PSIB group. The global food intake in the PSIB group was lower than that in the MSIB group. The PSIB group had a slightly better glucose-lowering effect than the MSIB and DSIB groups. The PSIB, MSIB, and DSIB groups all had improvement in insulin sensitivity at postoperative week 6. The MSIB group exhibited the best improvement in lipid homeostasis. Serum insulin and leptin levels were higher, and serum ghrelin levels were lower in the operated groups than in the sham group.
Conclusions
This study provides experimental evidence that PSIB surgery induces a better glucose-lowering effect than DSIB surgery, and MSIB induced the best improvement in lipid homeostasis, whereas DSIB was even more advantageous in terms of weight control in the STZ-induced diabetic rat model.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One of the initial metabolic/bariatric surgery (MBS) procedures developed in 1954 was jejunoileal bypass (JIB) [1, 2]. However, JIB was replaced with newer techniques due to its serious side effects [3, 4]. At certain medical centers, the JIB procedure is performed with modifications [5, 6] or in combination with other bariatric surgeries, such as sleeve gastrectomy (SG) [7] or gastric clip [8]. These kinds of procedure are still investigational, and data in humans are very limited. Based on the classic JIB, we designed a new jejunoileal bypass procedure that we termed “side-to-side jejunoileal bypass plus proximal loop ligation” or SSJIBL. Our previous studies showed that side-to-side jejunoileal bypass (SSJIB) resulted in better glucose-lowering effects than end-to-side jejunoileal bypass [9], and single-path (with ligation of the first portion of the bypassed loop) SSJIB showed better lowering effects than dual-path (without ligation) SSJIB [10].
To the best of our knowledge, no systematic comparisons of the improvement in glycemic control after exclusion of different segments of the small intestine have been performed. We developed three experimental modified jejunoileal bypass procedures using streptozotocin (STZ)-induced type 2 diabetic rats: proximal small intestinal bypass (PSIB), middle small intestinal bypass (MSIB), and distal small intestinal bypass (DSIB). All anastomoses in the three procedures were performed in a single-path side-to-side manner that has been established previously [11]. The three intestinal procedures (PSIB, MSIB, and DSIB) were compared to determine the contributions of these regions to weight control, glucose lowering, lipid homeostasis, and protection against liver damage after the surgical procedure.
Materials and Methods
Animal Model
The experiments were conducted in accordance with and were approved by the Ethical Committee on Animal Experimentation. A total of 60 male 7-week-old Sprague–Dawley (SD) rats weighing 264 ± 12 g were purchased from the Shanghai SLAC Laboratory Animal Co. Ltd. (Shanghai, China). The rats were acclimated for 1 week. Fed blood glucose (FEG) of these rats was measured before the STZ injection, and their FEG values were 4.4–8.4 mmol/L. Then, an intraperitoneal injection of 60 mg/kg streptozotocin was administered (STZ, Sigma, St. Louis, MO, USA). After 72 h, 40 of the 60 SD rats with FEG levels > 16.7 mmol/L (300 mg/dL) were considered diabetic rats and were selected for further analyses [12].
Experimental Protocol
Body weight, food intake, fasting blood glucose level (FBG), oral glucose tolerance (OGTT), and insulin tolerance (ITT) were measured before surgery.
The rats were randomly divided into groups of 10 rats after a week of acclimation for the following procedures: PSIB, MSIB, DSIB, and a sham operation.
Surgical Techniques
After fasting for 14 h, an operation was performed on the rats while they were under anesthesia (isoflurane, 4% for induction and 2% for maintenance). The abdomen was shaved carefully to avoid any scuffing of the epidermis, and the peritoneal cavity was accessed via a 4-cm midline incision.
Both sides of the abdominal incision were covered with saline-soaked gauze, and the entire small intestine was carefully lifted out of the abdominal cavity with a cotton swab and arranged in order. The length of the small intestine was measured along its mesangial margin with 0 silk sutures, ranging from 102 to 126 cm, with an average length of approximately 115 cm.
Proximal Small Intestinal Bypass
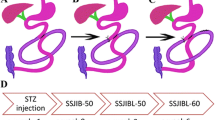
For the PSIB group, the reference point was 35 cm proximal to the ileocecal valve. An isoperistaltic side-to-side anastomosis between the proximal jejunum and the ileum was used to restore the bowel continuity by bypassing approximately 60% of the length of the small bowel from this point to 5 cm distal to the Treitz ligament (Fig. 1A). Enteric anastomoses were performed with a single layer of interrupted 6–0 nylon sutures (Hangzhou Huawei Medical Treatment Articles Co., Ltd). The length of the anastomosis was approximately 4 mm, and the number of suture needles was approximately 16–20. Luminal occlusion was performed using ligation of 0 silk sutures at the first portion of the bypassed segment. Furthermore, the abdominal cavity was closed with 3–0 silk sutures (ETHICON SA84G, Johnson & Johnson) after no bleeding or leakage was detected (Fig. 1D–F).
Surgical procedures. Schematic and operative photograph of the small intestinal bypass (PSIB, MSIB, or DSIB) model. A Proximal small intestinal bypass (PSIB). The PSIB procedure preserved 5 cm of the proximal jejunum and 35 cm of the ileum. B Middle small intestinal bypass (MSIB). The MSIB procedure preserved 20 cm of the proximal jejunum and 20 cm of the distal ileum. C Distal small intestinal bypass (DSIB). The DSIB procedure preserved 35 cm of the jejunum and 5 cm of the terminal ileum. D An isoperistaltic side-to-side anastomoses between jejunum and ileum. E Luminal occlusion was performed using ligation of 0 silk sutures at the first portion of the bypassed segment. F Operative photograph of side-to-side jejunoileal bypass with proximal loop ligation. G The design and grouping of the study
Middle Small Intestinal Bypass
For the MSIB group, a point 20 cm proximal to the ileocecal valve was used as the reference point. Starting proximally from this point to 20 cm distal to the Treitz ligament, approximately 60% of the length of the entire small bowel was bypassed, and bowel continuity was restored by side-to-side anastomosis between the proximal jejunum and the ileum. Luminal occlusion was performed at the first portion of the bypassed segment using ligation of 0 silk sutures (Fig. 1B).
Distal Small Intestinal Bypass
For the DSIB group, a point 5 cm proximal to the ileocecal valve was used as the reference point. Starting proximally from this point to 35 cm distal to the Treitz ligament, approximately 60% of the length of the entire small bowel was bypassed, and bowel continuity was restored by side-to-side anastomosis between the proximal jejunum and the ileum. Luminal occlusion was performed at the first portion of the bypassed segment using ligation of 0 silk sutures (Fig. 1C).
Sham Operation
For the rats in the sham group, the abdomen was accessed through a 4-cm midline incision, and the intestine was exposed as in the surgery groups. The abdominal cavity was closed using 3–0 silk sutures. The operative time was prolonged to induce a degree of anesthetic stress comparable to that experienced by the operated rats. All rats were given 10 mL sterile saline subcutaneously after surgery, and the animals were placed in individual cages to recover from anesthesia.
Postoperative Care
All rats fasted for 24 h. Free access to tap water was allowed from the first postoperative day to the end of the experiment, and standard rodent chow was provided 1 day after surgery.
Measurement of Different Intestinal Regions to Weight Control
All rats were fed a standard diet. Body weight and food intake were measured at baseline and postoperative weeks 1, 2, 3, 4, 5, and 6.
Measurement of Different Intestinal Regions to Glucose Lowering
Glucose levels were measured using an electronic glucometer (Accu-Chek Performa®, Roche Diagnostic, Switzerland) in blood obtained from the tail vein of conscious rats. For FBG, food was taken away at 8:00 AM, and after 6 h of fasting, the glucose level was measured at 2:00 PM before surgery and at 1, 2, 3, 4, 5, and 6 weeks postoperatively.
An OGTT was performed before surgery and at 2 and 6 weeks postoperatively. The animals fasted overnight, and glucose measurements were performed on blood obtained immediately before and 15, 30, 60, 90, 120, and 180 min after the administration of 1 g/kg glucose (20% dextrose) via oral gavage.
An ITT was performed before surgery and at 2 and 6 weeks postoperatively by measuring glucose levels before and 15, 30, 45, and 60 min after intraperitoneal injection of 0.5 IU/kg human insulin (Wanbang Biopharmaceuticals, Jiangsu, China).
Measurement of Different Intestinal Regions to Lipid Homeostasis
The rats were euthanized in the morning with 14-h fasting, and blood was withdrawn from the portal vein and placed into a biochemical tube containing coagulant. After centrifugation at 3000 rpm for 15 min at 4 °C, the isolated serum was immediately transferred to a new test tube and stored at − 80 °C until analysis. Serum total cholesterol (CHOL), triglyceride (TG), high-density lipoprotein (HDL), low-density lipoprotein (LDL), non-high-density lipoprotein (NHDL), and non-esterified fatty acid (NEFA) were measured by automatic biochemical analyzer. The tests and analyses were conducted in the biochemical laboratory.
Measurement of Different Intestinal Regions to Protection Against Liver Damage and the Nutrition Status
Total protein (TP), albumin (ALB), total bilirubin (TBIL), direct bilirubin (DBIL), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) were measured by automatic biochemical analyzer. The tests and analyses were conducted in the biochemical laboratory.
Measurement of Different Intestinal Regions to Changes of Gastrointestinal Hormones
Serum insulin, glucagon-like peptide-1 (GLP-1), peptide YY (PYY), leptin, and ghrelin levels were measured in blood obtained through the portal vein. The blood samples were immediately centrifuged at 3000 rpm for 13 min. The serum was decanted immediately and stored at − 80 °C until analysis. All serum indexes were detected by enzyme-linked immunosorbent assay kits (Merck Millipore, USA).
Statistical Analysis
The data are expressed as the means ± standard errors of the mean (SEMs). All analyses were performed using GraphPad Prism version 8.0, and the level of significance was set at 0.05. The area under the curve (AUC) was calculated using trapezoidal integration. The differences between groups were analyzed by analysis of variance (ANOVA). Body weight, food intake changes, FBG, OGTT, and ITT over time were analyzed using two-way ANOVA. The Tukey test was performed for pairwise comparisons between groups.
Results
Body Weight Control
From the second to the sixth postoperative week, all rats in the operated and sham groups had a slight weight gain, and rats that underwent the DSIB operation exhibited a lower weight gain of their preoperative weight than those in the PSIB and sham groups (both P < 0.01) (Fig. 2A).
Daily Intake of Food
Rats in all the operated groups had a significantly lower global food intake than those in the sham group (all P < 0.001), and the rats who underwent the PSIB operation consumed significantly less food than those in the MSIB group throughout the postoperative period (both P < 0.05) (Fig. 2B).
Fasting Blood Glucose Level Control
Figure 3A shows the FBG of the operated and sham group rats after surgery. All rats in the PSIB, MSIB, and DSIB groups exhibited lower FBG levels than the sham rats at postoperative weeks 1 to 6 (all P < 0.001), and rats in the PSIB group exhibited lower FBG levels than the DSIB rats (P < 0.05). There was no difference between the MSIB and DSIB groups (P > 0.05).
Fasting blood glucose (FBG) and OGTT. A Average FBG levels of rats in all groups before and after surgery. B Area under the curve of the OGTT values. C Oral glucose tolerance test (OGTT) values 2 weeks after surgery. D OGTT values 6 weeks after surgery. *All P < 0.01 for the PSIB, MSIB, and DSIB groups versus the sham group, #P < 0.05 for PSIB versus DSIB
Oral Glucose Tolerance Test
There was no significant difference in OGTT between rats allocated to the different surgery and sham groups before surgery. At postoperative weeks 2 and 6, the rats in the PSIB, MSIB, and DSIB groups exhibited significant improvements in glucose tolerance compared with the sham operation group, as exhibited by the lower 30- or 60-min peak glucose levels in response to glucose gavage (Fig. 3C and Fig. 3D) and lower AUCs for the OGTT values (Fig. 3B) (all P < 0.001). However, no significant difference was observed among the PSIB, MSIB, and DSIB groups at postoperative weeks 2 and 6 (all P > 0.05).
Comparisons of Insulin Sensitivity
Figure 4 shows the insulin tolerance of the operated and sham group rats. After surgery, only rats in the MSIB group exhibited improvement in insulin sensitivity compared with the sham group at postoperative week 2, as demonstrated by the greater 45-min glucose disappearance rate during the intraperitoneal ITT (P < 0.05) (Fig. 4A) and lower AUCs for ITT values (Fig. 4C) (P < 0.05). However, at postoperative week 6, all rats in the PSIB, MSIB, and DSIB groups exhibited improvement in insulin sensitivity compared with the sham group, as demonstrated by greater 60-min glucose disappearance rates during the intraperitoneal ITT (all P < 0.05) (Fig. 4B). There was no significant difference among the PSIB, MSIB, and DSIB groups (all P > 0.05).
A–C Insulin tolerance test (ITT). Mean blood glucose concentrations (expressed as a percentage of baseline values ± SEM) after intraperitoneal administration of insulin (0.5 IU/kg). *P < 0.05 for the MSIB group versus the sham group, #P < 0.05 for the MSIB group versus the DSIB group. **All P < 0.05 for the PSIB, MSIB, and DSIB groups versus the sham group, ***All P > 0.05 for the PSIB, MSIB, and DSIB groups versus the sham group
Comparisons of Serum Lipids Levels
At postoperative week 6, all rats in the PSIB, MSIB, and DSIB groups exhibited lower serum CHOL, LDL, NHDL, and NEFA levels than the sham group (all P < 0.05), and only the rats in the MSIB group exhibited lower serum TG levels than the sham group (P < 0.01). Rats in the MSIB group exhibited lower serum CHOL, HDL, and NHDL levels than those in the PSIB group (all P < 0.05) (Fig. 5).
Protection Against Liver Function Injury and the Assessment of the Nutrition Status
At postoperative week 6, all rats in the PSIB, MSIB, and DSIB groups experienced significant improvements in liver function injury compared with those in the sham operation group, as exhibited by the lower serum ALT and DBIL levels (all P < 0.05). There was no significant difference in serum ALT, AST, TBIL, or DBIL levels among the three operated groups (all P > 0.05) (Fig. 6).
The rats in the PSIB, MSIB, and DSIB groups showed no significant decrease in serum ALB levels compared with those in the sham group (all P > 0.05), and those in the PSIB group had higher serum TP and ALB levels than those in the MSIB group (both P < 0.05) (Fig. 6).
Measurement of Serum Insulin and Gut Hormones Levels
Serum fasting insulin and gut hormone levels at 6 weeks postoperatively are shown in Fig. 6. Only the PSIB group exhibited higher serum insulin and leptin levels than the sham group (both P < 0.01). There were no significant differences among the groups in serum GLP-1 or PYY concentration. All rats in the PSIB, MSIB, and DSIB groups exhibited lower ghrelin concentrations than the sham group (all P < 0.05). No significant difference was observed in insulin, GLP-1, PYY, or ghrelin secretion among the PSIB, MSIB, and DSIB groups (all P > 0.05). Furthermore, Spearman correlation analysis showed that there was a positive correlation between serum insulin levels and serum leptin levels. And serum insulin, GLP-1, leptin, and ghrelin were significantly correlated with FBG and AUC-OGTT.
Discussion
This study is the first systematic work to compare the effects of different intestinal bypass surgical procedures on weight control and improvement in glucose tolerance and lipid homeostasis in a nonobese diabetic rat model. All three different intestinal bypass surgical procedures have specific advantages. Our results provide experimental evidence that exclusion of the foregut, midgut, or hindgut mediates changes in weight control and glucose homeostasis. However, the proximal small intestine played a greater role in glucose metabolism; the midgut played a greater role in lipid metabolism; and the distal small intestine played a greater role in body weight homeostasis in the nonobese diabetic rats.
Four possible rationales were developed to explain the different postoperative glucose-lowering effects of the surgical procedures: (a) weight loss hypothesis, (b) caloric ingestion, (c) gastrointestinal hormones, and (d) islet β-cell function.
In this study, we found that PSIB exhibited significantly lower FBG levels without lower body weight compared with the sham group. Furthermore, our previous study demonstrated that the significant effects on diabetes control after mid-to-distal small bowel resection were independent of alterations in body weight [13]. These results suggest that weight loss does not contribute to changes in glucoregulatory mechanisms during intestinal bypass procedures. Moreover, we also found that the rats in the DSIB group showed significantly lower global body weights than those in the PSIB group. The lower body weight in the DSIB group could be associated with nutrient malabsorption. Previous studies have shown that jejunectomy has a relatively small impact on nutrient absorption because the retained ileum has a good compensatory capacity, which can compensate for the nutritional digestion and malabsorption caused by jejunectomy. In contrast, after ileectomy, the remaining jejunum cannot compensate for the specific digestive and absorption functions of the ileum [14]. These may be the reasons why the effect of DSIB is better on weight loss than that of PSIB.
The better hypoglycemic effect of PSIB compared with MSIB and DSIB may have been related to less food intake (Fig. 2). The proximal small intestine is the main part of nutrient digestion and absorption; thus, the PSIB operation, which bypasses the proximal small intestine, may induce better control of the diet in STZ-induced diabetic rats. Compared with the sham group, the three bypass procedure groups exhibited significantly decreased food intake postoperatively, accompanied by a decrease in the level of serum ghrelin. However, there was no significant correlation between the decreased level of serum ghrelin hormone and food intake. In addition, the change in food intake may also be related to the level of GLP-1 secreted by the intestinal tract [3, 15]. Our results showed that there was a significant negative correlation between food intake and serum GLP-1 (Fig. 7). Food intake after small bowel exclusion is regulated by many factors, and the regulatory mechanism of bypass in different parts of the small intestine for food intake needs to be further studied.
A Serum gut hormones for all groups at 6 weeks after surgery. B Correlation of serum insulin and leptin levels. C Heatmap analysis of the Pearson correlation of gut hormones and metabolic syndrome–related indexes. Red represents a positive correlation, and blue indicates a negative correlation. Error bars are expressed as means ± SEMs. Statistical significance was determined by one-way ANOVA with Tukey tests for multiple-group comparisons. GLP-1, glucagon-like peptide-1; PYY, peptide YY. *P < 0.05, **P < 0.01
Our results showed that PSIB significantly improved serum insulin levels in STZ-induced diabetic rats, and the level of insulin was positively correlated with the level of serum leptin (Fig. 7). Previous studies have shown that elevated leptin can promote insulin resistance in patients with liver cirrhosis [16], whereas another study showed that leptin can induce the expression of insulin signal-related genes in the jejunum of obese patients [17]. A longitudinal study in Guatemala showed that leptin lowered blood sugar levels in women [18]. Our study found that there was a significant negative correlation between serum leptin levels and FBG levels in rats. Whether leptin mediates the improvement of islet β-cells and hypoglycemic effects in diabetic rats remains to be further studied.
Regrettably, we did not have histological measurements of islet β-cells from the diabetic rats in the different operation groups, and therefore, there was no direct evidence to determine whether different intestinal bypass procedures have different effects on islet β-cell function in diabetic rats. However, the OGTT is a dynamic assessment of β-cell function, measuring the insulin secretion response to a standardized dose of oral glucose. Our animal experiments showed that PSIB induced significantly better improvement in glucose tolerance than DSIB, which might be due to the confounding effect of anatomical differences between the surgical procedures, but this needs to be further studied.
Generally, ghrelin is mainly secreted in the fundus of the stomach, and the mechanism affecting ghrelin secretion in the body is still unknown. Some scholars have speculated that the physiological secretion of ghrelin may be related to the secretion of gastrointestinal hormones such as cholecystokinin (CCK), GLP-1, and peptide YY (PYY) and the movements of the small intestine [19]. In our experiments, the small intestinal bypass procedures produced observable changes in the concentration of gastrointestinal hormones (such as insulin and leptin), which may be related to the decreased level of ghrelin in the experimental group. Although the influence of gastrointestinal surgery on postoperative ghrelin secretion was inconsistent in many experiments, the secretion level of ghrelin in most gastrointestinal bypass surgery groups (including SG, JIB, SSJIBL) decreased in the short-term compared with the control group [20, 21]. In our experiment, short-term changes were mainly observed; however, long-term changes in postoperative gastrointestinal hormones need to be further investigated experimentally.
Our results showed that all three modified JIB procedures had significant efficacy in lowering total cholesterol and free fatty acids, and MSIB and DSIB had better efficacy in lowering serum lipids than PSIB. However, in terms of TG level, MSIB was superior to PSIB and DSIB (Fig. 5). Diabetes mellitus is often associated with dyslipidemia, characterized by elevated TG levels, decreased HDL cholesterol levels, and increased small dense LDL production [22], and high TG levels lead to insulin resistance [23]. Studies have shown that low-dose statin interventions can significantly improve blood lipid metabolism and decrease insulin resistance in patients [24]. Therefore, we believe that improvement in glucose metabolism in the STZ-induced diabetic rats after the modified JIB surgeries may be related to the decreased serum lipid level. The present animal study revealed that MSIB provided better efficacy in lowering serum lipid levels than PSIB and DSIB. Further clinical studies are expected to confirm the superiority of MSIB.
In addition, it is interesting to note that the modified JIB procedures also significantly improved liver function in STZ-induced diabetic rats. Our results showed that serum ALT, AST, TBIL, and DBIL levels in the PSIB, MSIB, and DSIB groups were significantly lower than those in the sham group (Fig. 6). However, the serum TP and ALB levels were decreased in the MSIB group compared with the PSIB and sham groups. The small intestine is the primary digestive and absorptive organ. Previous studies have shown that piglets, which have a longer small intestine length, showed higher serum ALB levels [25]. There have been no systematic studies on nutritional status after bypass (or resection) with different parts of the intestine, and our data suggest that bypass over a certain proportion (60%) of the length of the middle intestine may cause the risk of hypoproteinemia. The middle intestine may play a more important role than the proximal or distal small intestine in protein absorption. Our findings will provide a theoretical basis for the clinical application of modified JIB in the treatment of obesity and T2DM.
We did not perform histological examinations of the intestines for rats in any of the groups. Some studies have demonstrated that changes in intestinal glucose absorption are an important mechanism for improving glucose metabolism after bariatric surgery. Saeidi et al. reported that reprogramming of intestinal glucose metabolism is triggered by the exposure of the Roux limb to undigested nutrients, which renders the intestine a major tissue for glucose disposal, contributing to the improvement in glycemic control after RYGB [26]. Another study also showed that RYGB increases intestinal glucose disposal, whereas SG delays glucose absorption; both contribute to observed improvements in glycemia [27]. Clearly, more work is needed to clarify the relationship between long-term changes in compensatory hypertrophy of the retained small bowel loop and subsequent intestinal glucose uptake contributing to improvements in glucose metabolism after different partial small bowel bypass surgeries.
Our experimental design had one limitation. The nonobese diabetic rat model in this study was induced using high-dose STZ, which may have caused gradual weight control and increased consumption during the period of observation as well as a gradual increase in blood glucose. Because the rats did not have obesity, the short-term effects on weight control and food restriction post-intestinal bypass surgery could not be well studied.
These results demonstrate that PSIB surgery induced slightly better glucose-lowering effects than MSIB and DSIB and that all three modified JIB surgeries yielded equivalent stable improvements in glucose tolerance and insulin sensitivity in a nonobese diabetic rat model. MSIB induced better lipid-lowering effects, whereas DSIB had a greater effect on weight control than PSIB. Our results should be confirmed with further rodent and clinical studies.
References
Griffen WO Jr, Young VL, Stevenson CC. A prospective comparison of gastric and jejunoileal bypass procedures for morbid obesity. Ann Surg. 1977;186(4):500–9.
Singh D, Laya AS, Clarkston WK, et al. Jejunoileal bypass: a surgery of the past and a review of its complications. World J Gastroenterol. 2009;15(18):2277–9.
Thirlby RC. Jejunoileal bypass: can the mistake be corrected? Gastroenterology. 1990;98(6):1710–1.
Lutrzykowski M. Vertical gastric resection (sleeve gastrectomy) in a morbidly obese patient with past jejunoileal bypass. Obes Surg. 2007;17(3):423–5.
Melissas J, ErenTaskin H, Peirasmakis D, et al. A Simple food diverting operation for type 2 diabetes treatment. Preliminary results in humans with BMI 28–32 kg/m2. Obes Surg. 2016;30(10):4533–8.
Machytka E, Bužga M, Zonca P, et al. Partial jejunal diversion using an incisionless magnetic anastomosis system: 1-year interim results in patients with obesity and diabetes. Gastrointest Endosc. 2017;86(5):904–12.
Hassn A, Luhmann A, Rahmani S, et al. Medium-term results of combined laparoscopic sleeve gastrectomy and modified jejuno-ileal bypass in bariatric surgery. Obes Surg. 2016;26(10):2316–23.
Chao SH, Lin CL, Lee WJ, et al. Proximal jejunal bypass improves the outcome of gastric clip in patients with obesity and type 2 diabetes mellitus. Obes Surg. 2019;29(4):1148–53.
Duan J, Tan C, Xu H, et al. Side-to-side jejunoileal bypass induces better glucose-lowering effect than end-to-side jejunoileal bypass on nonobese diabetic rats. Obes Surg. 2015;25(8):1458–67.
Ren Q, Duan J, Cao J. Rapid improvement in diabetes after simple side-to-side jejunoileal bypass surgery: does it need a ligation or not? Obes Surg. 2018;28(7):1974–9.
Duan J, Yuan L, Zhou J. Side-to-side jejunoileal anastomosis plus proximal loop ligation: a novel intestinal bypass model for diabetic rats. Obes Surg. 2014;24(1):141–2.
Breen DM, Rasmussen BA, Kokorovic A, et al. Jejunal nutrient sensing is required for duodenal-jejunal bypass surgery to rapidly lower glucose concentrations in uncontrolled diabetes. Nat Med. 2012;18(6):950–5.
Duan J, Zhou J, Ren F, et al. Mid to distal small bowel resection with the preservation of the terminal ileum improves glucose homeostasis in diabetic rats by activating the hindgut-dependent mechanism. J Gastrointest Surg. 2014;18(6):1186–93.
Tappenden KA. Pathophysiology of short bowel syndrome: considerations of resected and residual anatomy. JPEN J Parenter Enteral Nutr. 2014;38(1 Suppl):14s–22s.
Gribble FM, Reimann F. Function and mechanisms of enteroendocrine cells and gut hormones in metabolism. Nat Rev Endocrinol. 2019;15(4):226–37.
Košuta I, Mrzljak A, Kolarić B, et al. Leptin as a key player in insulin resistance of liver cirrhosis? A cross-sectional study in liver transplant candidates. J Clin Med. 2020;9(2):560.
Gutierrez-Repiso C, Ho-Plagaro A, Santiago-Fernandez C, et al. Jejunal insulin signalling is increased in morbidly obese subjects with high insulin resistance and is regulated by insulin and leptin. J Clin Med. 2020;9(1):196.
He S, Le NA, Ramirez-Zea M, et al. Leptin partially mediates the association between early-life nutritional supplementation and long-term glycemic status among women in a Guatemalan longitudinal cohort. Am J Clin Nutr. 2020;111(4):804–13.
Steinert RE, Feinle-Bisset C, Asarian L, et al. Ghrelin, CCK, GLP-1, and PYY(3–36): secretory controls and physiological roles in eating and glycemia in health, obesity, and after RYGB. Physiol Rev. 2017;97:411–63.
Wang K, Zhou X, Quach G, et al. Effect of sleeve gastrectomy plus side-to-side jejunoileal anastomosis for type 2 diabetes control in an obese rat model. Obes Surg. 2016;26:797–804.
Zhong MW, Liu SZ, Zhang GY, et al. Effects of sleeve gastrectomy with jejuno-jejunal or jejuno-ileal loop on glycolipid metabolism in diabetic rats. World J Gastroenterol. 2016;22:7332–41.
Wu L, Parhofer KG. Diabetic dyslipidemia. Metabolism. 2014;63(12):1469–79.
van Stee MF, Graaf AA, Groen AK. Actions of metformin and statins on lipid and glucose metabolism and possible benefit of combination therapy. Cardiovasc Diabetol. 2018;17(1):94.
Gruzdeva O, Uchasova E, Dyleva Y, et al. Effect of different doses of statins on the development of type 2 diabetes mellitus in patients with myocardial infarction. Diabetes Metab Syndr Obes. 2017;10:481–9.
Wang M, Yang C, Wang QY, et al. The growth performance, intestinal digestive and absorptive capabilities in piglets with different lengths of small intestines. Animal. 2020;14(6):1196–203.
Saeidi N, Meoli L, Nestoridi E, et al. Reprogramming of intestinal glucose metabolism and glycemic control in rats after gastric bypass. Science. 2013;341(6144):406–10.
Cavin JB, Couvelard A, Lebtahi R, et al. Differences in alimentary glucose absorption and intestinal disposal of blood glucose after Roux-en-Y gastric bypass vs sleeve gastrectomy. Gastroenterology. 2016;150(2):454-64.e9.
Funding
This study was supported by the National Natural Science Foundation of China (Grant Nos. 81500652, 81760156, 81960154, and 82060161) and the Natural Science Foundation of Jiangxi Province (Grant Nos. 20171ACB21062, 2018ACB21040, 20203BBGL73185, 20212BAB206020).
Author information
Authors and Affiliations
Contributions
Designed the studies: JD. Conducted experiments: CT, ZZ, FT, XL, QP, JC, and JD. Analyzed the data: CT, ZZ, FT, XL, QP, and JD. Wrote the manuscript: CT and JD. All authors approved the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
All animal studies were approved by the Laboratory Animal Ethics Committee of Nanchang University. Standard animal care and laboratory guidelines were followed according to the ARRIVE guidelines.
Informed Consent Statement
Informed consent does not apply.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key Points
1. Proximal small intestinal bypass induces a better glucose-lowering effect.
2. Middle small intestinal bypass exhibits a greater improvement in lipid homeostasis.
3. Distal small intestinal bypass has a stronger effect on weight control.
Rights and permissions
About this article
Cite this article
Tan, C., Tao, F., Zheng, Z. et al. Bypassing Different Parts of the Small Intestine Determines Different Metabolic Effects in Streptozotocin-Induced Diabetic Rats. OBES SURG 32, 671–681 (2022). https://doi.org/10.1007/s11695-021-05785-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-021-05785-0