Abstract
Background
Recent developments of magnetic resonance imaging (MRI) and spectroscopy have made it possible to quantify lipid deposited in different tissues. To what extent an improvement of glucose tolerance shortly after Roux-en-Y gastric bypass surgery (RYGBP) is reflected in lipid levels in liver and skeletal muscle, markers of insulin resistance, has not been clarified.
Methods
Whole-body MRI and MR spectroscopy (MRS) of liver and muscle and measurements of biochemical markers of glucose and lipid metabolism were performed at baseline and 1, 6, and 12 months following surgery in seven morbidly obese women. Volumes of adipose tissue depots and liver and muscle lipids were assessed from the MRI/MRS data.
Results
At 1 month postoperatively, body mass index and visceral and subcutaneous adipose tissues were reduced by 9%, 26%, and 10%, respectively, whereas no reductions in intrahepatocellular or skeletal intramyocellular lipid concentrations were found. Free fatty acid and beta-hydroxybutyrate levels were elevated two- and sixfold, respectively; glucose and insulin levels were lowered, indicating increased insulin sensitivity. Further weight loss up to 1 year was associated with reductions in all investigated lipid depots investigated, with the exception of the intramyocellular compartment.
Conclusion
RYGBP causes rapid lipid mobilization from visceral and subcutaneous adipose depots and enhanced free fatty acid flux to the liver. An exceptional disconnection between liver fat and insulin sensitivity occurs in the early dynamic phase after surgery. However, in the late phase, the energy restriction imposed by the surgical procedure also reduces the liver lipids, but not the intramyocellular lipids.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bariatric surgery is proven to be one of the most effective treatments for morbid obesity. It is accompanied by sustained weight loss and improvements in obesity-associated morbidities, as recently well documented in a large 10-year follow-up [1]. Remarkably, rapid improvement in glucose tolerance, even reversal of type 2 diabetes, can be seen already at a time when the effect on weight loss is minor [2–4]. This is most pronounced after Roux-en-Y gastric bypass (RYGBP) and after biliopancreatic diversion (BPD). The improvement in glucose tolerance is mainly due to improved insulin sensitivity rather than increased insulin secretion, as both fasting glucose and fasting insulin levels fall after surgery. The increased insulin sensitivity has also been demonstrated by the use of euglycemic–hyperinsulinemic clamps in patients operated on with BPD [3, 4]. The mechanism behind the reversal of insulin resistance after gastric surgery is not fully understood and is the focus of much current interest, including studies in experimental animals [5].
In morbid obesity, the body fat mass is expanded by excess lipid deposition in adipose tissues, as well as in other organs. Visceral fat is metabolically more active than subcutaneous fat [6] and is considered to play a main role for the development of dysmetabolic state with insulin resistance and hyperlipidemia [7, 8]. Fat deposited in liver [9] and skeletal muscle [10, 11] also seem to be of importance in this regard and are surrogate markers of insulin resistance. In obesity and type 2 diabetes, increased liver fat is commonly found and is related to insulin resistance, dyslipidemia, and visceral adiposity [12].
Recent developments in magnetic resonance imaging (MRI) and spectroscopy have made it possible to quantify lipid deposited in different tissues. The purpose of this study was, therefore, to investigate the relationship between alterations in lipid depots and markers of insulin resistance following RYGBP.
Materials and Methods
Seven morbidly obese women, mean age 35 years (range 22–47), mean weight 122 kg (range 97–137), and mean body mass index (BMI) 43.7 kg/m2 (range 38–48), were recruited from the obesity program at Samariterhemmet Hospital in Uppsala, Sweden. None of the subjects had a specific hepatic disease disorder resulting in fatty liver or a history of alcohol abuse. Written consent was obtained from all subjects. The patients underwent RYGBP at the Department of Surgery at Uppsala University Hospital. The surgical procedure was performed by creating a small proximal gastric pouch at the lesser curvature of the stomach, which was totally separated. The jejunum was divided 20–30 cm distal to the ligament of Treitz, and a 70-cm Roux-limb was made. The small bowel continuity was restored by a jejunojejunostomy. The Roux-limb was placed retrocolic, retrogastric, and anastomosed to the small gastric pouch directly below the esophagus [13]. Thus, the main stomach and duodenum are bypassed and all ingested food will pass directly into the proximal jejunum. Preoperatively, a whole-body MRI scan was done, blood was taken, and body height and weight were measured to determine the BMI. At 1, 6, and 12 months postoperatively, these procedures were repeated.
Blood Analyses
Blood was collected after an overnight fast and sera and plasma were prepared and stored at −70°C until analysis. Fasting plasma glucose, total cholesterol, low-density lipoprotein (LDL)-cholesterol, high-density lipoprotein (HDL)-cholesterol, triglycerides, and free fatty acids (FFAs) were measured with routine clinical chemistry laboratory techniques. Insulin was analyzed with an AutoDELFIA automatic immunoassay system (Wallac Oy, Turku, Finland). All analyses were performed at Uppsala University Hospital. Beta-hydroxybutyrate was spectrophotometricaly determined in plasma with an enzymatic end-point method [14]. Leptin and adiponectin were analyzed in plasma samples as described [15]. The homeostasis model assessment (HOMA) index was calculated by multiplying fasting plasma glucose and fasting serum insulin and then dividing by 22.5 [16].
Magnetic Resonance Imaging
Whole-body imaging was performed using an axial T1-weighted spoiled gradient echo acquisition [repetition time (TR)/echo time (TE) = 180/4.6 ms] with two different flip angles (30 and 80 degrees), and T1-mapping was performed prior to segmentation of the adipose tissue, as described previously [17]. Visceral and subcutaneous adipose tissues were manually segmented in a volume centered at the L4–L5 interface and extended +/−8 cm in the cranial/caudal direction. Total adipose tissue was automatically segmented from the T1-map and included all adipose tissue in the body. Single volume localized 1H-spectroscopy of the liver was performed using a 3 × 3 × 3-cm3 volume of interest positioned in the right lobe of the liver avoiding major vessels and bile ducts using a TR/TE of 3,000/30 ms with 16 excitations without water suppression and 64 with water suppression. The analysis was performed using MRUI (version 2.2; reference [18]) using water as internal reference giving intrahepatocellular lipid levels as output in percent. High spatial resolution spectroscopic imaging of the calf muscles was performed assessing the intramyocellular (IMCL) and extramyocellular (EMCL) lipid levels (volume %). The acquisition and analysis techniques used have been described previously [19].
Statistics
The data obtained at 1, 6, and 12 months were subtracted from the baseline values for all measurements in the study. To test if the sample significantly differed from zero, a one-sample analysis was performed using a two-tailed t test for each sample at each time. The p values for each sample were calculated.
Results
Biochemistry at Baseline and Following Surgery
RYGBP led to a mean reduction in body weight of 34% at 1 year after surgery and was accompanied by marked changes in glucose and lipid metabolism, as detailed in Table 1. At 1 month, the glucose levels were lowered in 6/7 and the insulin levels in 7/7 of the women from 5.84 to 5.4 mmol/L (mean values) and from 23.6 to 15.3 mU/L, respectively. The mean BMI was reduced by 9%. FFA and 3-hydroxybutyrate levels were elevated by 103% and 578%, respectively. The beta-hydroxybutyrate levels then gradually fell and approached preoperative levels at 1 year, indicating that the patients at this time point were close to weight-stable. HDL levels tended to increase and the LDL levels to decrease after surgery; the ratio was significantly altered at 6 and 12 months, and total cholesterol was not changed. Triglycerides levels tended to be reduced at the end of the study compared to baseline. Leptin fell at 1 month by 35% and further thereafter. Adiponectin concentrations were not different at 1 month, but they were increased at 1 year. The relative changes in biochemical variables are detailed in Table 2 and, in part, are illustrated in Fig. 1.
Changes in Lipid Depots After Surgery
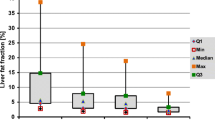
The visceral fat depot was reduced by 26% at 1 month, 50% at 6 months, and 73% at 1 year, and the subcutaneous fat by 10%, 37%, and 53%, respectively. Hepatic lipid levels were reduced at 6 and 12 months, but not at 1 month. EMCL, but not IMCL, was reduced at 6 and 12 months. At 6 months, mean liver volume was diminished by 21% and by 30% at 1 year. Estimated reductions of total lipid content in the liver were 13%, 75%, and 93%, respectively. The dynamics of the changes in the various lipid depots are illustrated in Fig. 2. It is notable that, at 1 month, the marked reduction in visceral fat contrasted with no change in hepatic lipid concentration. A surface reconstruction from the MRI data of the whole body at all time points showing subcutaneous adipose tissue is displayed in Fig. 3.
Discussion
We observed a rapid reduction in visceral and subcutaneous fat with elevations of FFA and beta-hydroxybutyrate levels, due to enhanced lipid turnover following RYGBP surgery. At 1 month postoperatively, fasting glucose was lowered by 7% and insulin was lowered by 34%, and HOMA, surrogate marker of insulin resistance, was reduced to a mean of 4.0. Such values corresponded to normal insulin sensitivity determined by euglycemic, hyperglycemic clamp in healthy individuals [20]. In accordance, improved insulin sensitivity has been shown in morbidly obese patients by HOMA and intravenous glucose tolerance test 6 days after RYGBP [2] and by hyperinsulinemic glucose clamping 10 days after BPD [3]. In the present study at the 1-month MR examination, no reduction in liver fat concentration was determined. Conceivably, this resulted from high hepatic exposure to FFAs due to lipid mobilization from predominantly the visceral depot. In support of this notion is an increase in liver fat observed in overweight subjects fed an isocaloric diet containing 56% fat, whereas an isocaloric diet with 16% fat reduced the initial fat content [21]. These changes in liver fat were accompanied by corresponding changes in circulating insulin levels, in good agreement with the close relationship between liver fat and insulin sensitivity repeatedly observed in studies of normal subjects [22] and different patient populations [23]. The present findings at 1 month on liver fat and insulin levels demonstrate that the typical relationship between fat content and insulin sensitivity does not apply to the early, dynamic phase after gastric bypass surgery.
Reductions in hepatic lipid levels upon diet-induced weight loss have been reported in overweight, obese, and morbidly obese individuals (Table 3). In the recent study of obese subjects [24], 3 months of a hypocaloric diet reduced body weight by 6% and intrahepatic lipid (IHL) by 5%. Larson-Meyer et al. [25] studied overweight subjects over a 6-month period with 10–14% weight loss and noted 29–40% reduction in IHL. In obese, nondiabetic women, 8% reduction in body weight after 5 months of a hypocaloric diet was associated with 39–49% reduction in IHL [26]. The most marked reduction in hepatic lipids has been reported in patients with type 2 diabetes—8% weight loss after 7 weeks of a low-calorie diet was associated with 81% reduction in IHL [27].
Reduced liver volume, in particular, the left lobe, is sought after by many surgeons to facilitate the operation, as an enlarged liver may obscure the view and, in fact, prevent placement of the liver retractor in laparoscopic procedures. Traction to a fatty liver may also cause trauma with significant bleeding. Preoperative weight loss programs are therefore frequently employed and claimed to facilitate surgery to some extent [28–30]. A very-low-calorie diet in morbidly obese subjects for 2 weeks resulted in a 5% reduction in liver size, as estimated by repeated ultrasound measurements of the left liver lobe [31]. The same diet for 6 weeks led to about 15% reduction in liver size, determined by MRI [32]. In a third study, the same very-low-calorie diet over 12 weeks led to a reduction in liver volume by a mean of 18.7%, range 20 to −51.6%, determined by computed tomography; 80% of the volume reduction took place during the first 2 weeks [33].
Thus, it seems that liver volume can be reduced by a few weeks of a very-low-calorie diet. Subjects with the largest IHL, who typically are insulin-resistant [23], are likely to obtain the largest effect, as indicated from the report of identical weight loss producing different extents of liver fat reduction, in a high-fat group from 9.4% to 4.8% and in a low-fat group from 3.2% to 2.0% [26]. The present study is, to our knowledge, the first prospective report on hepatic and myocellular lipid content after bariatric surgery. Previously, Busetto et al. [34] have noted a reduction in liver size 8 weeks after gastric banding; hepatic lipid concentration was not determined (cf. data summarized in Table 3).
IMCL lipid content correlates with insulin sensitivity [35], and short-term starvation is associated with IMCL accumulation [36, 37]. No reduction in IMCL lipid during weight loss on hypocaloric diet was observed [24], and likewise, no lowering of soleus muscle fat was found in type 2 diabetes after weight reduction [27]. We did not observe depletion of IMCL up to 1 year after RYGBP. In contrast, IMCL fat reduction was observed 6 months after BPD [38], possibly explained by the marked lipid disturbance and malabsorption that occurs after this type of surgery.
In short, RYGBP excludes the stomach and duodenum from the passage of food, induces massive weight loss, and is accompanied by altered secretion of gastrointestinal peptides such as GLP-1, PP, and PYY [5, 39, 40]. The energy restriction causes mobilization of fat from visceral and subcutaneous depots and enhanced FFA flux to the liver. In the early phase after surgery, hepatic lipid levels are not reduced, although, as reflected in lowered glucose and insulin levels, insulin sensitivity is enhanced. Possibly altered gastrointestinal signaling contributes to this exceptional outcome, the details of which remain to be clarified.
References
Sjöström L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683–93.
Wickremesekera K, Miller G, Naotunne TD, et al. Loss of insulin resistance after Roux-en-Y gastric bypass surgery: a time course study. Obes Surg. 2005;15:474–81.
Mari A, Manco M, Guidone C, et al. Restoration of normal glucose tolerance in severely obese patients after bilio-pancreatic diversion: role of insulin sensitivity and beta cell function. Diabetologia. 2006;49:2136–43.
Guidone C, Manco M, Valera-Mora E, et al. Mechanisms of recovery from type 2 diabetes after malabsorptive bariatric surgery. Diabetes. 2006;55:2025–31.
Rubino F, Forgione A, Cummings DE, et al. The mechanism of diabetes control after gastrointestinal bypass surgery reveals a role of the proximal small intestine in the pathophysiology of type 2 diabetes. Ann Surg. 2006;244:741–9.
Arner P. Human fat cell lipolysis: biochemistry, regulation and clinical role. Best Pract Res Clin Endocrinol Metab. 2005;19:471–82.
McGarry JD. Banting lecture 2001: dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes. 2002;51:7–18.
Nielsen S, Guo Z, Johnson CM, et al. Splanchnic lipolysis in human obesity. J Clin Invest. 2004;113:1582–8.
Yki-Järvinen H, Westerbacka J. The fatty liver and insulin resistance. Curr Mol Med. 2005;5:287–95.
Kelley DE, Goodpaster BH, Storlien L. Muscle triglyceride and insulin resistance. Annu Rev Nutr. 2002;22:325–46.
Sinha R, Dufour S, Petersen KF, et al. Assessment of skeletal muscle triglyceride content by (1)H nuclear magnetic resonance spectroscopy in lean and obese adolescents: relationships to insulin sensitivity, total body fat, and central adiposity. Diabetes. 2002;51:1022–7.
Kelley DE, McKolanis TM, Hegazi RA, et al. Fatty liver in type 2 diabetes mellitus: relation to regional adiposity, fatty acids, and insulin resistance. Am J Physiol Endocrinol Metab. 2003;285:E906–16.
Sundbom M, Gustavsson S. Randomized clinical trial of hand-assisted laparoscopic versus open Roux-en-Y gastric bypass for the treatment of morbid obesity. Br J Surg. 2004;91:418–23.
Wildenhoff KE. A micro-method for the enzymatic determination of acetoacetate and 3-hydroxybutyrate in blood and urine. Scand J Clin Lab Invest. 1970;25:171–9.
Holdstock C, Edén Engström B, Öhrvall M, et al. Ghrelin and adipose tissue regulatory peptides: effect of gastric bypass surgery in obese humans. J Clin Endocrinol Metab. 2003;88:3177–83.
Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9.
Kullberg J, Angelhed JE, Lönn L, et al. Whole-body T1 mapping improves the definition of adipose tissue: consequences for automated image analysis. J Magn Reson Imaging. 2006;24:394–401.
Naressi A, Couturier C, Devos JM, et al. Java-based graphical user interface for the MRUI quantitation package. MAGMA. 2001;12:141–52.
Weis J, Johansson L, Courivaud F, et al. Quantification of intramyocellular lipids in obese subjects using spectroscopic imaging with high spatial resolution. Magn Reson Med. 2007;57:22–8.
Lind L, Berne C, Lithell H. Prevalence of insulin resistance in essential hypertension. J Hypertens. 1995;13:1457–62.
Westerbacka J, Lammi K, Häkkinen AM, et al. Dietary fat content modifies liver fat in overweight nondiabetic subjects. J Clin Endocrinol Metab. 2005;90:2804–9.
Westerbacka J, Cornér A, Tiikkainen M, et al. Women and men have similar amounts of liver and intra-abdominal fat, despite more subcutaneous fat in women: implications for sex differences in markers of cardiovascular risk. Diabetologia. 2004;47:1360–9.
Yki-Järvinen H. Fat in the liver and insulin resistance. Ann Med. 2005;37:347–56.
Sato F, Tamura Y, Watada H, et al. Effects of diet-induced moderate weight reduction on intrahepatic and intramyocellular triglycerides and glucose metabolism in obese subjects. J Clin Endocrinol Metab. 2007;92:3326–9.
Larson-Meyer DE, Heilbronn LK, Redman LM, et al. Effect of calorie restriction with or without exercise on insulin sensitivity, β-cell function, fat cell size, and ectopic lipid in overweight subjects. Diabetes Care. 2006;29:1337–44.
Tiikkainen M, Bergholm R, Vehkavaara S, et al. Effects of identical weight loss on body composition and features of insulin resistance in obese women with high and low liver fat content. Diabetes. 2003;52:701–7.
Petersen KF, Dufour S, Befroy D, et al. Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes. 2005;54:603–8.
Alami RS, Morton JM, Schuster R, et al. Is there a benefit to preoperative weight loss in gastric bypass patients? A prospective randomized trial. Surg Obes Relat Dis. 2007;3:141–5.
Alvarado R, Alami RS, Hsu G, et al. The impact of preoperative weight loss in patients undergoing laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2005;15:1282–6.
Liu RC, Sabnis AA, Forsyth C, et al. The effects of acute preoperative weight loss on laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2005;15:1396–402.
Fris RJ. Preoperative low energy diet diminishes liver size. Obes Surg. 2004;14:1165–70.
Lewis MC, Phillips ML, Slavotinek JP, et al. Change in liver size and fat content after treatment with Optifast very low calorie diet. Obes Surg. 2006;16:697–701.
Colles SL, Dixon JB, Marks P, et al. Preoperative weight loss with a very-low-energy diet: quantitation of changes in liver and abdominal fat by serial imaging. Am J Clin Nutr. 2006;84:304–11.
Busetto L, Tregnaghi A, De Marchi F, et al. Liver volume and visceral obesity in women with hepatic steatosis undergoing gastric banding. Obes Res. 2002;10:408–11.
Krssak M, Falk Petersen K, Dresner A, et al. Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H NMR spectroscopy study. Diabetologia. 1999;42:113–6.
Stannard SR, Thompson MW, Fairbairn K, et al. Fasting for 72 h increases intramyocellular lipid content in nondiabetic, physically fit men. Am J Physiol Endocrinol Metab. 2002;283:E1185–91.
Johnson NA, Stannard SR, Rowlands DS, et al. Short-term suppression of plasma free fatty acids fails to improve insulin sensitivity when intramyocellular lipid is elevated. Diabet Med. 2006;23:1061–8.
Greco AV, Mingrone G, Giancaterini A, et al. Insulin resistance in morbid obesity: reversal with intramyocellular fat depletion. Diabetes. 2002;51:144–51.
Borg CM, le Roux CW, Ghatei MA, et al. Progressive rise in gut hormone levels after Roux-en-Y gastric bypass suggests gut adaptation and explains altered satiety. Br J Surg. 2006;93:210–5.
Morinigo R, Moize V, Musri M, et al. Glucagon-like peptide-1, peptide YY, hunger, and satiety after gastric bypass surgery in morbidly obese subjects. J Clin Endocrinol Metab. 2006;91:1735–40.
Acknowledgements
We thank Margareta Ericson for expert technical assistance and Elisabeth Olsson for excellent care and samplings. This study was supported by Uppsala University, the Novo Nordisk Fund, and Ernfors Fund.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Johansson, L., Roos, M., Kullberg, J. et al. Lipid Mobilization Following Roux-en-Y Gastric Bypass Examined by Magnetic Resonance Imaging and Spectroscopy. OBES SURG 18, 1297–1304 (2008). https://doi.org/10.1007/s11695-008-9484-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-008-9484-0